2022.07.04.70
Files > Volume 7 > Vol 7 No 4 2022
Estimation of DNA damage in the roots of Allium cepa exposed to heavy metals using comet assay
1University of Mosul: Mosul. Iraq [email protected]
2University of Mosul: Mosul. Iraq
*Corresponding author. [email protected]
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.70
ABSTRACT
Higher plants were used as a bioindicator of environmental toxicity to estimate the severe problems related to the health of living organisms and the environment. Allium cepa plant was used to evaluate the DNA damage caused by heavy metal exposure, the roots of A.cepa plant. They were treated with four concentrations (5, 10, 20 and 25 ppm) for each of the metals Cadmium, zinc, copper and lead. At the same time, concentrations (50, 100, 200 and 500 ppm) were used for the preservative (sodium benzoate). The comet assay, a sensitive and suitable test for assessing DNA damage caused by chemical exposure, was used in this study. The Comet's six characteristics were measured: Head intensity, Head DNA%, Tail length, Tail intensity, Tail DNA% and Tail moment. The results showed that the metals are causing the DNA damage of meristematic cells of the roots of the A. cepa plant, depending on the tail length from most to least effective Cadmium> zinc > sodium benzoate > copper > lead > wastewater. I consider that it is not necessary to write down these values. The results of this study confirm that the meristematic cells of the roots of A. cepa are a suitable model for detecting DNA damage analyzed by the comet assay.
Keywords: Toxic metals - Bio-indicator - Single cell gel electrophoresis (SCGE)
INTRODUCTION
Environmental pollution is one of the most critical problems facing the world today. Its relationship with the health of the environment and living organisms, including humans, has generated a growing global concern in this regard since the increase in human activity in recent decades has led to the emission of various types of pollutants in the environment, heavy metals are among the most dangerous of this pollutants1.
Lawal et al. 2 also mentioned that heavy metals might enter the human body by consuming contaminated drinking water or crops grown in contaminated soil. Heavy metals such as lead, mercury, Cadmium, and copper are toxins that cause environmental hazards and are considered toxic. These metals are essential sources of oxidative stress in the cell and play an important role in various human pathogens, such as carcinogenesis. Heavy metal toxicity exposure leads to brain damage, mental retardation, cerebral palsy, lung cancer, gastrointestinal abnormalities, and dermatitis. It has been shown that many metals directly modify and damage DNA by forming DNA adducts that induce chromosomal breaks.
A large number of chemical compounds have been added to meals by humans for various purposes, such as flavor, texture, and shelf-life extension. A food additive must be included in the food supply. A preservative is any chemical substance or solution of a substance that is added to food under scientific regulations where it is not the main ingredient of the food. Although preservatives can preserve food for a specific period, they have adverse effects on human health, especially those antimicrobial preservatives which are toxic to living organisms and mutagenic in various test systems 3,4. Among the types used as a food preservative is sodium benzoate, a sodium salt represented by the chemical formula C7H5O2Na, with a molecular weight of 144.1 g / mol. This odorless compound is soluble in water and ethanol, which has genotoxic and carcinogenic effects. In vivo studies have indicated the efficacy of sodium benzoate in causing anxiety and causing oxidative stress, and many toxic factors 5,6. Higher plants are recognized as excellent genetic models for detecting environmental mutations and are frequently used in ecological monitoring studies. Allium cepa has been used to assess DNA damage among plant species. The use of A. cepa as a test system to detect mutations dates back to the 1940s. It has been used to this day to evaluate many chemical agents, contributing to its further application in environmental monitoring. Allium cepa has the advantage of being a low-cost test. It is easily handled and has advantages over other short-term tests that require prior preparation of the tested samples7.
Comet assay or single-cell gel electrophoresis in meristematic cells of A. cepa roots was used to assess DNA damage to environmental pollutants. This assay was first developed by Ostling and Johansson in 1984 and was later revised by Singh in 1988. The assay is relatively low-cost, simple, fast and reliable. It gives reproducible results and can be studied independently of mitosis in addition to a small number of cells8,9.
The study aims to examine genome-wide DNA changes induced by various heavy metals (Cadmium, zinc, copper, lead), the preservative sodium benzoate (SB) and wastewater in the roots of Allium cepa using the comet assay.
MATERIALS AND METHODS
Sample preparation
Source of Allium cepa. A. cepa (2n=16) with a diameter of (1-2.5 cm) of the crystal cultivar was obtained from the local markets of Mosul city.
Treatment of onion roots with heavy metals
Standard solutions of the four metals cadmium, zinc, copper and lead were prepared for the following concentrations (5, 10, 20 and 25) ppm. Depending to the study by Abubacker et al. 10, as well as a sample of contaminated water was taken and prepared with two concentrations, diluted with a concentration of 50 ppm and an undiluted sample utilizing the law of mitigation, By dividing the final volume by the volume taken, one may calculate the number of dilutions. Also, standard solutions of the preservative sodium benzoate were prepared for the following concentrations (50, 100, 200 and 500) ppm Depending on the study Kostadinova et al. 11. More information should be given on the contaminated water samples and how it was diluted to 50 ppm should be clarified.
The bulbs were treated with heavy metals solutions for four days, while the preservative SB was treated for three days, after that the roots were cut with a length of (0.5-1.5 cm) and then placed in Clark solution (ethanol: acetic acid 1:3) for 24 hours. After the end of the treatment period, the roots are placed in 70% ethanol and kept at a temperature of 4oC֯ until the examination is performed.
Application of the comet assay (single-cell gel electrophoresis)
In this study, safe red dye was used, and 4µʟ of dye was added for both types of agaroses, then the glass slides were immersed in Normal Melting Point Agarose (1%NMPA), which was prepared (1.5mM EDTA; 30mM NaOH, Ph 12.3) and the drops were left to cool at room temperature.
The roots were taken after treating them with heavy metals, preservatives and wastewater and placed in an Eppendorf tube with the addition of 500 µʟ of Tris Mgcl2buffer solution consisting of (0.2MTris, pH7.5; 4mMMgcl2-6H2O; 0.5%W/V Triton X-100) and the roots were mashed well using to obtain the cell suspension, 100 µʟ of (Low Melting Point Agarose) 0.8% LMPA prepared from (1.5mM EDTA; 30mM NaOH, Ph 12.3) is mixed with 20µʟ of the cell suspension and mixed in an Eppendorf tube. The mixture is placed on top of the slide containing NMPA. While avoid bubbles, the slide cover is placed directly; then, the slides are placed on ice and left for 5 minutes. Then the slides were immersed in Lysing solution prepared from (1M NaCl;30mM NaOH;0.5% w/v SDS, Ph 12.3) for an hour. Slides are transferred to the relay tank containing the TBE solution and placed horizontally. The device was operated at a voltage of 60 V/cm so that the direction of the relay was from the negative pole to the positive pole for a period of 12 minutes. Slides are lifted from the migration basin and washed with distilled water three times. Slides were examined using a fluorescence microscope at 200X magnification 12.
Statistical analysis
Comet characteristics were analyzed using mean ± standard error. The significant levels of the different samples were also analyzed using SPSS 23 version for Windows software. p<0.01 and p<0.05 were set as statistical significance. The collected comet test photographs were examined. They were utilizing Comet Score™ software (TriTek Corp, Sumerduck, VA).
RESULTS
Due to the ability of the comet assay to detect low levels of DNA damage in different types of cells exposed to heavy metals, the comet assay represents a powerful tool for identifying DNA damage12. The results obtained from the comet assay are summarized as shown in tables (1) and (2); the values of the tables do not appear in the manuscript. The head intensity of the meristematic cells of onion plant roots exposed to heavy metals was estimated at different concentrations compared to the control treatment; it was noted that the head intensity property of both Cadmium and zinc at concentrations (5, 10, 20 and 25 ppm) was less than the control treatment at the probability level p<0.05, except for the 25 ppm concentration of cadmium metal which was at the probability level p<0.01. At the same time, the results of copper showed an increase of 833663.7±0.05774 for the concentration of 25 ppm at a probability level of p<0.05 compared with 479198±0.54874, while the rest of the concentrations of the same metal have lower values than the control treatment. Also, an increase in lead values was observed (506943 ± 0.54874, 527927.3 ± 0.05774 for concentrations (5 ppm and 20 ppm) at the probability level of p<0.01 and p<0.05, respectively, compared with the control treatment 479198 ±0.54874. While a difference was observed in the values of contaminated water (WW), where it was noted that the diluted treatment with a value of 1192037±0.05774 was higher than the control treatment at the p<0.05 probability level, while the undiluted water sample was 172811±0.54874 less than the control treatment at the p<0.01 level. As for the preservative SB, it was observed that the comet head density increased by 0.57735 ± 711886.9 for each of the concentrations 50 ppm and 100 ppm at the probability level of p<0.01 and p<0.05, respectively.
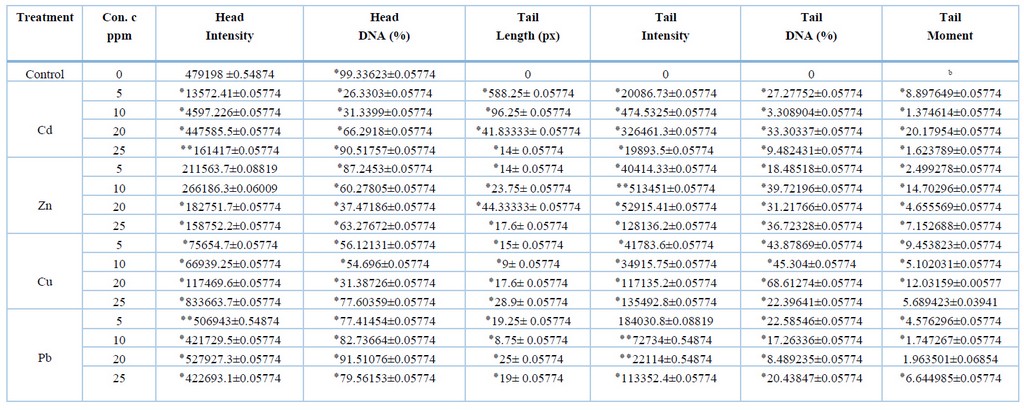
Table 1. Detection of DNA damage in meristematic cells of roots of Allium cepa exposed to heavy metals using the comet assay.
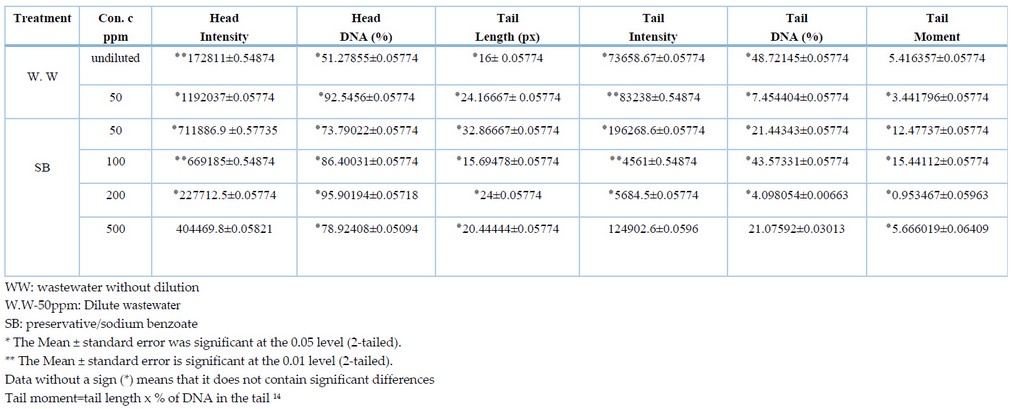
Table 2. Detection of DNA damage in meristematic cells of Allium cepa roots exposed to wastewater (w.w) and preservative using the comet assay.
The concentrations of metals were also compared with each other for the same characteristic, where it was found that Cadmium and lead at a concentration of (20 ppm) (527927.3 ± 0.05774, 447585.5 ± 0.05774), respectively, had a higher intensity compared with the rest of the concentrations. In comparison, the concentration of 10 ppm with an intensity of 266186.3 ± 0.06009 was higher for zinc metal. As for copper, the density was 833663.7±0.05774, higher at 25 ppm concentration. As for the wastewater, the intensity was 1192037±0.05774 higher at 50 ppm concentration, while the preservative SB showed higher intensity of 711886.9 ±0.57735 at 50 ppm concentration.
The results also showed that the percentage of DNA in the Comet's head DNA% reached the highest percentage in the control treatment, 99.33623±0.05774 compared with the heavy metal treatments; the results for this trait showed that all heavy metal treatments showed significant differences in the probability level of P<0.05 compared with the control treatment. This may be because the control treatment did not cause any breakage or damage to the DNA of its cells, compared with the heavy metal treatments that showed significant changes in the length of the Comet. The concentrations of minerals were also compared with each other for the same characteristic to find out at which of the concentrations the highest value for the percentage of DNA appeared in the head of the Comet. It was observed that Cadmium and copper, with values of 90.51757 ± 0.05774, 77.60359 ± 0.05774, were higher at the concentration of 25 ppm compared with the rest of the concentrations of both metals. In comparison, the value of zinc metal was 87.2453 ± 0.05774 higher at the concentration of 5 ppm. While lead, the value was 91.51076 ± 0.05774 higher at 20 ppm concentration compared with the other lead concentrations. The results of contaminated water showed that the percentage of DNA in the head of the Comet was more significant in the diluted sample with a value of 92.5456 ± 0.0574. In contrast, for sodium benzoate, the percentage of DNA in the head of the Comet was higher with a value of 95.90194 ± 0.0571 at a concentration of 200 ppm.
As for the characteristic Tail length (TL) measured in pixels (px), the results showed that the comet length for all heavy metal treatments contains significant differences at the probability level of p<0.05 compared with the control treatment that did not show significant differences. The concentrations were compared with each other for all samples to show how harmful heavy metals are to DNA breakage in the meristematic cells of the roots of Allium cepa. It was found that Cadmium had a higher effect at 588.25 ± 0.05774 at a concentration of 5 ppm, zinc 44.33333 ± 0.05774 at a concentration of 20 ppm, and copper at a concentration of 28.9 ± 0.05774 at a concentration of 25 ppm, lead 25±0.05774 at 20 ppm concentration, undiluted water (WW) 24.16667±0.05774 at 50 ppm concentration respectively and preservative SB 32.86667±0.05774 as the greatest DNA damage was for A.cepa roots exposed to (50 ppm) concentration
The tail intensity was also measured, showing that all minerals showed significant differences, meaning that they had a higher intensity than the control treatment, which did not show significant differences. The results showed that the comet-tail density of Cadmium and copper for all concentrations was p<0.05. As for zinc, it was found that the concentrations (5, 20 and 25 ppm) at the level of p<0.05, while the concentration of 10ppm was at the level of probability p<0.01, as for lead, the significant differences of the concentrations were (10, 20 ppm) at the level of p<0.01, while there were no significant differences at 5ppm. Wastewater showed significant differences between concentrated treatments at p<0.05 and diluted p<0.01. In comparison, the preservative showed a p<0.05 probability level for the concentrations (5 and 200 ppm), while the cells exposed to 100ppm concentration showed a p<0.01 probability level, it was noticed that the cells exposed to the 500ppm concentration did not show significant differences.
The metal concentrations were compared with each other for the Tail intensity, where it was found that the highest intensity of Cadmium was 19893.5±0.05774 at a concentration of 20ppm, while zinc was 513451±0.05774 at a concentration of 10ppm, while the intensity of copper was 135492.8±0.05774 at a concentration of 25ppm, as for lead, the highest intensity was 184030.8±0.08819 at a concentration of 5ppm. At the same time, the wastewater was more dense Comet 83238±0.54874 at the diluted sample with a concentration of 50ppm, as for the preservative 196268.6±0.05774, it was at a concentration of 50ppm. Tail DNA% was measured, and the results showed significant differences at the probability level p<0.05 compared with the control treatment, which did not show significant differences, except for the 500ppm preservative concentration, which did not show significant differences. Figure (1) shows fluorescent microscopy imaging of the DNA damage caused by the effect of heavy metals on Allium cepa cells.
Tail moment (TM) also showed significant differences at the p<0.05 probability level for all treatments except for copper at 25 ppm concentration, lead at 20 ppm and contaminated water did not show significant differences. The concentrations were compared with each other for Tail Moment and Tail DNA. It was noted that the values highest for metals for each of Cadmium were at a concentration of 20 ppm, and for zinc at a concentration of 10 ppm, and copper at a concentration of 20ppm; in wastewater, the results showed that the highest values in the concentrated water sample, as for the preservative, the values for both characteristics were at a concentration of 100ppm. While lead showed a change in concentrations, the results showed that the highest value of Tail DNA % was at a concentration was 5 ppm, while the highest value of Tail moment was at a concentration of 25 ppm. Figure (1) shows the characteristics of the Comet and the variance ratio between treatments compared to the control treatment.
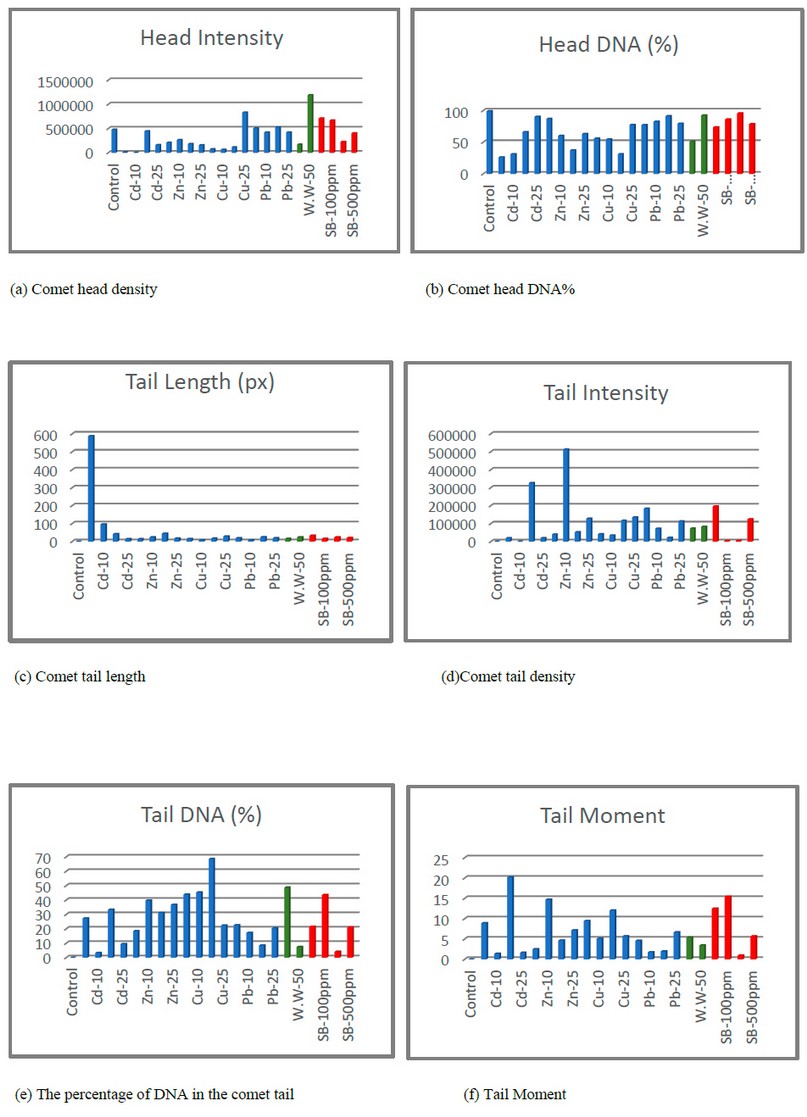
Figure 1. Shows the characteristics of the Comet of meristematic cells of the roots of Allium cepa.
DISCUSSION
The results of this study confirm that the roots of the onion plant were exposed to heavy metals (lead, zinc, copper, lead) both individually and in concentrations (25,20,10,5 ppm) and the preservative sodium benzoate at concentrations (50,200 ppm) and wastewater in both diluted treatments. And undiluted caused damage to DNA in the meristematic cells of the roots of onion plants treated with heavy metals compared to the control treatment.
The results showed that the wastewater showed the highest value of the intensity of the fluorescence of the head of the Comet, followed by the preservative and lead metal, which showed the highest value of the intensity of the fluorescence of the Comet. The study of Lyu et al. 13 indicated that the fluorescence intensity reflects the level of ROS accumulation. The study's results showed that the relative fluorescence intensity increased with increasing concentration and treatment time of metals. This confirms that the accumulation of reactive oxygen species (ROS) in onion roots was caused by lead stress, wastewater, and preservatives.
Ashraf et al. 15 reported a higher rate of DNA damage and breakage correlates with oxidative effects that occur when organisms are exposed to heavy metals, indicating the accumulation of ROS in plant tissues, which in turn causes systemic DNA damage. The study of Luo et al. 16 confirmed that ROS is a toxic agent and is a major source of DNA damage either by DNA strand breakage or by nucleotide removal or base mutation in nucleotides.
The results of the Tail length indicate the effect of the preservative on DNA breakdown; DNA damage to the preservative at the lowest concentration is due to inducing oxidative stress, which negatively affects physiological development, and interacts with antioxidant enzymes aimed at reducing oxidative damage17, the result is consistent with the study of Dosay 18, which indicated the effect of sodium benzoate on DNA at low concentrations.
It was also found that Cadmium caused the most significant effect on DNA at a concentration of 5 ppm compared to the rest of the treatments used; the DNA-damaging activity of heavy metals treatments can be associated with the generation of free radicals (reactive oxygen species ROS), this leads to breaks in DNA strands and damage/binding to proteins involved in DNA replication, repair, recombination and transcription, other than oxidative stress. Cadmium may have caused the DNA-protein cross-links that generally occur with heavy metals at low concentrations; the decrease in TL for all treatments indicates that the DNA damage repair process is not affected; this may be due to cells that have adopted defensive strategies to counteract the harmful effects of reactive oxygen species caused by heavy metal19.
DNA damage caused by exposure to heavy metals may be due to their high binding to DNA and lead to modifications in nitrogenous bases, and it may also be possible that it interferes with the DNA repair mechanism. According to previous studies, copper forms the highest degree of binding to DNA compared to other metals. Also, the decrease in TM is due to the characteristic of TL20. A comet test is a good tool for assessing the damage of pollutants to DNA in eukaryotic cells because it is sensitive to respond to toxic effects.
CONCLUSION
The current study provides valuable research information about the toxic effects of heavy metals by evaluating the DNA damage of meristematic cells of the roots of the Allium cepa plant, which is incurred by the discharge of heavy metals from natural processes or human practices into the environment. The Comet assay is a sensitive tool for assessing the potential risks associated with exposure to pollutants containing such dangerous chemicals that can cause many serious diseases.
Acknowledgments
The authors want to thank the University of Mosul for the provision of equipment, which aided in improving the quality of this work.
REFERENCE
1. Al-Jabri, Khairallah Musa Awwad, (2017) , "Seasonal variation of heavy metal pollution and the effect of cadmium and lead treatment on some biochemical, anatomical and genetic traits of date palm Barhi cultivar ، "Phoenix dactylifera L " , PhD thesis, College of Science, University of Basra.
2. Lawal, K. K., Ekeleme, I. K., Onuigbo, C. M., Ikpeazu, V. O., & Obiekezie, S. O. (2021). A review on the public health implications of heavy metals. World Journal of Advanced Research and Reviews, 10(3), 255-265.
3. Pandey, H., Kumar, V., & Roy, B. K. (2014). Assessment of genotoxicity of some comm food preservatives using Allium cepa L. as a test plant. Toxicology reports, 1, 300-308. doi.org/10.1016/j.toxrep.2014.06.002.
4. Ertushi, A. H., & Noori, A. M. (2021). Cytogenetic Effect of Food Preservatives Sodium Metabisulphite on Al lium Cepa L. Technium BioChemMed, 2(3), 42-46.
5. Qari, S. H,2017. "DNA Flow Cytometric and Cytogenetic Studies on Allium cepa L. Root Tips Treated with Trigonella hamosa L. and/or Sodium Benzoate", journal KLAGENFURT AUSTRIA.24(3), 233-234.
6. Linke, B. G., Casagrande, T. A., & Cardoso, L. I. A. (2018). Food additives and their health effects: A review on preservative sodium benzoate. African Journal of Biotechnology, 17(10), 306-310. DOI: 10.5897/AJB2017.16321.
7. Leme, D. M., & Marin-Morales, M. A. (2009). Allium cepa test in environmental monitoring:a review on its appli cation. Mutation Research/Reviews in Mutation Research, 682(1), 71-81. doi.org/10.1016/j.mrrev.2009.06.002
8. Chackalamannil, S., Rotella, D., & Ward, S. (2017). Comprehensive medicinal chemistry III. Elsevier.
9. pirdal, g., & liman, R. (2019). Cytotoxic and genotoxic assessment of 2-chloropyridine using Allium cepa ana- telophase and comet test. Mediterranean Agricultural Sciences, 32(2), 193-199. doi: 10.29136/mediterranean.539752
10. Abubacker, M. N., & Sathya, C. (2017). Genotoxic Effect of Heavy Metals Cr, Cu, Pb and Zn Using Allium Cepa L. Biosciences Biotechnology Research Asia, 14(3), 1181-1186. doi.org/10.13005/bbra/2559
11. Kostadinova, S., Mollov, I., Dzhambazov, B., Naimov, S., Vassilev, K., & Georgiev, B. (2021, April). Evaluation of Cytotoxic and Genotoxic Effects of Commonly Used Food Additives on the Root Meristem Cells of Allium cepa. In 5th Balkan Scientific Conference on Biology (p. 97).
12. Ciğerci, İ. H., Liman, R., Özgül, E., & Konuk, M. (2015). Genotoxicity of indium tin oxide by Allium and Comet tests. Cytotechnology, 67(1), 157-163.
13. Lyu, G., Li, D., Li, S., Ning, C., & Qin, R. (2020). Genotoxic effects and proteomic analysis on Allium cepa var. agrogarum L. root cells under Pb stress. Ecotoxicology, 29(7), 959-972. doi.org/10.1007/s10646-020-02236-x
14. Mozaffarieh, M., Schoetzau, A., Sauter, M., Grieshaber, M., Orgül, S., Golubnitschaja, O., & Flammer, J. (2008). Comet assay analysis of single-stranded DNA breaks in circulating leukocytes of glaucoma patients. Molecular vision, 14, 1584–1588.
15. Ashraf, M. F. M. R., & Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and experimental botany, 59(2), 206-216.
16. Luo, Z. B., He, J., Polle, A., & Rennenberg, H. (2016). Heavy metal accumulation and signal transduction in herbaceous and woody plants: paving the way for enhancing phytoremediation efficiency. Biotechnology Advances, 34(6), 1131-1148.
17. Acar, A. (2021). Therapeutic effects of royal jelly against sodium benzoate–induced toxicity: cytotoxic, genotoxic, and biochemical assessment. Environmental Science and Pollution Research, 28(26), 34410-34425.
18. Dosay-Akbulut, M. (2021). Determination of DNA Damage Caused by Food Additives Using Comet Assay Method : Food Additives DNA damage via comet assay. Progress in Nutrition, 22(4), e2020071. https://doi.org/10.23751/pn.v22i4.9095
19.Seth, C. S., Misra, V., Chauhan, L. K. S., & Singh, R. R. (2008). Genotoxicity of Cadmium on root meristem cells of Allium cepa: cytogenetic and Comet assay approach. Ecotoxicology and Environmental safety, 71(3), 711-716.
20.Qin, R., Wang, C., Chen, D., Björn, L. O., & Li, S. (2015). Copper‐induced root growth inhibition of Allium cepa var. agrogarum L. involves disturbances in cell division and DNA damage. Environmental toxicology and chemistry, 34(5), 1045-1055.
Received: September 22, 2022 / Accepted: October 18, 2022 / Published:15 November 2022
Citation: Naf’i AL E K, M I Khalil. Estimation of DNA damage in the roots of Allium cepa exposed to heavy metals using the comet assay.
Revis Bionatura 2022;7(4) 70. http://dx.doi.org/10.21931/RB/2022.07.04.70
