2022.07.04.62
Files > Volume 7 > Vol 7 No 4 2022
Polyene macrolide antibiotic nanoemulsion: a proposal for the treatment of cutaneous leishmaniasis
Lilian Sosa1*, Lupe Carolina Espinoza2, Jhunior Marcia Fuentes3, Jorge Alberto Siwady4, Fredy Rodríguez Rivas5, María Rincón Díaz6
1 Pharmaceutical Technology Research Group, Faculty of Chemistry and Pharmacy, National Autonomous University of Honduras (UNAH), Tegucigalpa, Honduras; [email protected]
2 Departamento de Química y Ciencias Exactas, Universidad Técnica Particular de Loja (UTPL), Loja, Ecuador. [email protected]
3 Faculty of Technological Sciences, National University of Agriculture (UNAG), Honduras. [email protected]
4 Department of Pharmaceutical Technology, Faculty of Chemical Sciences and Pharmacy, National Autonomous University of Honduras (UNAH), Tegucigalpa, Honduras. [email protected]
5 Faculty of Chemical Sciences and Pharmacy, National Autonomous University of Honduras (UNAH), Tegucigalpa, Honduras. [email protected]
6Department of Materials Science and Physical Chemistry, Faculty of Chemistry, University of Barcelona (UB), Barcelona; Spain. [email protected]
*Corresponding author: [email protected], teléfono (504) 9450-5228
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.62
ABSTRACT
Leishmaniasis is a neglected tropical disease that requires timely and inexpensive treatment. For this purpose, a nanoemulsion with a polyene macrolide antibiotic, or amphotericin B (NE-AmB), was developed. This study quantified the amount of drug permeated and retained in intact and lacerated human skin, simulating cutaneous leishmaniasis (CL) processes. Toxicity in macrophage and keratinocyte cell lines, activity against promastigotes and amastigotes of Leishmania tropica, in vivo irritant activity, and histological evidence was evaluated. Results. The amount of drug retained in intact and damaged skin was 750.18 ± 5.43 and 567.97 ± 8.64 µg/g/cm2, respectively. There was no permeation. No apparent toxic effect was observed in HaCaT cell lines. The IC50 of NE-AmB found for promastigotes and amastigotes was 0.26 ± 0.09 and 0.37 ± 0.05 µg/mL, respectively. NE without AmB did show antiparasitic activity. The formulation showed lower IC50 values on both parasite stages than the AmB solution. There was no skin irritation, and histology showed skin improvement with treatment. We suggest that this NE-AmB may be a candidate for in vivo studies in CL patients.
Keywords. Leishmaniasis, Amphotericin B, ex vivo permeation studies, in vitro cytotoxicity, in vitro leishmanicidal activity, Draize test, histology.
INTRODUCTION
Leishmaniasis is a parasitic disease caused by protozoa of the genus Leishmania. It is estimated that about one million new cases and more than 20,000 deaths occur annually due to this parasitosis.1 In European, Asian, and African countries, this disease is transmitted by biting female sandflies (vectors) belonging to the genus Phlebotomus. However, in the American region, it is caused by Lutzomyia, specifically Psychodidae.2 Leishmania has two primary cell morphologies: promastigotes in the vector and amastigotes in the mammalian host. Its life cycle begins with promastigotes inoculated into the host, which are phagocytosed by macrophages. Once inside the macrophages, they lose their flagella and become amastigotes that multiply by binary fission. The infected macrophages then "explode" and release their amastigotes to infect other macrophages, thus reproducing the cycle.3
There are three types of leishmaniasis: cutaneous (CL), mucocutaneous (MCL), and visceral (VL), with CL being the most common form of the disease. Depending on the infecting species, this disease can cause anything from skin ulcers, which may heal independently, to deep wounds that can spread to other body parts.4 Antimonials, such as sodium stibogluconate (Pentostam®) or meglumine antimoniate (Glucantime®), are the first choice for treating all leishmaniasis. However, their use presents problems such as invasive administration (intravenous, intramuscular, and intralesional) and adverse effects: musculoskeletal pain, renal failure, and hepatic and cardiac toxicity. Other therapeutic options include intramuscular pentamidine, paromomycin ointment alone, or imiquimod (in combination with paromomycin). Azithromycin, miltefosine, antifungal drugs (all oral administration), and, in particular, itraconazole and fluconazole could be considered an alternative in complex lesions or with possible mucosal involvement. However, despite the existence of a vast therapeutic arsenal, the increase in resistance to antimonials, their high toxicity, high cost, and prolonged treatment regimens, especially in immunocompromised patients, has generated that drugs such as amphotericin B (AmB) have become the most appropriate treatment alternative for LC.5
AmB is a polyene macrolide antifungal agent isolated from Streptomyces nodosus and collected in 1955 from the Orinoco River in Venezuela. AmB was rapidly introduced into clinical medicine and received Food and Drug Administration (FDA) approval in 1958, even without elucidating its chemical structure. This molecule binds explicitly to ergosterol in the parasite cell membrane, establishing aggregated transmembrane pores, and causing membrane depolarization.6
AmB deoxycholate (AmB-Deox) has been used during the last few years due to the increasing number of immunosuppressed patients. However, it has been associated with a high rate of side effects, especially renal toxicity. For this reason, other formulations have been developed: a lipid formulation (liposomal, AmBisome®, Gilead Sciences, Foster City, CA, USA), a lipid complex (Abelcet®, Sigma Tau Pharmaceuticals, Pomezia, Italy), and a colloidal suspension (Amphocil®, Penn Pharmaceuticals, Ltd, Tredegar, UK), which share the same spectrum of action but differ in efficacy and toxicity, in addition to being administered invasively and having a high cost, making them difficult to acquire in developing countries.7
Given the above, topical application of AmB could be an alternative for treating LC. Topical administration has several advantages: it is a simple technique, quite comfortable, is not painful for the patient and allows self-administration, direct application to the site of infection, and could prevent systemic effects caused by the drug, ensuring its local effect and retention of a sufficient amount on the skin. 8 To date, there is no marketed drug containing AmB for topical administration, so there is a need to develop new formulations of AmB against leishmaniasis which are more effective, safe, and affordable.
Taking into account the above, in the previously published work by Sosa et al., a nanoemulsion with Amphotericin B (NE-AmB) was developed to treat LC. For this purpose, castor oil as the oil phase, Transcutol® P as the aqueous phase, and an emulsifier system composed of a combination of labrasol®/plurol oleic acid® were used as excipients. In the present study, permeation and retention analysis evaluated the amount of AmB retained and permeated in healthy and damaged human skin. In addition, the cytotoxic effect of NE-AmB was studied in three cell lines: HaCaT, RAW 264.7, and J774A.1, and the leishmanicidal activity against both parasite stages (promastigotes and amastigotes) of Leishmania tropica. Finally, a Draize test was performed on New Zealand rabbits, and histological analysis of rabbit skin was done to test dermal irritation and its effect at the microscopic level.
MATERIALS AND METHODS
Materials
The AmB used for the study was obtained from Acofarma (Barcelona, Spain). Dimethyl sulfoxide (DMSO), methanol, castor oil, and acetonitrile were obtained from Sigma-Aldrich (Darmstadt, Germany). Transcutol®P, Labrasol®, and Plurol Oleico® were kindly provided by Gattefossé (Barcelona, Spain). The water used in all experiments was obtained from a Milli-Q® Plus system (Millipore Co., Burlington, MA, USA). All chemicals and reagents were of analytical grade.
Parasite strains and cultures
Leishmania tropica species was isolated from a patient with LC in Barcelona, Spain (MHOM/ES/2010/BCN-809). Promastigote growth curves were performed by counting the number of parasites daily for six days to determine their kinetics and establish the logarithmic growth phases. The promastigotes obtained were seeded at 26 °C in a Shneider culture medium at pH 7.0, supplemented with heat-inactivated 20% fetal bovine serum, 25 µg/mL gentamicin solution (Sigma, St. Louis, MO, USA), and 1% penicillin (100 IU/mL) with streptomycin solution (100 mg/mL) (Sigma, St. Louis, MO, USA).
Preparation of NE-AmB
First, a selection of solvents was tested to determine the solubility of AmB. DMSO was selected as the best solubilizing component of AmB. The oily component, aqueous component, and emulsifying system were chosen. Subsequently, a ternary phase diagram was performed to find the best combination that was a single phase and had the smallest droplet size and the lowest polydispersion. The results of this formulation have been reported in a previous study.9
Ex vivo permeation studies
Franz diffusion cells were used to perform the permeation assays, placing the studied sample on human skin from a donor undergoing plastic surgery at the Barcelona-SCIAS Hospital. The patient gave prior written informed consent by the provisions of the Barcelona Hospital Ethics Committee (ethical experimental, protocol reference number: BEC/001/16, Barcelona, Spain). Human skin was brought to the laboratory, cut with a Zimmer® dermatome (Ohio, USA) in 400 µm thick samples, and the integrity of the skin was verified by measuring transepidermal water loss (TEWL) parameters.
The stratum corneum of healthy skin was partially removed by applying an adhesive tape seven times (thus simulating damaged skin in leishmaniasis processes). Transcutol®P was used as the receptor médium, and a temperature of 32 ± 0.5 °C was maintained with constant agitation. A total of 300 µL of NE-AmB was deposited in the compartment with the donor skin and placed towards the stratum corneum (Figure 1).
To extract the drug retained in the skin, the pieces of skin that had contact with the drug were cut, weighed, and washed with a 0.05% sodium lauryl sulfate solution and then with distilled water. Extraction was performed by puncturing the skin pieces 70 times with a sterile needle, and after the skin pieces were weighed, 1 mL of DMSO was added and placed in an ultrasonic bath for 30 minutes and cold. The amount of AmB from the permeation samples and that extracted from the skin pieces were determined using the High-Performance Liquid Chromatography (HPLC) method, previously validated and described by Sosa et al.
AmB was determined using HPLC, with a Waters® 515 chromatograph, a 717 Plus autosampler, and a 2487 dual absorbance detector (Waters®, Milford, MA, USA). The assay was performed with a Kromasil® Eternity C18 (250 mm x 4.6 mm x 5 µm, Teknokroma, Barcelona, Spain). The mobile phase was a mixture of acetonitrile, acetic acid, and water (52:4.3:43.7 v/v/v) and was pumped through the C18 column at a flow rate of 0.5 mL/min. A 10 µL per sample was injected, and finally, the fluid was analyzed at 406 nm. All measurements were performed at room temperature and under isocratic elution conditions. Calibration curves were prepared with freshly prepared AmB stock solutions in a concentration range of [0.39 to 200] µg/mL. The analytical method was accurate, with a coefficient of variation between 0.02% and 8.79%, relative percentage error between -1.16% and 3.46%, and linear within the concentration range used [0.39-200] µg/mL, with a p-value corresponding to ANOVA applied to the mean values of 0.05.9

Figure 1. The skin used in permeation and retention studies. (A) It has intact human skin. (B) Lacerated human skin. Source: own elaboration.
In vitro cytotoxicity test
Three cell lines were used to establish the cytotoxic effect of NE-AmB: two macrophage cell lines, RAW 264.7 and J774A.1, and one keratinocyte cell line (HaCat) (Eppelheim, Germany). A suspension of 5.0 x 104 cells/mL of each cell line was seeded in 96-well plates (Costar 3596, Corning Incorporated, NY, USA) and incubated at 37 °C, 5% CO2 atmosphere and in RPMI-1640 complete medium supplemented with 20% fetal bovine serum, thermoinactivated, and 1% penicillin (100 U/mL)-streptomycin (100 mg/mL) solution for 24 h. After that, serial double dilutions of the NE-AmB, NE without AmB, and AmB solution were added, and incubation conditions were maintained for another 24 h. Finally, 10% WST-1 reagent (Roche Diagnostics GmbH) was added to all Wells, and these were incubated for four h under the same conditions of temperature and CO2 atmosphere. Cultures were included as positive control and negative control. Absorbance was read at 450 nm (Multiskan EX, ThermoElectron Corporation, Shanghai, China). Different concentrations of AmB were assayed, from 150 µg/mL to 0.14 µg/mL. The following equation was used to determine the % cell viability:

In vitro leishmanicidal activity in promastigotes
Promastigote cultures were performed as explained in section 2.2. To evaluate the antimicrobial activity of NE-AmB, serial double dilutions of NE-AmB, drug-free NE and AmB solution were made using Schneider culture medium and plated in a 96-well plate (Costar 3596, Corning Incorporated, NY, USA). To the dilutions, a suspension of 1 x 106 promastigotes/mL (in log phase) was added and incubated at 26 °C for 48 h. Briefly, the samples were lysed, and alkalinization carried out the enzymatic reaction with p-nitrophenyl phosphate. The optical density was read at 405 nm (Multiskan EX, ThermoElectron Corporation, Shanghai, China). Cultures were included as positive control and negative control. The IC50 (the concentration inhibiting 50% of parasite growth) was calculated by variable transformation analysis and linear regression using Excel version 2019, and experiments were performed in triplicate. Different concentrations of AmB in the range of [150-0.14] µg/mL were tested.
In vitro leishmanicidal activity on amastigotes
RAW 264.7 cell line was used to study the activity of NE-AmB, drug-free NE, and AmB solution against amastigotes. A concentration of 5 × 104 cells/mL was seeded on a LabTek 8-well chamber slide system (Nunc, Rochester, NY, USA) and incubated for 24 h at 37 °C in a 5% CO2 atmosphere. Leishmania tropica promastigotes were added to the cells in a 1:10 ratio (macrophages: parasites) and incubated for 24 h under the same conditions. After removal (washing with sterile 0.1 M PBS) of free promastigotes, fresh RPMI-1640 was added as culture médium. Then NE-AmB, drug-free NE, and AmB solution were added, making double dilutions. Plates were incubated for 48 h at 37 °C in a 5% CO2 atmosphere. Cultures were included as the positive and negative control. Slides were fixed and stained with Giemsa stain and counted to assess the number of amastigotes in 300 macrophages to determine the percentage of infected cells (in triplicate). The IC50 was expressed as the percentage of growth inhibition concerning untreated controls.
Draize test
Adult male New Zealand albino rabbits weighing 1.9 and 2.1 kg (San Bernardo farm, Spain) were used. The dorsal area of the trunk was shaved with an electric shaver 24 h before the start of the test, checking that the chosen animals had completely healthy skin. Four 2-cm-long scarifications were made with a lancet, 2 cm apart. The backs of the rabbits were divided into four zones with the help of adhesive tape. In rabbits number 1, 2, and 3, 0.5 mL of NE without active substance was applied on the upper left side, 0.5 mL of the NE-AmB was applied on the lower left side, and no formulation was applied on the upper right and lower right sides, taking them as a control. After 48 h of applying the different formulations, the substances under study were removed to score from 0 to 4 (from lowest to highest) in each area, both the formation of edema and irritation on the exposed skin. Scoring was repeated at 72 h. The mean value of the individual primary dermal irritation index was determined for each rabbit by summing the edema and erythema scores at 48 h and 72 h and dividing the result by 4. The PDI is the average value of the individual indices for each rabbit. According to the obtained PDI value, the products are classified as "non-irritant" (PDI<0.5), "mildly irritant" (0.5 <PDI<2), "moderately irritant" (2 <PDI<5), and "severely irritant" (PDI > 5). Three animals were used for each formulation. Ethical approval for the handling of experimental animals was obtained from the Institutional Animal Ethics Committee (AICE) of the University of Barcelona.
Histological analysis of rabbit skin
Rabbits were anesthetized and sacrificed with sodium pentobarbital. For histological analysis, skin samples from the back of the rabbits were cut and placed for 24 h on plastic disks immersed in 4% buffered formaldehyde at room temperature. After fixation, all samples were embedded in kerosene blocks cut into 5 µm sections and mounted on microscope slides. Subsequently, the samples were stained with hematoxylin and eosin and finally viewed under a light microscope (Olympus BX41 and Olympus XC50 camera) at 100X magnification to evaluate tissue structure.
RESULTS
Preparation of NE-AmB
The physicochemical characterization of NE-AmB has been published, presenting a low viscosity (12.20 ± 0.02 mPa.S) and a Newtonian flow (r=1). It remained chemically stable for at least three months, and the pH remained between 5 and 6. Accelerated stability studies using Turbiscan®Lab showed that this formulation would remain stable (physically) for at least six months. The 55:05:40 composition was finally chosen, i.e., 55% Labrasol®/Plurol Oleic® (5:1), 5% castor oil, and 40% Transcutol®P. NE-AmB presented a droplet size of 112.90 ± 10.15 and a polydispersity index of 0.22 ± 0.02 (Figure 2).9
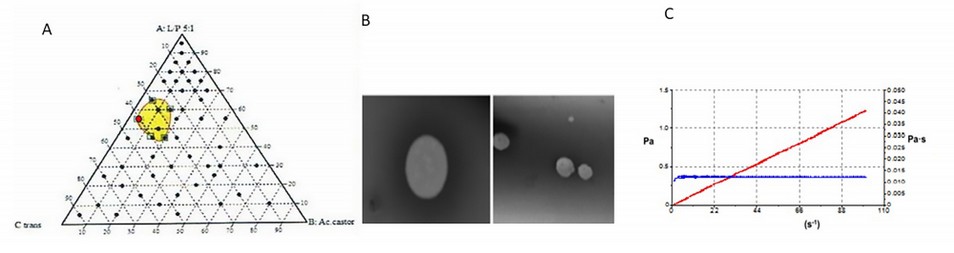
Figure 2. (A) Ternary phase diagram where the dots are the tested mixtures and the nanoemulsion zones in yellow. The red dot indicates the composition selected for the preparation of the NE-AmB. (B) The image taken by Transmission Electron Microscopy (TEM) can also be seen. (C) The rheological behavior of the NE-AmB.
Ex vivo permeation studies
The amount of AmB retained in both healthy and damaged skin is shown in Table 1. No AmB was found in the receptor medium of the Franz cell, demonstrating that the drug could not permeate through either healthy or damaged skin. Furthermore, 567.97 µg/g/cm2 was found to be retained in damaged skin and 750.18 µg/g/cm2 in intact skin.

Table 1. AmB Amount permeated and retained in human skin.
Cytotoxicity assay
Regarding the HaCaT cell line, these are human keratinocytes, so it is essential to determine toxicity in these cells since NE-AmB would be placed directly on the skin. No cytotoxic effect of this formulation or its excipient was observed. We only observed toxicity with the AmB solution at concentrations of 150 µg/mL (Figure 3A).

Figure 3. HaCaT keratinocytes cell toxicity. (A) AmB solution (µg/mL) (B) NE-AmB (µg/mL) (C) NE without the drug (%v/v).
On the other hand, the cellular toxicity of AmB, NE-AmB, and NE solutions without drug was studied in two macrophage lines, considering that AmB could be absorbed into the bloodstream when the LC is ulcerated. In these cases, it is essential to determine the concentration at which the drug to be tested would be toxic, although, in the permeation studies on damaged skin, we did not find AmB in the receptor medium. The AmB solution showed imminent toxicity at 150 and 75 µg/mL concentrations in the RAW 264.7 and J771A.1 cell lines, respectively. In contrast, at concentrations of 37.5 µg/mL, cell viability above 80% was shown (Figure 4B). NE alone shows toxicity only in the J774A. One cell line (Figure 4B) and NE-AmB showed similar behavior as the excipient (Figure 4C). NE-AmB and NE observe no cell toxicity without the drug in the RAW 264.7 cell line (Figure 4B and 4C).

Figure 4. Cell toxicity in RAW 264.7 (pink) and J774A.1 (purple) macrophages (A) AmB solution (µg/mL) (B) NE-AmB (µg/mL) (C) NE without AmB (%v/v).
In vitro leishmanicidal activity
Table 2 summarizes the activity on promastigotes and amastigotes. The IC50 of NE-AmB for promastigotes and amastigotes was 0.26 ± 0.09 and 0.73 ± 0.02 µg/mL, respectively. The IC50 of the AmB solution on promastigotes and amastigotes was 0.73 ± 0.08 and 1.79 ± 0.02 µg/mL, respectively. The drug-free NE showed activity against both parasite stages: 0.16 ± 0.03% (promastigotes) and 0.39 ± 0.02% (amastigotes). Likewise, Figure 5 shows the infected and untreated macrophages (5A) and the cultures treated with NE-AmB (5B). Table 2. IC50 values in promastigotes and amastigotes of the genus Leishmania tropica.

Table 2. In vitro leishmanicidal activity

Figure 5. Effect of NE-AmB on infection of RAW 264.7 macrophages with Leishmania tropica. (A) Infected and untreated control cultures (B) NE-AmB-treated cultures. Arrows indicate the amastigotes.
Draize test
48 h after the application of NE-AmB and NE without the drug on the back of the rabbits, no discomfort was observed on the part of the animal throughout the test. No apparent changes (to the naked eye) were detected in the skin of the rabbits, and no edema, erythema, or irritation phenomena were observed (Figure 6).

Figure 6. Draize test with emulsified systems at t0, 24 h, and 48 h. (A) NE = nanoemulsion without AmB. (B) NE AmB = nanoemulsion with AmB. (C) Cº = control (scarification skin without drug).
Histological analysis of rabbit skin
Regarding histological evaluation, micrographs revealed that provoked scarification or control without AmB (scarifications only) showed histological alterations and the presence of nonspecific inflammatory cells in the skin (Figure 7B). Topical application of NE without AmB (Figure 7C) caused some improvement, but inflammatory cells were still observed. However, NE-AmB (Figure 7D) markedly repaired this alteration, resulting in a less pronounced inflammatory process than the control. Figure 7A shows skin without scarification.
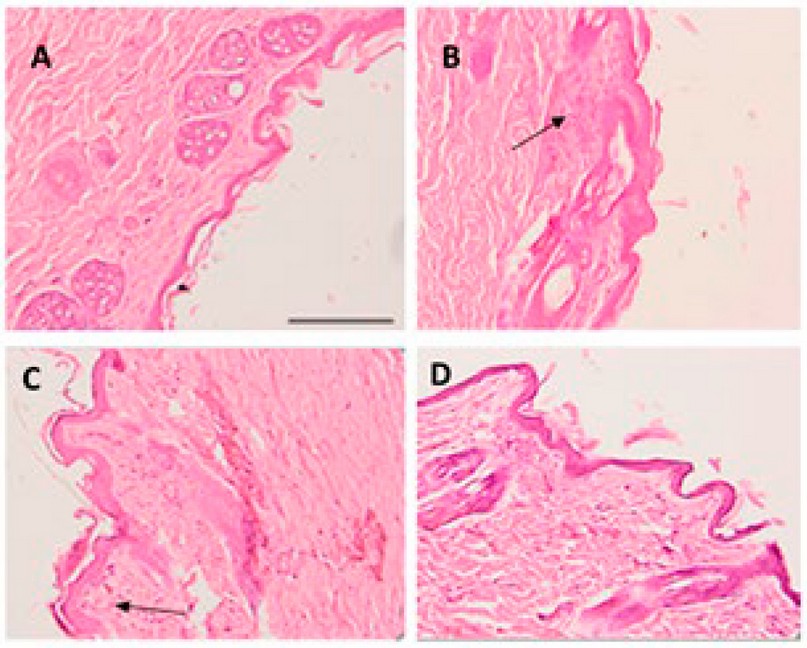
Figure 7. (A) Skin without scarification. (B) Skin with scarification and without drugs. (C) NE without AmB. (D) NE-AmB. The arrow indicates the inflammatory process—bar scale of 200 µm.
DISCUSSION
Leishmaniasis is a parasitic infection of high medical, social and economic importance. The absence of effective vaccines and the limitations of current treatment's search for effective therapies is a real need, especially in developing countries, where there is little accessibility to antimony salt treatments, considered the first line.10 AmB is considered an option for antimony salts treatment. 11 To this end, we propose an alternative, an easily prepared and applied NE-AmB for LC treatment.
Ex vivo permeation studies provide valuable information to predict the in vivo behavior of the formulation. Human skin used for testing has been lacerated to simulate skin in leishmaniasis processes. In this investigation, in the permeation tests, AmB was not quantified in the receptor compartment of the Franz cell, indicating that there was probably no absorption through the skin. Previous studies have shown that AmB does not tend to be absorbed. 9,12-14 This can be explained because AmB has a high molecular weight (926 Da), high hydrophobicity, and liposolubility, which would limit the passage of the drug through the dermis due to the aqueous structure of the dermis. Moreover, it can penetrate the skin without reaching the systemic circulation, which is confirmed by a large amount of drug retained in the tissue, which is indicative that the formulation, having a more local effect on the target area without side effects, is useful in the topical treatment of ulcers present in the LC. Other studies show that AmB can be absorbed in small amounts. However, the absorption tests in these studies were performed on rat skin, which is more permeable than human skin. The quantities found in the receptor medium were minimal, below the detection limit of the methodical used.15,16
As we can see in Table 1, there was less AmB retained in the lacerated skin. This could be explained by the fact that AmB, which is more lipophilic, is more contained in the stratum corneum in the epidermis. In addition to being a high molecular weight drug, it takes longer to be absorbed by the dermis.17 In a previous study by Berenguer et al. (2020), in which they prepared an AmB gel, they also found a lower amount of the drug in the lacerated skin. However, it is essential to note that in leishmaniasis processes, nodules and papules may contain epidermis, so AmB would be retained in both layers of the skin, exerting an eminently local effect.13
Cellular toxicity studies are essential as they indicate that a formulation can be administered into the body. The AmB solution showed similar toxicity in both macrophage cell lines. However, NE-AmB and NE alone showed more significant toxicity in J774A.One than in Raw 264.7 cell lines. The latter cell line is known to exhibit multiple drug resistance properties caused by the presence of P-glycoprotein in the structure of these cells.18
Regarding toxicity in keratinocytes, it was observed that only AmB solution presented toxicity at a concentration of 150 and 75 µg/mL. This could be because AmB is dissolved in DMSO, which is the cause of toxicity in this solvent. This is evidence that we can place NE-AmB on the skin without presenting any toxic effect. On the other hand, Leishmania tropica was used because it is one of the species that causes LC. It is characterized by causing relapsing leishmaniasis and is usually more resistant to drugs. The IC50 values of NE-AmB found in this study, both in amastigotes and promastigotes, were less than 1.00 µg/mL, lower than those found for the AmB solution, so at the in vitro level, it would be considered more effective. This could be because NE alone produced an effect against the parasite. However, being non-toxic, it is regarded as a suitable vehicle.
Previous research has demonstrated the efficacy of various AmB-based formulations in a range of IC50 values between 0.2-1.62 µg/mL, similar to those found in this study (Table 2). However, the values vary because they depend on the infecting species.19 In addition, the level of AmB retained in the injured skin (567.97 ± 8.64 µg/g/cm2) and the intact skin (750.18 ± 5.43 µg/g/cm2) was higher than the AmB IC50 values found in both parasite stages, amastigotes (0.73 ± 0.02 µg/mL) and promastigotes (0.26 ± 0.09 µg/mL). Therefore, after topical application of the formulation, the drug found in the dermis is phagocytosed by infected macrophages that hydrolyze the molecule through acidic lysosomal enzymes, releasing AmB where they live. Then Leishmania parasites multiply. 20
Finally, in the Draize test, no signs of irritation, itching, or edema were evident in the animal. Histological studies showed that NE alone caused a decrease in inflammation, but not entirely. Nevertheless, NE-AmB generated a considerable improvement in the inflammatory process of the rabbit skin compared to the positive control (or only with bedsores). This would indicate that NE-AmB could be applied directly to the skin. This can be observed in a previous study, where the obtained values of transepidermal water loss and stratum corneum hydration (SCH) evidenced no problems directly applying this formulation on human skin. 9,12
CONCLUSIONS
The low IC50 values found in this study for both parasite stages show that NE-AmB is promising for treating LC. The amounts of drug retained in the skin (intact or damaged) would be sufficient to eliminate the parasite from the site of infection, in this case, the skin. No drug was quantified in the Franz cell receptor compartment, showing no permeation or that amounts that might permeate are undetectable. This indicates that AmB will probably not be absorbed into the bloodstream. Both the excipient and the formulation with the drug did not show cytotoxicity on keratinocytes, so we can assume that its application on human skin is safe.
Furthermore, the formulation was non-irritant and did not produce edema or allergy during the Draize test. The NE-AmB showed microscopic improvements in scarified skin, which would benefit the level of skin leishmaniasis. The low viscosity of NE would allow its easy spray application, avoiding direct contact with the ulcer and preventing concomitant infections. Tests on infected animals are recommended to continue the study and ensure the efficacy of NE-AmB at the in vivo level.
Funding: “The name of IN2UB, grant number [2017.3.IN2UB.2] funded this research,”
Conflicts of Interest: "The authors declare no conflict of interest."
REFERENCES
1. World Health Organization (WHO). Leishmaniasis. 2022. Available online: https://www.who.int/ leishmaniasis/en/ (accessed on April 23 2022).
2. Chongo Alfaro, M. L. García Echegoyen, R. Leishmaniasis y transfusión. Artículo de revisión. Rev. Mex. Med. Tran., 2010, 3(1), S42-S47.
3. Kato, H., Gómez, E.A., Cáceres, A.G., Uezato, H., Mimori, T., Hashiguchi, Y. Molecular epidemiology for vector research on leishmaniasis. Int J Environ Res Public Health., 2010, 7(3), 814-26.
4. Rajni, E., Ghiya, B.C., Singh, S., Shankar, P., Swami, T., Jadon, DS, Negi, S.R., Malik, M., Khatri, P.K. Cutaneous leishmaniasis in Bikaner, India: Clinico epidemiological profile; parasite identification using conventional, molecular methods and CL DetectTMrapid test, a new food, and drug administration-approved test. TropParasitol., 2019, 9(2), 115-123.
5. Souto, E.B., Días-Ferreira, J., Craveiro, S.A., Severino, P., Sánchez-López, E., García, M.L., Silva, A.M., Souto, S.B., Mahant, S. Therapeutic interventions for countering leishmaniasis and Chagas's disease: From traditional sources to nanotechnological systems. Pathogens., 2019, 1;8(3),119.
6. Cohen, BE. The role of signaling via aqueous pore formation in resistance responses to Amphotericin B. ASM Journals, 2016, 60, 5122–5129.
7. Botero, M.C., Puentes-Herrera, M., Cortés, J.A. Lipid formulations of amphotericin. Rev. Chil. Infectol., 2014, 31, 518–527.
8. Atiyeh, B.S., Gunn, S.W., Hayek, S.N. State of art in burn treatment. World J. Surg., 2005, 29, 131–148.
9. Sosa, L., Clares, B., Alvarado, H.L., Bozal, N., Domenech, O., Calpena, A.C. Amphotericin B is releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomedicine, 2017,13(7), 2303–2312.
10. Chavez-Fumagalli, M.A. New delivery systems for amphotericin B applied to the improvement of leishmaniasis treatment. Rev. Soc. Bras. Med. Trop., 2015, 48(3):235-242.
11. Apa, H., Devrim, İ., Bayram, N., Deveci, R., Demir-Özek, G., Cartı, Ö.U. Liposomal amphotericin B versus pentavalent antimony salts for visceral Leishmania in children. Turk J Pediatr., 2013, 55(4), 378-83.
12. Sosa, L., Calpena, A.C., Silva-Abreu, M., Espinoza, L.C., Rincón, M., Bozal, N.; Domenech, O., Rodríguez-Lagunas, M.J., Clares, B. Thermoreversible gel-loaded amphotericin B for the treatment of dermal and vaginal candidiasis. Pharmaceutics, 2019, 11, 312.
13. Berenguer, D., Alcover, M.M., Sessa, M., Halbaut, L., Guillén, C., Boix-Montañés, A., Fisa, R., Calpena-Campmany, A.C, Riera, C, Sosa, L. Topical Amphotericin B Semisolid Dosage Form for Cutaneous Leishmaniasis: Physicochemical Characterization, Ex Vivo Skin Permeation and Biological Activity. Pharmaceutics, 2020, 12;12(2)
14. Moraes Santos, C., Barbosa de Oliveira, R., Arantes, V.T., Rodríguez-Caldeira, L., De Oliveira, M.C., Tabosa-Egito, E.S., Miranda-Ferreira, L.A. Amphotericin B-loaded nanocarriers for topical treatment of cutaneous leishmaniasis: Development, characterization, and in vitro skin permeation studies. J. Biomed. Nanotechnol., 2012, 8, 322–329.
15. Hussain, A., Singh, S., Webster, T.J., Ahmad, F.J. New perspectives in the topical delivery of optimized amphotericin B loaded nanoemulsions using excipients with innate antifungal activities: A mechanistic and histopathological investigation. Nanomedicine., 2017, 1117–1126.
16. Jaafari, M.R., Hatamipour, M., Alavizadeh, S.H., Abbasi, A., Saberi, Z., Rafati, S., Taslimi, Y., Mohammadi, A.M., Khamesipour, A. Development of a topical liposomal formulation of Amphotericin B for the treatment of cutaneous leishmaniasis. Drugs Resist., 2019, 11, 156–165.
17. Anderson, B.D., Raykar, P.V. Solute structure-permeability relationships in human stratum corneum. J. Investig. Dermatol., 1989, 93, 280–286.
18. Pujol, A., Urbán, P., Riera, C., Fisa, R., Molina, I., Salvador, F., Estelrich, J., Fernández-Busquets, X. Application of quantum dots to the study of liposome targeting in leishmaniasis and malaria. Int. J. Theoret. Appl. Nanotech., 2014, 2, 1–8.
19. Rodrigues Caldeira, L., Ribeiro Fernández, F., Ferreira Costa, D., Frézard, F., Crocco Alfonso, L.C., Miranda Ferreira, L.A. Nanoemulsions loaded with Amphotericin B: A new approach for the treatment of leishmaniasis. Eur. J. Pharm., 2015; 70:125-31.
20. Huang, Z., Jaafari, M.R., Szoka, F.C. Disterolphospholipids: nonexchangeable lipids and their application to liposomal drug delivery. Angew. Chem., 2009, 121:4210–4213.
Received: September 22, 2022 / Accepted: October 18, 2022 / Published:15 November 2022
Citation: Sosa L, Espinoza L C, Fuentes J M, Siwady J A, Rivas F R, Díaz M R. Polyene macrolide antibiotic nanoemulsion: a proposal for the treatment of cutaneous leishmaniasis. Revis Bionatura 2022;7(4) 62. http://dx.doi.org/10.21931/RB/2022.07.04.62
