2019.04.01.11
Files > Volume 4 > Vol 4 No 1 2019
REVISION/REVIEW
Unravelling the endometrium: a pictorial review of saline infusion sonohysterography in the evaluation of abnormal uterine bleeding.
Saika Amreen1, Naseer A2. Choh, Yawar Yaseen3, Cimona Lyn Saldanha4, Manjeet Singh5, Tariq A. Gojwari6, Feroze Shaheen7. Irfan Robbani8, Sheikh Riaz Rasool
Available from: http://dx.doi.org/10.21931/RB/2019.04.01.11
ABSTRACT
This article describes the diagnosis of causes of abnormal uterine bleeding with experience of the biggest medical institute in Kashmir, India. We work in a low resource setting where unavailabity of hysteroscopy made us acknowledge the accuracy and efficacy of saline infusion sonohysterography in diagnosis of patients with AUB thus helping guide their management.
INTRODUCTION
Abnormal uterine bleeding (AUB) is defined as an alteration in the volume, pattern, and duration of menstrual blood flow and is the most common reason for gynecologic referrals(1). In India, the prevalence of AUB is reported to be around 17.9%(2). Women with abnormal uterine bleeding have a considerably lower quality of life than the average population with a multitude of symptoms. (3)
The PALM-COEIN Classification System for causes of Abnormal Uterine Bleeding
P PolypsA AdenomyosisL LeiomyomaM MalignancyC CoagulopathyO Ovulatory disordersE EndometrialI IatrogenicN Not classified(4)
In about 25% of patients, abnormal uterine bleeding is the result of a well defined organic abnormality(5)
For the initial assessment of possible gynecologic abnormalities, pelvic ultrasonography is most often used. (6) The introduction of saline infusion sonohysterography (SIS) has been a significant advance in the evaluation of the endometrial cavity. SIS provides an unparalleled, clear, enhanced view of the endo myometrial complex that cannot be obtained with transvaginal sonography (TVS) alone. (7) Sonohysterography is a technique in which the endometrial cavity is distended with saline, allows evaluation of the single layer of the endometrial lining and enables the radiologist to reliably distinguish focal from diffuse endometrial pathologic conditions.(8) SIS was first described in 1981 by Nannini et al.(9)
a. 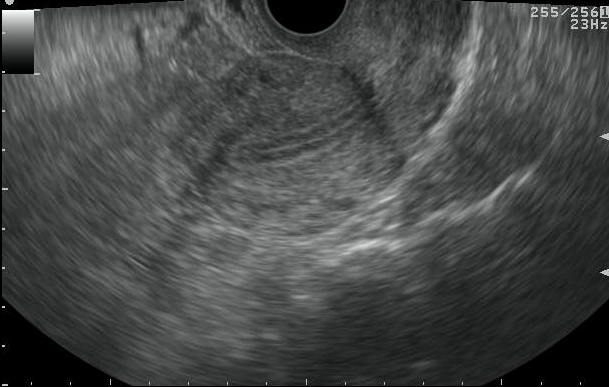
b 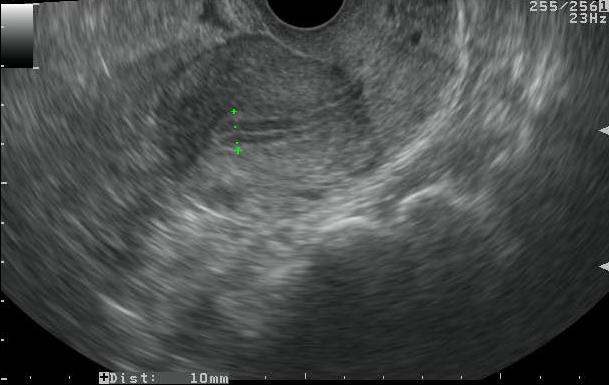
Figure 1. a. Visualization of endometrium as a trilaminar echo on TVS.
b. Placement of electronic calipers for measurement of endometrial thickness on TVS
TECHNIQUE OF SALINE INFUSION SONOHYSTEROGRAPHY
Performance of SIS after induced or natural menses minimizes confusion because at that time functions should be entirely shed, leaving only the basalis layer. (10) In premenopausal women, the best time to perform SIS is in the proliferative phase (first half) of the menstrual cycle. In postmenopausal women taking HRT, the SIS should be scheduled approximately six days after the last progestin pill when the endometrium is thinnest. (11) The baseline ultrasound study (transabdominal and transvaginal) is performed first.(10) A speculum is used to allow visualization of the cervix. After cleansing the external os, the cervical canal is catheterized using aseptic technique, and normal saline is instilled slowly using manual injection under real-time sonographic imaging into the endometrial cavity. (12) The saline distends the cavity, pushing the opposed walls of the endometrium apart. The anechoic fluid is then juxtaposed against the echogenic endometrium, giving exquisite detail of the uterine lining. (13)
a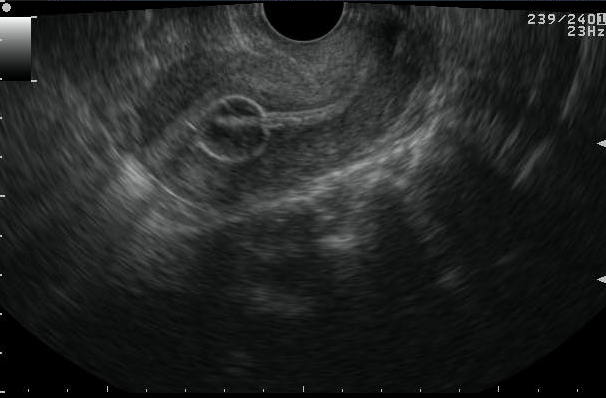
b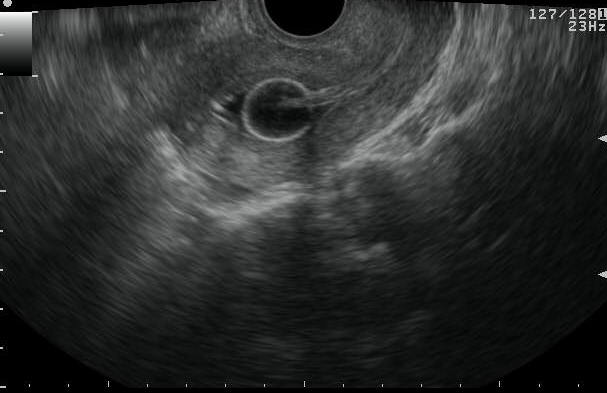
c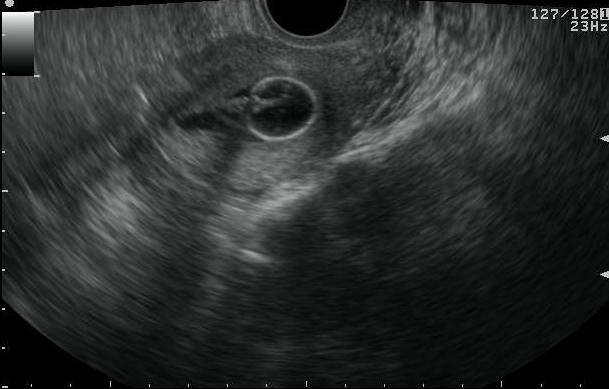
d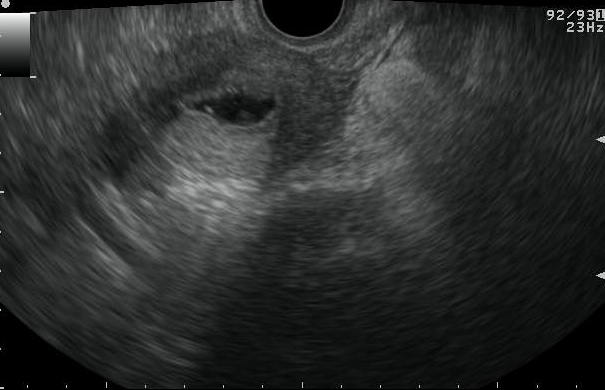
Figure 2 Saline infusion sonohysterography
a. TVS image revealing catheter in situ with bulb inflated with salineb. Saline infusion is started and begins filling the endometrial cavityc. d. Endometrial layers separated by saline
EVALUATION OF ENDOMETRIUM
Endometrial thickness should be measured on a sagittal (long-axis) image of the uterus, and the measurement should be performed on the thickest portion of the endometrium, excluding the hypoechoic inner myometrium. It is a ‘‘double-thickness’’ measurement from basalis to basalis.(11) If the fluid is seen within the endometrial cavity, the individual wall thicknesses of the 2 sides of the endometrium are summed, excluding the intervening fluid.(13) This central quality assurance reading provides a consistent evaluation of endometrial thickness on US image.(14)
THE NORMAL ENDOMETRIUM
Normal endometrium should be uniform in thickness, homogeneous in echotexture, and not displaced by any submucosal, myometrial abnormality.(8) A thin endometrium of 5 mm or less had a high negative predictive value.(13) In premenopausal patients, a thickness greater than 8mm during proliferative phase or more excellent than 16mm during the secretory phase is considered abnormal. (15)
P - ENDOMETRIAL POLYPS
Endometrial polyps are a common gynecologic condition associated with symptoms of AUB. They account for 39% and 21% to 28% of pre- and postmenopausal women, respectively(1).
Endometrial polyps may be visualized at transvaginal ultrasound as nonspecific endometrial thickening (16). Preservation of the endometrial-myometrial interface and echogenic appearance have been described as features of a typical polyp. (8) Some of the atypical features of polyps include cystic components, multiplicity, a broad base, and hypoechogenicity or heterogeneity that may indicate hemorrhage, infarction, or inflammation within the polyp. A small percentage of endometrial polyps may contain malignant foci or foci of endometrial hyperplasia. (17)(17)
Addition of Color Doppler to sonohysterography may help distinguish an endometrial polyp, which usually has a single feeding vessel – the pedicle artery, from an intracavitary submucosal fibroid, which usually has several vessels arising from the inner myometrium. (18)
a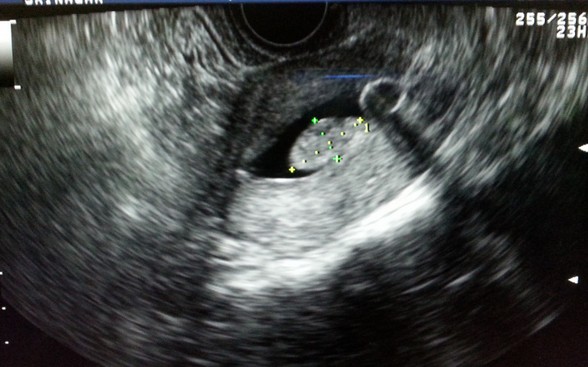

b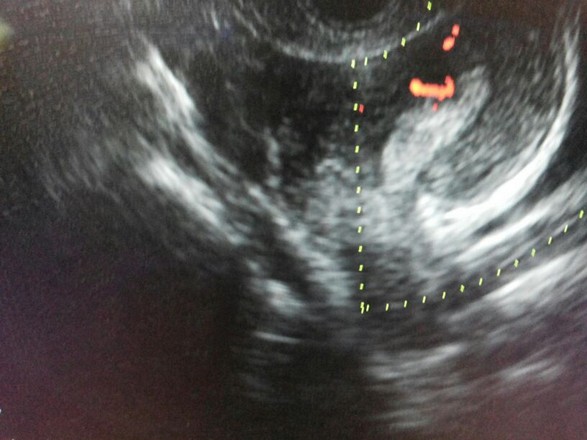

c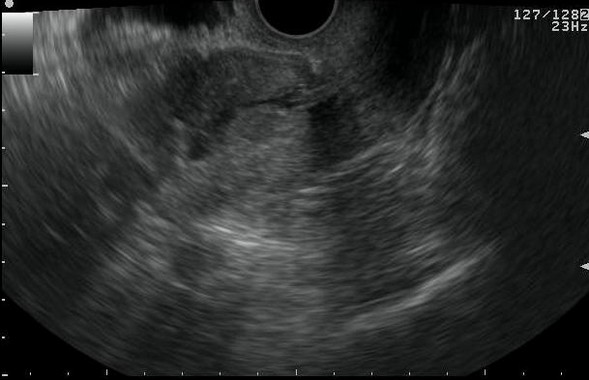
d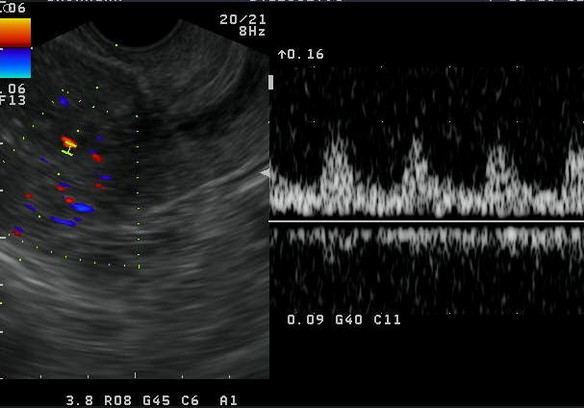
e 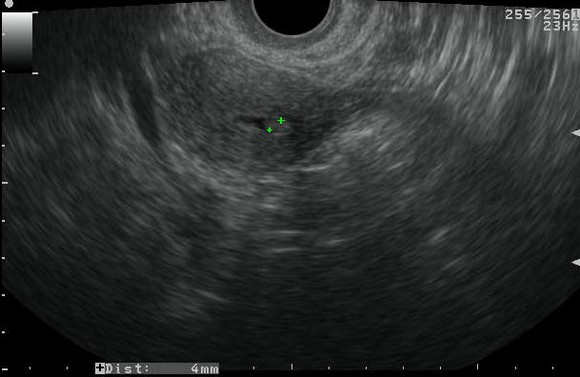
f 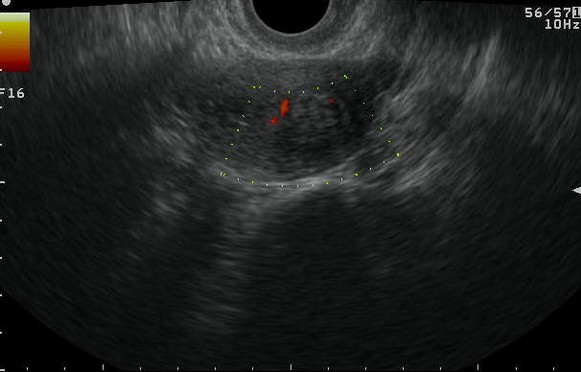
Figure 3. Endometrial Polyp
a. Saline infusion reveals broad based, echogenic lesion on posterior aspect of endometrial cavity – polyp.b. Demonstration of the pedicle artery by color doppler examinationc. Another patient with SIS revealing an echogenic polyp within the endometrial cavityd. Arterial waveform obtained from pedicle artery of the above endometrial polype. A very small endometrial polyp identified by SIS in a patient with metrorrhagia with otherwise normal imagingf. Identification of pedicle artery by power doppler in the same patient
A - ADENOMYOSIS
Adenomyosis is a common benign disease of the uterus characterized by ectopic endometrial glands and stroma within the myometrium associated with surrounding smooth-muscle hypertrophy(19). Approximately 70% of patients with adenomyosis have symptoms of AUB; 30% have symptoms of dysmenorrhea; and 19% present with both(1). Diffuse globular uterine enlargement is a common sonographic manifestation of adenomyosis(17).
The reported frequency of adenomyosis varies widely, ranging from 5 to 70% (20). The most common sonographic finding in adenomyosis on SIS is asymmetric myometrial thickening and heterogeneous echotexture. The second most common findings include myometrial cracks and myometrial cysts followed by an indistinct endo-myometrial junction(19).
a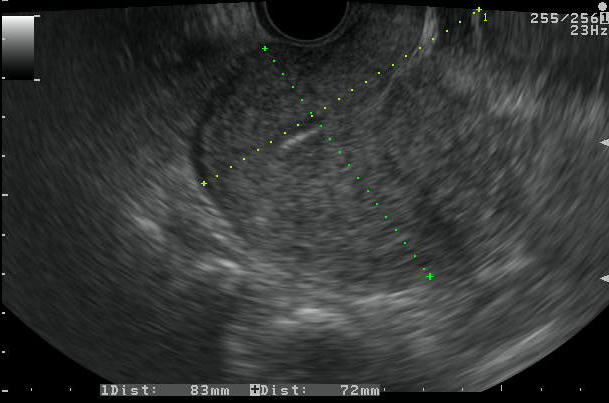
b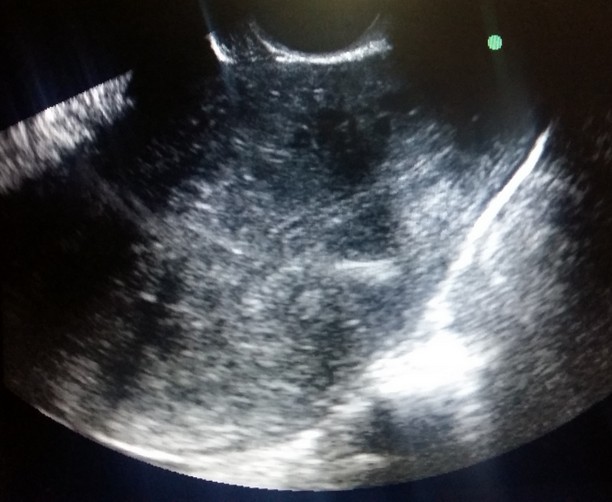

Figure 4. Adenomyosis
a. TVS images revealing enlarged, globular uterine morphology with heterogenous myometrium and thick irregular endomyometrial junction. SIS demonstrated normal endometrium will poor distensibilityb. Another patient with thick EMJ and multiple myometrial cysts with SIS showing normal endometrium with diagnosis of adenomyosis
L - LEIOMYOMAS
Uterine fibroids are the most common benign tumor of the female genital tract(1). The PALM-COEIN system adds categorization of intramural and subserosal myomas as well as a category that includes lesions (‘‘parasitic’’) that appear to be detached from the uterus(4). Submucous myomas are most likely to cause menorrhagia(21).
Fibroids are commonly identified at US as hypoechoic solid masses, but they may be heterogeneous or hyperechoic, depending on the degree of degeneration and calcification. Fibroids tend not to interrupt the endometrium unless they are submucosal in location. Submucosal fibroids may distort the uterine cavity with varying degrees of intracavitary extension and are best visualized at sonohysterography(16). The addition of SIS not only shows hypoechoic, smooth rounded masses with an overlying echogenic endometrium and some degree of acoustic shadowing, but also clearly demonstrates the wall of origin, the exact localization (intramural or submucosal), and the extension into the uterine cavity (22). This feature is important because only those fibroids in which at least 50% of the mass projects into the endometrial cavity may be removed hysteroscopically.(23)
Differentiation of endometrial polyp from submucosal fibroid at sonohysterography is most effectively done by echotexture assessment and identification of an overlying echogenic endometrium. The classic submucosal fibroid is hypoechoic with shadowing and similar in texture to the myometrium with an overlying echogenic endometrium defining the subendometrial location. Because a submucosal fibroid may present almost entirely within the endometrial cavity as does a polyp, location is not a reliable distinguishing feature. (9)
a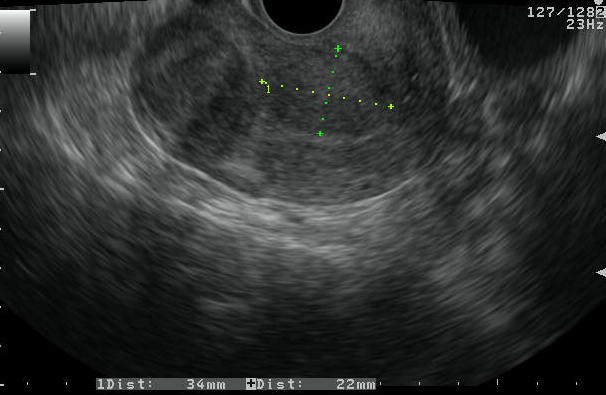
b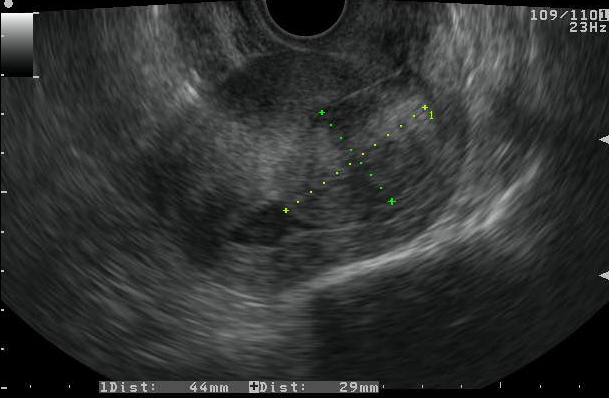
c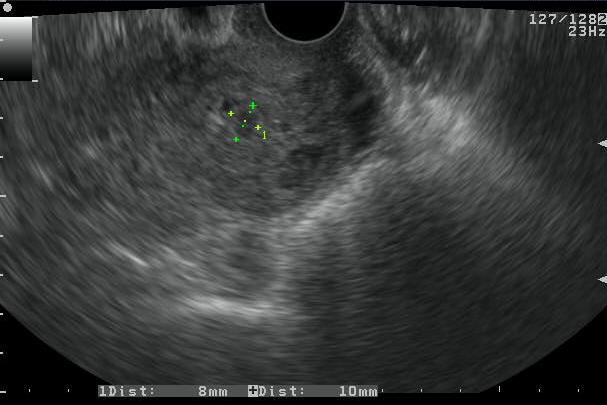
d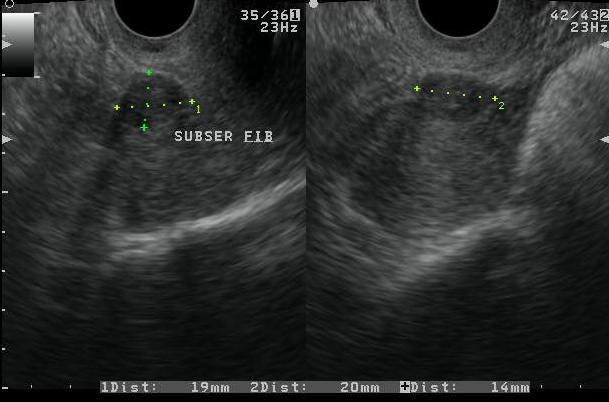
Figure 5. Leiomyomas
a. TVS images showing patient with intramural and submucous fibroids.
b. A large submucous fibroid shown on SIS image with significant protrusion into the endometrial cavity
c. A small submucous fibroid identified in a patient with SIS
d. TVS images showing subserosal fibroids in a patient
E- ENDOMETRIAL HYPERPLASIA
Endometrial hyperplasia is an abnormal proliferation of endometrial stroma and glands and represents a spectrum of endometrial changes ranging from glandular atypia to frank neoplasia. A definitive diagnosis can be made only with biopsy, and imaging cannot reliably allow differentiation between hyperplasia and carcinoma(16). It is the result of unopposed estrogens on the endometrium. It is most commonly encountered in perimenopausal women. (24)
On saline infusion sonohysterography, endometrial hyperplasia has been described as focal or diffuse thickening of the endometrium without a focal mass(25). A meta-analysis of 85 published studies that included 5892 women showed that an endometrial thickness of greater than 5 mm identified 96% of endometrial cancer. (26).Definitive criteria for distinguishing between endometrial hyperplasia and carcinoma have not been established, and both present with endometrial thickening.(25) The diagnosis of endometrial carcinoma should be suspected when the single layer of the endometrium is thicker than 8 mm, irregular, broad based, or poorly marginated or when the endometrial-myometrial interface is disrupted. However, endometrial thickness measurements often overlap in benign and malignant conditions(8).
Smaller endometrial thicknesses markedly reduce but do not exclude, the possibility of abnormalities such as polyps, hyperplasia, and cancer. (27)
a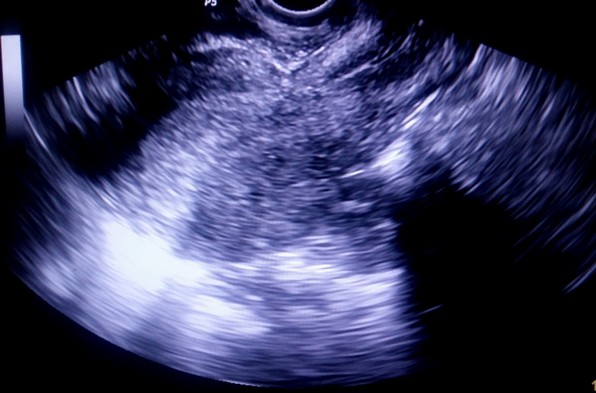

b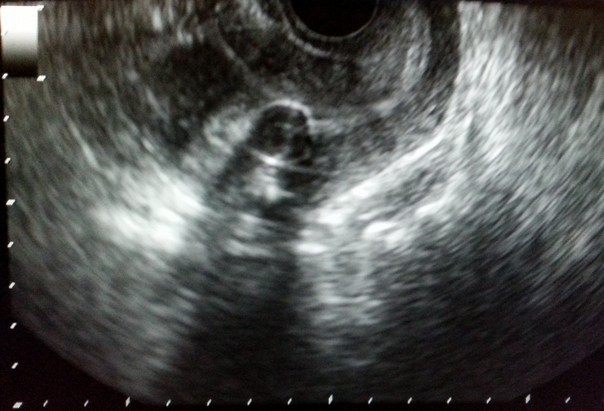

c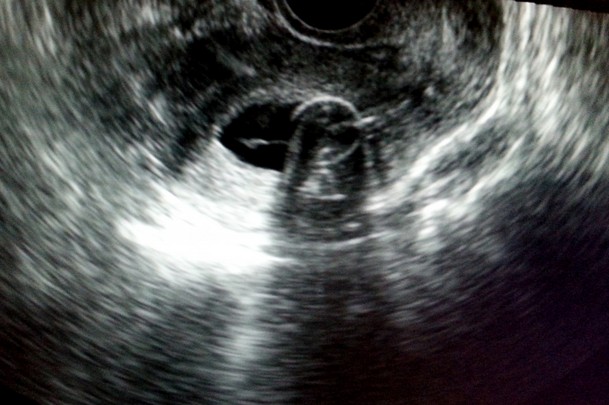

Figure 6. Endometrial hyperplasia
a. Sagittal section TVS image of uterus demonstrating increased endometrial thicknessb. c. Endometrial cavity distended with saline revealing no intraendometrial lesion, and smooth and regular endometrial contour.
SIGNIFICANCE
Initially, curettage was the ‘‘gold standard’’ for assessment of endometrium. First described in 1843, its performance in the hospital became the most common operation performed on women in the world. As early as the 1950s, a review of 6907 curettage procedures found the technique missed endometrial lesions in 10% of cases. Of these, 80% were polyps. (11)
Hysteroscopic evaluation for abnormal uterine bleeding is an option providing direct visualization of cavitary pathology and facilitating directed biopsy. (24) It is both sensitive and specific but is generally performed in an operating room under anesthesia, and requires specialized and highly skilled personnel, which increases the risks and costs. (28)
A saline hysterosonogram depicting focal abnormalities such as endometrial polyps or submucosal leiomyomas can guide the hysteroscopic resection of these masses. A saline hysterosonogram depicting myomas in a solely intramural location may be used to determine that an abdominal myomectomy is the optimal surgical approach for their resection. A saline hysterosonogram with specific negative findings can be extremely informative and obviate unnecessary procedures, and distinguish women who require medical therapy and investigation from those who require surgery. A normal endometrium on saline hystero sonography combined with a regular uterine appearance will direct the clinician to search for causes of vaginal bleeding other than anatomic causes, such as ovulatory versus anovulatory dysfunctional uterine bleeding or systemic disease. (25)
Safety and short-term effectiveness of hysteroscopy are now accepted for lesions limited to the uterine cavity. Submucous myomas up to 30 mm diameter and polyps can be resected endoscopically, allowing short hospital stay and quick recovery. (29) SIS on comparison outpatient diagnostic hysteroscopy is both less invasive to the patient and less expensive.(30) An approach using endometrial thickness measurement by TVS and reserving SIS for patients who have an endometrial thickness greater than 5 mm or an intracavitary abnormality visualized by TVS would be the effect to reduce the number of hysteroscopies.(31)
REFERENCES
1. Garza-Cavazos A, de Mola JRL. Abnormal Uterine Bleeding New Definitions and Contemporary Terminology. Female Patient (Parsippany). 2012;37(August):1–9.
2. Sharma A DY. Trends of AUB in tertiary centre of Shimla hills. J Midlife Health. 2013;4(1):67–8.
3. Fraser IS, Langham S, Uhl-Hochgraeber K. Health-related quality of life and economic burden of abnormal uterine bleeding. Expert Rev Obstet Gynecol. 2009;4(2):179–89.
4. Munro MG, Critchley HOD, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertil Steril. Elsevier Ltd; 2011;95(7):2204–8.e3.
5. Brenner P. Differential diagnosis of abnormal uterine bleeding. Am J Obstet Gynecol. 1996;175:766–9.
6. Langer JE, Oliver ER, Lev-toaff AS, Coleman BG. Imaging of the Female Pelvis through the Life Cycle 1. RadioGraphics. 2012;
7. Bradley LD, Falcone T, Magen AB. Radiographic imaging techniques for the diagnosis of abnormal uterine bleeding. Obstet Gynecol Clin North Am. 2000 Jun;27(2):245–76.
8. Davis PC, Neill MJO, Yoder IC, Lee SI, Mueller PR. Sonohysterographic Findings of Endometrial and Subendometrial Conditions. RadioGraphics. 2002;22:803–16.
9. Wei a. Y, Schink JC, Pritts E a., Olive DL, Lindheim SR. Saline contrast sonohysterography and directed extraction, resection and biopsy of intrauterine pathology using a Uterine Explora Curette. Ultrasound Obstet Gynecol. 2006;27(2):202–5.
10. Parsons AK. Saline infusion sonohysterography. Medica Mundi. 2001;45(July):29–41.
11. Goldstein SR. Abnormal Uterine Bleeding: The Role of Ultrasound. Ultrasound Clin. 2006;1(2):415–24.
12. AIUM Practice Guideline for the Performance of Sonohysterography. J ultrasound Med. 2012;
13. Berridge DL, Winter TC. Saline Infusion Sonohysterography. J ultrasound Med. 2004;23:97–112.
14. Bredella MA, Feldstein VA, Filly RA, Goldstein RB, Callen PW, Genant HK. Measurement of Endometrial Thickness at US in Multicenter Drug Trials : Value of Central Quality Assurance Reading 1. Radiology. 2000;217:10–2.
15. Jorizzo JR, Chen MYM, Carr JJ. Sonohysterography : The Next Step in the Evaluation of the Abnormal Endometrium. RadioGraphics. 1999;19:5117–30.
16. Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the Endometrium : Disease and Normal Variants. RadioGraphics. 2001;21:1409–24.
17. Yang T, Pandya A, Marcal L, Bude RO, Platt JF, Bedi DG, et al. Sonohysterography: Principles, technique and role in diagnosis of endometrial pathology. WJR. 2013;5(3):81–7.
18. Verrotti C, Benassi G, Caforio E, Nardelli GB. Targeted and tailored diagnostic strategies in women with perimenopausal bleeding: Advantages of the sonohysterographic approach. Acta Biomed l’Ateneo Parm. 2008;79(2):133–6.
19. Verma SK, Lev-toaff AS, Baltarowich OH, Bergin D, Verma M, Mitchell DG. Adenomyosis: Sonohysterography with MRI Correlation. AJR. 2009;192(April):1112–6.
20. Bromley B, Shipp TD, Benacerraf B. Adenomyosis: sonographic findings and diagnostic accuracy. J Ultrasound Med. 2000;19(8):529–34; quiz 535–6.
21. Becker E, Lev-toaff AS, Kaufman EP. The Added Value of Transvaginal Sonography Alone in Women With Known or Suspected Leiomyoma. J ultrasound Med. 2002;21:237–47.
22. Nass Duce M, Öz U, Özer C, Yildiz A, Apaydin FD, Çil F. Diagnostic value of sonohysterography in the evaluation of submucosal fibroids and endometrial polyps. Aust New Zeal J Obstet Gynaecol. 2003;43(6):448–52.
23. Williams PL, Laifer-Narin SL, Ragavendra N. US of Abnormal Uterine Bleeding. RadioGraphics. 2003;23:703–18.
24. Fairbanks J, Sams D. Abnormal Uterine Bleeding in Women. J Obstet ang Gynaecol Canada. 2013;35(5):S1–25.
25. Laifer-Narin S, Ragavendra N, Lu DSK, Perrella RR, Grant EG. Transvaginal saline hysterosonography: Characteristics distinguishing malignant and various benign conditions. AJR. 1999;172(June):1513–20.
26. Goldstein RB, Bree RL, Benson CB, Carlos R, Fleischer AC. Evaluation of the Woman With Postmenopausal Bleeding. J ultrasound Med. 2001;20:1025–36.
27. Timmerman D, Verguts J, Konstantinovic ML, Schoubroeck DVAN, Deprest J. The pedicle artery sign based on sonography with color Doppler imaging can replace second-stage tests in women with abnormal vaginal bleeding. Ultrasound Obstet Gynecol. 2003;22(May):166–71.
28. Bernard J., Lecuru F, Darles C, Robin F, Bievre P de, Taurelle R. Saline contrast sonohysterography as first-line investigation for women with uterine bleeding. Ultrasound Obstet Gynecol. 1997;10:121–5.
29. Bernard JP, Rizk E, Camatte S, Robin F, Taurelle R, Lecuru F. Saline contrast sonohysterography in the preoperative assessment of benign intrauterine disorders. Ultrasound Obstet Gynecol. 2001;17(2):145–9.
30. De Kroon CD, De Bock GH, Dieben SWM, Jansen FW. Saline contrast hysterosonography in abnormal uterine bleeding: A systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol. 2003;110(10):938–47.
31. Vries LD de, Dijkhuizen FPHLJ, Mol BWJ, Brolmann HAM, Moret E, Heintz PM. Comparison of transvaginal sonography, saline infusion sonography, and hysteroscopy in premenopausal women with abnormal uterine bleeding. J Clin Ultrasound. 2000;28(Jun):217–23.
Received: 16 December 2018
Accepted: 2 March 2019
Saika Amreen1, Naseer A2. Choh, Yawar Yaseen3, Cimona Lyn Saldanha4, Manjeet Singh5, Tariq A. Gojwari6, Feroze Shaheen7. Irfan Robbani8, Sheikh Riaz Rasool
Sher-I-Kashmir Institute of Medical Sciences. Soura. India
1Dr Saika Amreen. Senior Resident. Department of Radiodiagnosis & Imaging.SKIMS, Soura
2Dr Naseer Ahmad Choh. Associate Professor. Dept. Of Radiodiagnosis & Imaging.SKIMS, Soura
3Dr Cimona Saldanha. Associate Professor. Dept. Of Obstetrics & Gynaecology. SKIMS, Soura
4Dr Manjeet Singh. Professor.Dept. Of Radiodiagnosis & Imaging. SKIMS, Soura
5Dr Tariq Ahmad Gojwari. Professor Dept. Of Radiodiagnosis & Imaging. SKIMS, Soura
6Dr Feroze Shaheen. Professor Dept Of Radiodiagnosis & Imaging SKIMS, Soura
7Dr Irfan Robbani. Professor Dept. Of Radiodiagnosis & Imaging. SKIMS, Soura
8Dr Sheikh Riaz Rasool. Associate Professor. Dept. Of Radiodiagnosis & Imaging. SKIMS, Soura
