2023.08.03.112
Files > Volume 8 > Vol 8 No 3 2023
Description and determination of the nanocellulose components produced from acetic acid bacteria
1,2 Department of Biology / College of Science / University of Baghdad / Baghdad / Iraq
3Department of Biotechnology / College of Science / University of Baghdad / Baghdad / Iraq
*Corresponding Author: [email protected], [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.112
ABSTRACT
Some microorganisms can produce nanocellulose, which is known as bacterial nanocellulose (BNC); the most active bacterial producer is acetic acid bacteria (AAB), which is a gram-negative, motile and obligate aerobic belongs to the family Acetobacteraceae. Bacterial nanocellulose has excellent attention in medical (surgical domain), industrial and pharmaceutical fields because of its flexible properties, characteristics and advantages. So, in this study, the AAB (5AC) isolate was isolated from apple vinegar. The production of BNC was performed by using a natural medium called palm dates liquid medium, the produced bacterial Cellulose was purified by using the sodium hydroxide method; it was observed that the wet weight of the BNC was about (43.11gm), and its dry weight was about (2.2gm); also the bacterial nanocellulose was characterized by various techniques for detect the morphology of the surface area of it, these techniques are: FESEM technique apparatus which used to clarify the surface morphology of nanostructured bacterial thin films, the results of FESEM presented the presence of different nano-scale diameters of nanofibers. Also, Energy Dispersive X-ray Spectroscopy analysis (EDX) was performed. The results showed that the AAB bacterial cellulose membrane composed of the elements: C, O, H, N and Na (40.71%),(13.98%), (38.6%), (5.41%) and (1.3%) respectively, the FTIR analysis showed that the bacterial nanocellulose fiber functional groups and chemical bonds were observed at the region with wavelength (800-1700) cm−1. All these techniques provide an idea about surface morphology and the composition of BNC membrane, giving it many properties that consider BNC a safe bioproduct in many industrial fields.
Keywords: Purification; characterization; nanocellulose ; acetic acid bacteria
INTRODUCTION
The progress of nanotechnology in the last twenty years has focused much attention on the electrospinning process; this technique is used to construct nano-polymer important in the bio-medical sector because of its low cost, flexibility and simplicity [1]. Cellulose (C6H10O5)n consists of a D-glucose unit at one end and a C4-OH group at the non-reducing end, while the C1-OH is the terminating group [2]. Bacterial Cellulose (BC) is an extracellular polysaccharide created by specific bacterial genera of acetic acid bacteria such as Acetobacter, Agrobacterium, Gluconacetobacter, and other bacteria like Rhizobium, Achromobacter, Alcaligenes and Aerobacter; it could be characterized as an alternative to plants Cellulose for some fields of applications such as in the medical area, food and many industries [3]. The (AAB) acetic acid bacteria belong to the family Acetobacteracea. e, the (5- 6.5) is the optimum pH for the growth of AAB [4]. Vinegar is the product of acetic acid bacteria metabolism. It is an aqueous solution of acetic acid and other components, and it is consumed as a food preservative and seasoning. This product is the outcome of (2) stages of fermentation. Anaerobic fermentation is the first step, the alcoholic fermentation of sugars into ethanol caused by yeasts, and the second step is aerobic fermentation, the oxidation of ethanol into acetic acid caused by acetic acid bacteria (AAB) [5]. In organic vinegar that does not use preservative agents after opening the bottle, this could encourage the growth of cellulose-producing aerobic bacteria that are not removed entirely in the filtration procedure, which causes the creation of a pellicles layer on the surface medium of the product, which occurs even in conventional vinegar that contains preservative agents if the acetic acid bacteria are not removed ideally in the filtration method before bottling [6. Also, according to previous research, dates syrup (Alzahdy syrup) was used for BNC production from A. xylinum and showed high quantity of produced cellulose [7]. Presently, much research actions and attention are concentrated on the isolation and production of nano-cellulose fibers; the interest in nano-cellulose comes from the fact that the nano-cellulose collects the physical and chemical properties of Cellulose the ability to be chemically modified by a broad range of reactions and its hydrophilicity, with the features, of nanomaterial.
Ls high specific surface area and aspect ratio, also the microbial Cellulose has an excellent tensile strength in compared with plant cellulose [8]. The cellulose nanocrystals are prepared by the acid hydrolysis of BC, characterized as: nontoxic, environment-friendly, degradable, edible and biocompatible [9]. The (BNC) exhibits an overall amphiphilic configuration due to the high density of surface hydroxyl groups. In contrast, the hydrophobic interactions are initiated by the crystalline structure and extensive hydrogen bonds in polymer chains; for this reason, their amphiphilic properties could be applied to stabilize surfactant-free Pickering emulsions [10]. The Cellulose's poor solubility and low functionality hamper its application in various fields, such as in medical and pharmaceutical areas like wound healing and surgical methods; the inter-molecular and intra-molecular hydrogen bonds render the cellulose fibers insoluble in most solvents, different chemical modifications such as cross-linking, or graft- copolymerization, reactions were lately applied to prepare functionalized cellulose [11]. The produced bacterial nano-cellulose positive outcome of the nano-cellulose membrane or pellicles could be possibly observed as the formation of a gel-like mat that floats on the medium surface; this (BNC) produced by the bacterial isolate could be purified by (0.1N) of NaOH [12]. The structure of BNC, such as the determination of width, length, cross-linking, and chemical compositions of the Cellulose, could be characterized by using some methods like scanning electron microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDS) and Fourier transform infrared spectrophotometer (FTIR). Also, other essential parameters characterize the (BNC) such as the degree of polymerization (D, P) and crystallinity, which is measured by the XRD techniques [13]. In the present study, BNC, produced from acetic acid bacteria (AAB) isolated from vinegar, was purified and characterized,
MATERIALS AND METHODS
Bacterial isolates
The acetic acid bacteria (AAB) were isolated from apple vinegar and date palm vinegar using three culture media: GYC broth medium [14], GY broth medium, and GYC agar medium [15]. A total of 25 samples from different types of vinegar were collected under aseptic conditions from the local market in Baghdad city. About 5 isolates were characterized as AAB from the total samples according to procedures of [16, 14], and the identification of bacterial isolates was confirmed by the Real-Time Polymerase chain reaction (PCR) amplification technique [17], the bacterial isolate (5AC) was selected as BNC producer after screening.
Production medium
Palm dates liquid medium (pH= 6) was used as production medium, and it was prepared by mixing 15gm of palm dates (kind: Osta-Omran) waste with 100 ml of DW; this palm dates waste was blended by blender. After that, sifted in a sieve and then 1gm of glucose was added to each screwed cup of the medium [18].
Production of BNC from AAB:
A total of one liter of the production medium (palm dates liquid medium), was inoculated with overnight activated bacterial isolate (5AC) culture broth (the inoculum size 2%) and incubated at (30ºC) for (8-10 days), after this period, the BNC was performed in the medium, the (BNC) wet weight and dry weight then were determined.
Purification of (BNC) produced by Acetic acid bacteria (5AC):
The layers of (BNC) that formed, at the air-liquid interface in the production medium: (palm dates liquid medium) were taken and washed with water and soaked in (0.1M) NaOH at 80°C for (30 to 45 min) to remove the bacterial cells that probably attached to the BNC membrane. Then, the membranes were washed with distilled water several times to confirm the whole removal of the alkali; the membranes were left at neutral pH. The purified Cellulose was dried at room temperature until reaching a constant mass [3, 19].
Characterization of bacterial nanocellulose BNC:
After the purification and drying BNC membrane that formed by AAB (5AC), isolate, this dried membrane was characterized by:
Field Emission Scanning Electron Microscope (FESEM)
A piece of BNC layer was placed on a glass slide and allowed to dry, and then, a thin layer of gold was coated in the sample of BNC layer [20, 21]. The surface morphology and composition of thin films of this test were carried out in Alkhora company SEM lab/Baghdad using FEI (InspectTM F50) Field Emission Scanning Electron Microscope (FESEM) which was operated at a vacuum system; the operated accelerating voltage was kept in the range 200V to 30 kV.
Energy Dispersive X-ray Spectroscopy Scanning Electron Microscope (EDS-SEM or EDX-SEM):
To determine the elements composition of the sample, a piece of purified BNC layer and a small amount of manufactured cellulose suspension (as a standard) were placed on glass slide samples and left to dry, then analyzed by EDS-SEM device [21].
Fourier Transform Infrared Spectrophotometer (FTIR):
The characterization of functional groups of the BNC and the powder of Cellulose were investigated by FTIR analysis instrument (FTIR- 8400/Shimadzu-Japan), and the spectra were scanned in the range of (400–4000) cm−1. The samples were prepared by uniformly dispersing the dried BNC and the standard cellulose powder in a matrix of dry KBr in a mortar and grinding to mix well, then compressed to form an almost compact disc in a specific device under vacuum. KBr was used as a standard to analyze the samples [21].
RESULTS
Identification of Bacterial Isolates
Twenty-five samples were collected from different vinegar types: (apple and palm date vinegar), five isolates were characterized as acetic acid bacteria (AAB) from the total samples. The bacterial colony was aerobic, round, smooth, raised with white or off-white, and semi-translucent opacity. Microscopic examination of the isolates: displayed that they were all: Gram-negative, non-spore-forming and rod, (Figure 1). Results of biochemical tests showed that all isolates are negative for oxidase and positive for catalase, motile, and produce Cellulose. The results of the Real-Time PCR amplification technique confirmed the identification of these five isolates as acetic acid bacteria (AAB). The acetic acid bacteria (AAB) are Gram-negative, rod-shaped and can occur r as a single cell, in pairs or chains; they are motile due to the presence of flagella which can be either polar or peritrichous; they do not form endospores; they have an obligate aerobic metabolism, that the oxygen as terminal electron an acceptor [23, 24].
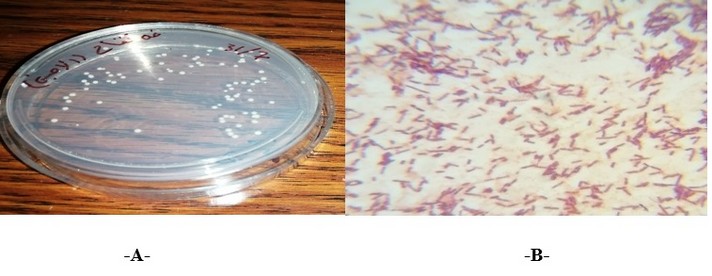
Figure 1. A- Acetic acid bacterial cells under the microscope (100X), B- Colonies of acetic acid bacteria (AAB) on GY agar medium
Production of BNC from AAB
After incubation of the AAB (5AC), a bacterial isolate that can produce bacterial nanocellulose (BNC) for (8-10) days in palm date liquid medium, the BNC was observed as a white pellicle with a diameter of approximately 48 mm covering the surface of the liquid medium (Figure 2), it was found that the (1Liter) of medium gave about (55.41gm) wet weight of BNC. Komagataeibacter xylinus (formerly Acetobacter xylinum) is the e most generally used species of bacteria for the production of bacterial Cellulose a (BC) since I it produces quite large amounts of this bacterial cellulose e from a wide range of carbon and nitrogen sources s in broth culture media [25]. For the production of BC c, carbon sources commonly used are agro-industrial wastes, for example, rotten fruit like pineapple peel juice, and sugar as a medium used to yield a quantity of bacterial cellulose [26]. The yield, of bacterial cellulose synthesis is up to (40) %, in, relation tow the starting carbon source in the large, ,scale production of BC [8]. The researchers found that the production of the bacterial nano-cellulose (BNC), representing, the assimilation, off sugar.s within the bacterial cells and the creation of β-1,4, glucan chains to lastly construct, the micro-structure of Cellulose, actually the BNC generated o on the wastes primarily presented analogous morphological features to the control BNC mainly a after washing [12]. Bacterial Cellulose could be industrially, produced and broadly, used in various, areas.. Doninii et al. (2010) [27] valuate that the, fermentation production of the bacterial Cellulose could achieve, a similar prroduction, efficacy with the growth, of plant cellulose, when the yield of bacterial Cellulose reaches up to (15) g/L in,500 hr [28].
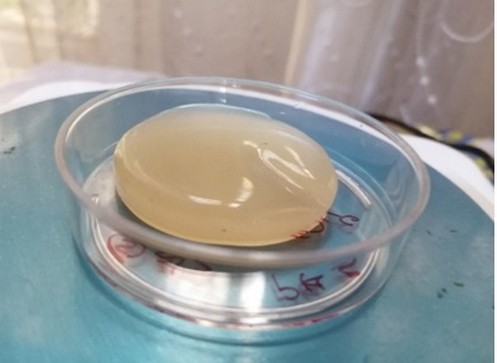
Figure 2. Bacterial nano-cellulose membrane: (wet membrane)
Purification of BNC produced by (5AC) Acetic acid bacteria:,
After the production of bacterial nanocellulose in (1L) of natural medium (palm date liquid medium), the BNC layers were harvested and purified by sodium hydroxide (0.1M NaOH). The wet weight of the BNC was measured after the purification and it was found about (43.11gm), while the dry weight of it was about (2.2gm) (Figure 3).

Figure 3. Bacterial nanocellulose membrane (dry membrane) of (5AC) Acetic acid bacteria
The BNC isolation and purification are relatively simple and do not need wide chemical o or any other types of treatments, in contrast with wood cellulose and plant cellulose [29]. The BNC membrane was separated and purified from the treatment medium with distilled water. (DW) and weak alkalin,e treatment of (0.5).M sodium hydroxide, where it was possible, to dry these membranes and characterize them by various techniques to investigate the membrane composition, crystallinity, and its fibrils morphology,, the results obtained from purification were acceptable, decreasing, the water band, of the biomaterial, increasing the crystallinity and also eliminating impurities, in the membrane such as: a bacteria and other, stuck materials and also with, the purification that performed, it will be possible to more additional study of the material for use as a product such as in: biomedical field and tissue engineering as it used as a biomaterial reinforcement, a [30]. The BNC purification using (1M) of sodium hydroxide ((.NaOH) solution is effective. And considered as eco-friendly with no signs of recalcitrant development as commonly found in plant cellulose purification steps [31].
Characterization of BNC, by
Field, Emission Scanning Electron, Microscope (FESEM,):
(FESEM) was used to clarify the surface morphology of nanostructured thin films. A cross-sectional view of the films could be taken, with the help of FESEM to get information about the thickness of the experimented film and also characterize the particle diameter in addition to the morphology of nanoparticles [22]. The morphology o of the BNC membrane, a was analyzed by the FESEM system. This technique of scanning electron microscope showed that the BNC consists from net of very fine fibers which made together the whole mat of bacterial Cellulose, the average diameter of the bacterial cellulose fibril was approximately (38.56 nm) (Figures 4 and 5).
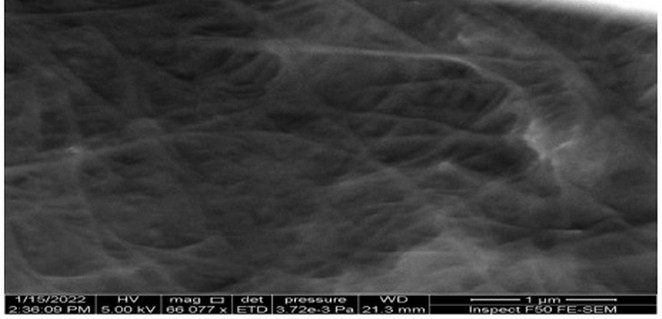
Figure 4. Bacterial nanocellulose (BNC) under Field Emission Scanning Electron Microscope (Top view)

Figure 5. BNC under, Field Emission Scanning Electron Microscope (FESEM) (Cross section of BNC).
The bacterial Cellulose structurally appears in SEM images as ribbon-like cellulose nanofibers that further interweave into 3D, reticulated net, so that it was characterized with high purity, high crystallinity and also high degree of polymerization [28]. The structure of Cellulose produced from bacteria (BC.) is the form of fiber with nano-sized width, analogous to silk, natural fiber formed from the silkworm; this bacterial Cellulose tends to be produced as nano-sized particles with high crystallinity during enzymatic or acidic methods, and these procedures are involved into the "gamification" of the bacterial Cellulose. [[32]. The SEM technique was used to study the surface morphology of the bacterial Cellulose, the BC dried samples were sputter, covered with a thin layer of evaporated gold, and a typical mesh of bacterial cellulose fibrils net was detected in the SEM images showed that the average fibril, diameter, of the bacterial Cellulose is 71 nm, which is a nanomaterial [33]. The bacterial cellulose membrane structure produced by K. maltaceti was examined by SEM analysis, the results showing that the bacterial cellulose membrane synthesized by K. maltaceti, after the cultivation, in HS medium at (30) °C, for about (21) dayss, this BC membrane consist of fibrils were condensed together into a reticular, or net structure with, porous size approximately (50–250), nm [24].
Characterization of BNC by EDS:
For detection and determination the elements composition of the bacterial dried (BNC) products from the acetic acid bacteria isolate (5AC), the Energy Dispersive X-ray Spectroscopy (EDS-SEM) or EDX devise was used, the results showed that AAB bacterial cellulose membrane composed of the elements: C (40.71%), O (13.98%), H (38.6%), N (5.41%) and Na (1.3%) as observed in (Figure 6) and as the results showed for the percentages of elements components of bacterial Cellulose which is could be attributed to the components of the medium that the bacterial isolate grown in it and the purification procedure for the bacterial cellulose membrane by (NaOH).
EDX spectroscopy was carried out to confirm the presence of the elements belonging to the functional groups for the Cellulose produced by Gluconacetobacter xylinus; the material was described using solid-state techniques because of the insolubility of Cellulose in common solvents as carbon and oxygen were already present in the structure of Cellulose, G. xylinus BC pellicles identified that the carbon content approximately (34.2%), oxygen content is about (38.35%) and the nitrogen content about (0.29%) [34
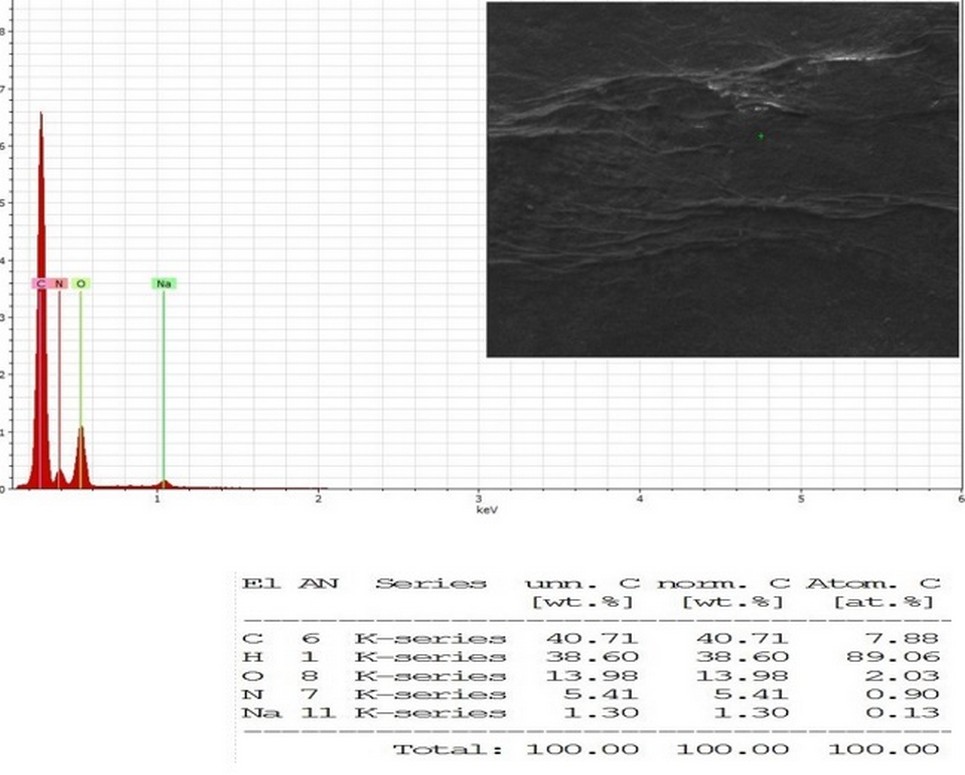
Figure 6. Energy dispersive X-ray spectroscopy (EDX-SEM) elemental mapping image of BNC from AAB (5AC) isolate
The BNC produced by Gluconacetobacter xylinus was analyzed by scanning electron microscopy (SEM) and for detection of the element's content using energy dispersive spectroscopy (EDX analysis), the elemental results disclose the basic component of BNC with their particular mass and atomic distribution within the BNC sample, the results are shown that the carbon content about (72.124%) and the oxygen content approximately (18.202%) 31.
Characterization of BNC by Fourier transform infrared spectrophotometer (FTIR):
The FT-IR analysis was performed in order to determine the functional groups of bacterial nanocellulose produced from acetic acid bacteria (5AC) isolate, the FTIR recorded for dried cellulose membrane sample at a wavelength range of 4000–400 cm−1, the bacterial nanocellulose fiber functional groups and chemical bonds was observed at the region with wavelength (800-1700) cm−1 very close peaks values with cellulose peaks values as showed in (Figure 7).
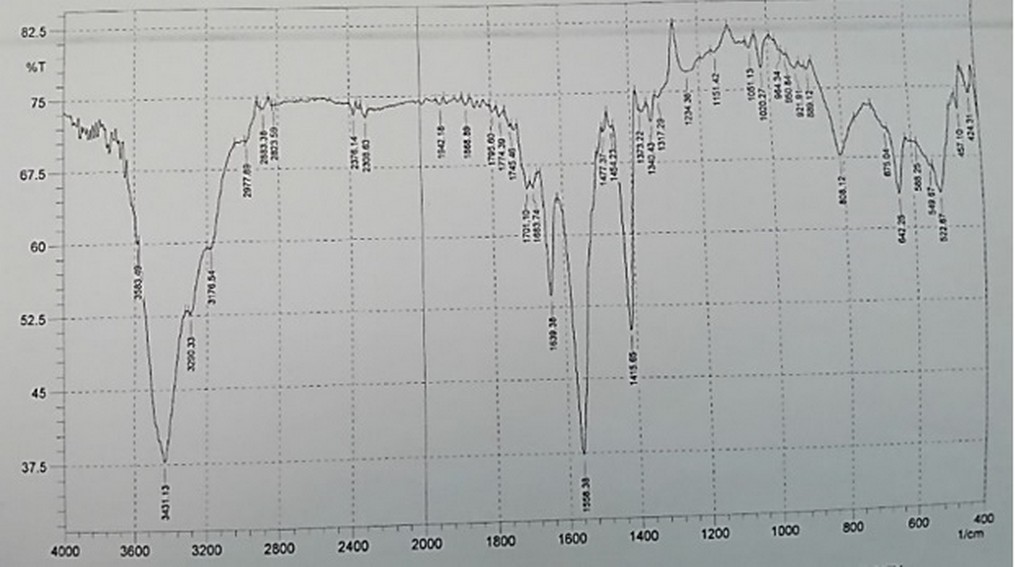
Figure 7. FTIR analysis for BNC produced from acetic acid bacteria (5AC) isolate
FTIR measurements were used in order to detect the presence of various functional groups in nanoparticle materials by using FTIR, spectrometer, FTIR spectra recorded in the solid case using the (KBr) pellets mechanism in the range of (4000-400) cm−1 [35,36]. These peaks (3431.13 and 3434.98) cm−1 may be expressed to the O-H stretching vibration mode [37]. The region with wavenumbers 800 and 1200 cm−1 supposed to be the fingerprint region for the carbohydrates also the wide vibration band at wavenumbers (3200–3400) cm-1 region could be allocated to the (OH) stretching vibration of the hydroxyl groups existing in the bacterial cellulose net fiber, the two comparatively smaller bands at (2800–2900) cm−1 may be considered to the stretching vibrations of CH, the peak at 1158 cm−1 was resultant from the (C–O–C) stretching vibration of the bacterial cellulose matrix [38]. The wavenumber of the peaks at the area of 1200–900 cm−1 ascended from the C–O and C–C stretching vibrations of the cellulose net fibers; the difference in the strengths of various bands could be due to the diverse thickness of the cellulose samples that used in the measurements of Fourier transform infrared spectrophotometer (FTIR) [39,38]. Due to the importance of the bacterial isolates, it was confirmed by (RT-PCR) amplification technique.17the molecular technique applied in different areas related to biology.40-62
CONCLUSION
The bacterial nanocellulose (BNC) synthesized by acetic acid bacteria (AAB) was efficaciously performed under cultural conditions using a natural medium, which is palm date liquid medium (pH 6) that was used as a production medium that incubated conditions at 30°C for (8-10) days. The BNC was purified by sodium hydroxide method, the BNC was described and different techniques determined its components, these techniques are: FESEM which used to clarify the surface morphology of nanostructured bacterial thin films, Energy Dispersive X-ray Spectroscopy (EDS) for detection and determination the elements composition of the bacterial dried (BNC) products and Fourier transform infrared spectrophotometer (FTIR) was performed in order to determine the functional groups of bacterial nanocellulose produced from acetic acid bacteria. The results from all these techniques and analyses gave the BNC more attention in many fields: medical, industrial and electrical applications, because of its valuable properties such as its nanofibers having a high ability to absorb liquids and also its high tensile strength and many other characteristics that make it essential in many applications.
Funding: Self-funded.
Acknowledgments: We appreciate the support of Al-Amal National Hospital for Cancer Management, Baghdad, Iraq.
Conflicts of Interest: No.
REFERENCES
1. Ali HM, Ali NA. Electrospinning of polycaprolactone/hydroxyapatite composites in wound dressing application. Iraqi Journal of Physics. 2022; 20(1): 14-25.
2. Zuppolini S, Salama A, Cruz-Maya I, Guarino V, Borriello A. Cellulose Amphiphilic Materials: Chemistry, Process and Applications. Pharmaceutics. 2022; 14(2): 386.
3. Rangaswamy BE, Vanitha KP, Hungund BS. Microbial cellulose production from bacteria isolated from rotten fruit. International Journal of polymer science. 2015; Hindawi.V.2015, ID 280784.
4. Trček J, Barja F. Updates on quick identification of acetic acid bacteria with a focus on the 16S–23S rRNA gene internal transcribed spacer and the analysis of cell proteins by MALDI-TOF mass spectrometry. Int. J. Food Microbiol. 2015; 196:137–44.
5. Du XJ, Jia SR, Yang Y, Wang S. Genome sequence of Gluconacetobacter sp. strain SXCC-1, isolated from Chinese vinegar fermentation starter. J Bacteriol. 2011; 193(13):3395-3396.
6. Sengun IY, editor. Acetic acid bacteria: Fundamentals and food applications. Boca Raton, 2015; USA: CRC Press.
7. Kamona ZK, Alshaikh Daher AA, Al Shamary EI. The use of Vitally Active Cellulose Membranes for the Reduction of Pathogenic Bacterial Count in White Cheese. Iraqi Journal of Science. 2021; 62(4): 1121-1127.
8. Klemm D, Kramer F, Moritz S, Lindstro¨m T, Ankerfors M Gray D, Dorris A. Nanocelluloses: a new family of nature-based materials. Angew Chem Int Edit. 2011; 50:5438– 5466.
9. Habibi Y, Lucia LA, Rojas OJ. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010; 110:3479–3500.
10. Yan H, Chen X, Feng M, Shi Z, Zhang W, Wang Y, Ke C, Lin Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloids and Surfaces B: Biointerfaces. 2019; 177:112–120.
11. Baldino L, Zuppolini S, Cardea S, Diodato L, Borriello A, Reverchon E, Nicolais L. Production of biodegradable superabsorbent aerogels using a supercritical CO2 assisted drying. J. Supercrit. Fluids. 2019; 156: 104681–104687.
12. Abol-Fotouh D, Hassan MA, Shokry H, Roig A, Azab MS, Kashyout AB. Bacterial nanocellulose from agro-industrial wastes: low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Scientific Reports nature research. 2020; 10:3491.
13. Kondo E, Rytczak P, Bielecki S. Chapter 4- Bacterial NanoCellulose Characterization, Editor(s): Gama, M.; Dourado, F. and Bielecki, S. Bacterial Nanocellulose, From Biotechnology to Bio-Economy. Elsevier.2016; P: 59-71.
14. Bellankimath A, Katti A, Hemalata VB, Meti BS. Isolation and characterization of the indigenous acetic acid bacteria from Western Ghats soil samples. Int. J. Curr. Microbiol. App.Sci. 2017; 6(9): 1255-1265.
15. Klawpiyapamornkun T, Bovonsombut S, Bovonsombut S. Isolation and Characterization of Acetic acid Bacteria from Fruits and Fermented fruit juices for Vinegar Production. Food and Applied Bioscience Journal. 2015; 3 (1): 30–38.
16. Zahoor T, Siddique F, Farooq U. Isolation and characterization of vinegar culture (Acetobacter aceti) from indigenous sources. British Food Journal. 2006; 108(6): 429-439.
17. Longin C, Guilloux-Benatier M, Alexandre H. Design and Performance Testing of a DNA Extraction Assay for Sensitive and Reliable Quantification of Acetic Acid Bacteria Directly in Red Wine Using Real Time PCR. Frontiers in Microbiology. 2016; 7:831.
18. Azeredo HMC, Barud H, Farinas CS, Vasconcellos VM, Claro AM. Bacterial Cellulose as a Raw Material for Food and Food Packaging Applications. Front. Sustain. Food Syst. 2019; 3:7.
19. Ahmed EF, Ali WS, Heider NH. Purification and Characterization of Bacterial Nanocellulose Produced by Gluconobacter 5AC Isolate from Apple Vinegar. Tropical journal of natural product research. 2023; 7(3): 2580–2585.
20. Yaaqoob LA. Effect of Using Zinc Oxide and Gold Nanoparticles on Experimentally Induced Diabetic Rats. Ph.D. Thesis. 2016; College of Science. University of Baghdad.
21. Krishnan VN, Ramesh KA. Synthesis and characterization of cellulose nanofibers from coconut coir fibers. Journal of applied chemistry. 2013; 6(3):18-23.
22. Ali IM. Investigation on the Effect of Manganese and Cerium on ZnS Nanoparticles Prepared by Microwave Irradiation. Ph.D. Thesis. 2015; College of Science. University of Baghdad.
23. Holt JM, Krieg NR, Sneath PHA, Staley JY, Williams ST. Genus Acetobacter and Gluconobaceter. In Bergery’s Manual of Determinative Bacteriology.9 th edn., Williams & Ilkens, Maryland, U.S.A. 1994; pp: 71-84.
24. Thongwai N, Futui W, Ladpala N, Sirichai B, Weechan A, Kanklai J, Rungsirivanich P. Characterization of Bacterial Cellulose Produced by Komagataeibacter maltaceti P285 Isolated from Contaminated Honey Wine. Microorganisms. 2022; 10: 528.
25. Zhong C, Zhang GC, Liu M, Zheng XT, Han PP, Jia SR. Metabolic flux analysis of Gluconacetobacter xylinus for bacterial cellulose production. Appl Microbiol Biotechnol. 2013; 97:6189–6199.
26. Jozala A, Pe´rtile R, dos Santos C, de Carvalho Santos-Ebinuma V, Seckler M, Gama F, Pessoa AJ. Bacterial cellulose production by Gluconacetobacter xylinus by employing alternative culture media. Appl Microbiol Biot. 2015; 99:1181–1190.
27. Donini ÍAN, Salvi DTBD, Fukumoto FK, Lustri WR, Barud HS, Marchetto R, Messaddeq Y, Ribeiro SJL. Biosynthesis and recent advances in production of bacterial Cellulose. Eclética Química. 2010; 35: 165–178.
28. Zhong C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020; 8:605374.
29. Seddiqi H, Oliaei E, Honarkar H, Jin J, Geonzon CL, Bacabac RG, Klein-Nulend J. Cellulose and its derivatives: towards biomedical applications. Cellulose. 2011; 28:1893–1931.
30. Sousa LP dos S B de, Leite PMSCM, Vieira AA, Faria AC, Vieira L. Effect of water and alkali on purification bacterial cellulose membrane from Kombucha. Research, Society and Development. 2021; 10(15): e526101523267.
31. Abba M, Abdullahi M, Md Nor MH, Chong CS, Ibrahim Z. Isolation and characterization of locally isolated Gluconacetobacter xylinus BCZM sp. with nanocellulose producing potentials. IET Nanobiotechnology. 2017; 12(1): 52-56.
32. Choi SM, Shin EJ. The nanofication and functionalization of bacterial Cellulose and its applications. Nanomaterials. 2020; 10:406.
33. Jia Y, Wang X, Huo M, Zhai X, Li F, Zhong C. Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomaterials and Nanotechnology. 2017; 7: 1–8.
34. Orlando I, Basnett P, Nigmatullin R, Wang W, Knowles JC, Roy I. Chemical Modification of Bacterial Cellulose for the Development of an Antibacterial Wound Dressing. Front. Bioeng. Biotechnol. 2020; 8:557885.
35. Ahmed SA, Hasan HM, Ahmed EF. Silver nanoparticles biosynthesis and their antimicrobial activity against wild and mutant isolates of different G-ve bacterial types. Bioscience Research. 2018; 15(4): 2997-3005.
36. Ali IM, Ibrahim IM, Ahmed EF, Abbas QA. Structural and Characteristics of Manganese Doped Zinc Sulfide Nanoparticles and Its Antibacterial Effect against Gram-Positive and Gram-Negative Bacteria. Open Journal of Biophysics. 2016; 6 (1): 1-9.
37. Abdulsattar ZS, Kadhim FJ. Synthesis and characteristics of electrochromic glass with multi-layer configuration based on glass/ITO/WO3/ZrO2/NiO/ITO/glass. Iraqi journal of applied physics. 2019; 15(4): 17-22.
38. Padmanabhan SK, Lionetto F, Nisi R, Stoppa M, Licciulli A. Sustainable production of stiff and crystalline bacterial Cellulose from orange peel extract. Sustainability. 2022; 14: 2247.
39. Czaja W, Romanovicz D, Brown RM. Structural investigations of microbial Cellulose produced in stationary and agitated culture. Cellulose. 2004; 11: 403-411.
40. Hashim ST, Fakhry SS, Rasoul LM, Saleh TH, Alrubaii BAL. Genotyping toxins of Clostridium perfringens strains of rabbit and other animal origins. Trop J Nat Prod Res. 2021; 5(4):613–616.
41. Al-Musawi, AHO, Aziz, HM, Khudair, S, Saleh, TH. Molecular characterization of HBB gene mutations in beta-thalassemia patients of Southern Iraq. Biomedicine. 2022; 42(5):1040-1043.
42. Rasin KH, Algabar FA, Al-Obaidi M, Krayfa AH. Evaluation of Medical waste management during the COVID-19 in Diyala Governorate. Environ Eng Manag J. 2022; 21(12):1931–1944.
43. Eyad HN, Adbulateed YA, Lafi SA. Abnormal presentation of TB patients: anthropological study. Ann Trop Med Public Health. 2019; 22(VI): S186.
44. Abdulrazaq, RA., Mahmood, WS., Alwan, B, Saleh, TH, Hashim, ST, Al-Rubaii, BAL. Biological study of protease produced by clinical isolates of Staphylococcus aureus. Res J Pharm Technol.2022; 15(12):5415-5420.
45. Rasoul LM, Allami RH, Alshibib AL, laftaah Al-Rubaii BA, Sale TH. Expression and cytotoxic effect of recombinant Newcastle Disease Virus (rNDV) vector expressing enhanced green fluorescent gene in JHH5 cell line. Biomedicine. 2023; 43(1):205-209.
46. Allami RH, Hassoon AH, Abdulateef YM, Ghani AA. Genetic Association of Angiotensin-converting enzyme 2 ACE-2 (rs2285666) Polymorphism with the Susceptibility of COVID-19 Disease in Iraqi Patients. Trop J Nat Prod Res. 2023;7(2):2346-2351.
47. Kadhim AL-Imam MJ, AL-Rubaii BAL. The influence of some amino acids, vitamins and anti-inflammatory drugs on activity of chondroitinase produced by Proteus vulgaris caused urinary tract infection. Iraqi J Sci. 2016; 57(4A):2412-2421.
48. Jawad NK, Numan AT, Ahmed AG, Saleh TH, Al-Rubaii BA. IL-38 gene expression: A new player in Graves’ ophthalmopathy patients in Iraq. Biomedicine. 2023;43(1):210-215.
49. Shehab ZH, AL-Rubaii BAL. Effect of D-mannose on gene expression of neuraminidase produced from different clinical isolates of Pseudomonas aeruginosa. Baghdad Sci J. 2019; 16(2):291–298.
50. Abdulla L, Ismael MK, Salih TA, Malik SN, Al-Rubaii BAL. Genotyping and evaluation of interleukin-10 and soluble HLA-G in abortion due to toxoplasmosis and HSV- 2 infections. Ann Parasitol. 2022; 68(2):385–390.
51. Jiad AL, Ismael MK, Muhsin SS, Al-Rubaii BAL. ND2 Gene Sequencing of Subfertile Patients Recovered from COVID-19 in Association with Toxoplasmosis. Bionatura. 2022; 7(3):45.
52. Rasoul LM, Nsaif MM, Al-Tameemi MT, Al-Rubaii BA. Estimation of primer efficiency in multiplex PCR for detecting SARS-Cov-2 variants. Bionatura. 2022; 7(3):48.
53. Al-Rubii, BAL. Cloning LasB gene of Pseudomonas aeruginosa eiastase 10104-2aI in E. coli BL21 and E.coli DH5α and investigated their effect on the stripping of vero cells. Pakistan J Biotechnol.2017; 14(4):697-705.
54. Saleh, T.; Hashim, S.; Malik, S.N.; Al-Rubaii, B.A.L. The impact some of nutrients on swarming phenomenon and detection the responsible gene RsbA in clinical isolates of Proteus mirabilis. Int J Pharm Sci Res. 2020;1(6):437-444.
55. Bresam S, Al-Jumaily RM, Karim GF, Al-Rubaii BA. Polymorphism in SNP rs972283 of the KLF14 gene and genetic disposition to peptic ulcer. Biomedicine. 2023; 43(1):216-220.
56. Shehab ZH, Laftah BA. Correlation of nan1 (Neuraminidase) and production of some type III secretion system in clinical isolates of Pseudomonas aeruginosa. Biomed Res. 2018;15(3):1729-1738.
57. Rasoul, LM., Marhoon AA, Albaayit SFA, Ali RW, Saleh,TH, Al-Rubaii BAL. Cytotoxic effect of cloned EGFP gene on NCI-H727 cell line via genetically engineered gene transfer system. Biomedicine. 2022; 42(5): 938-942.
58. Abdulateef Y. Role of PSA in Diagnosis of Chronic Prostatitis. Indian Journal of Forensic Medicine & Toxicology. 2020; 14(1):774-779.
59. Ali SM, Laftah BA, Al-Shammary AM, Salih HS. Study the role of bacterial neuraminidase against adenocarcinoma cells in vivo. In AIP Conference Proceedings 2021, 2372, 030009.
60. Hamoode RH, Alkubaisy SA, Sattar DA, Hamzah SS, Saleh TH, Al-Rubaii BAL. Detection of anti-testicular antibodies among infertile males using indirect immunofluorescent technique. Biomedicine. 2022; 42 (5):978-982.
61. Salih HS, Al-Shammari AM, Laftaah BA. Intratumoral co-administration of oncolytic newcastle disease virus and bacterial hyaluronidase enhances virus potency in tumor models. J Glob Pharma Technol. 2018; 10(10):303-310.
62. Abbas MS, Ahmed AG, Ali SQ, AL-Rubaii BA. Immunological inflammatory factors in patients diagnosed with COVID-19. Biomedicine. 2023; 43(1):230-235.
Received: 25 June 2023/ Accepted: 26 August 2023 / Published:15 September 2023
Citation: Faroun Ahmed E, Shawkat Ali W , Hasan Heider N. Description and determination of the nanocellulose components produced from acetic acid bacteria. Revis Bionatura 2023;8 (3) 112 http://dx.doi.org/10.21931/RB/2023.08.03.112
