2023.08.01.49
Files > Volume 8 > Vol 8 No 1 2023
Neutralizing antibodies as a correlate of protection against classical swine fever in Porvac® vaccinated pigs
Marisela Suárez-Pedroso1*, Yusmel Sordo-Puga1, María Pilar Rodríguez-Moltó1, Paula Naranjo-Valdés2, Danny Pérez-Pérez1, Iliana Sosa-Teste3, Carlos Montero-Espinosa1, Yohandy Fuentes-Rodríguez1, Talía Sardina-González1, Elaine Santana-Rodríguez1, Milagros Vargas-Hernández1, Ayme Oliva-Cárdenas1, Nemecio González-Fernández4, Eddy Bover-Fuentes4, Carlos A. Duarte1, Mario Pablo Estrada-García1.
1 Departamento de Biotecnología Animal. Centro de Ingeniería Genética y Biotecnología. Ave. 31 be/158 and 190, Cubanacán, Playa, Apdo 6162, La Habana 10600, Cuba
2 Unidad de Laboratorio Central para Salud Agropecuaria (ULCSA), La Habana 11400, Cuba
3 Centro de Toxicología Experimental (CETEX), Centro Nacional para la Producción de Animales de Laboratorio (CENPALAB), Mayabeque 10300, Cuba.
4 Departamento de Investigación Desarrollo. Centro de Ingeniería Genética y Biotecnología, Camagüey, Cuba
Corresponding author: *Marisela Suárez. Departamento de Biotecnología Animal, Centro de Ingeniería Genética y Biotecnología, Ave 31 and 158, Cubanacán, Playa. Apdo: 6162, La Habana 10600, Cuba. Tel: (53) 250 4420. Fax: (53-7) 33 1779. e-mail: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.49
ABSTRACT
Porvac is a classical swine fever (CSF) subunit vaccine. It is safe and induces a robust neutralizing antibody response, sterilizing immunity, and early protection, and it prevents vertical transmission in pregnant sows. The methodology to approve Porvac batches is a challenging experiment in pigs with a virulent CSF virus strain. However, there is an ethical reason to reduce, at minimum, the use of animals in these lethal experiments. The knowledge indicates that neutralizing antibody titers in the blood could be a good correlate of protection. The results of 22 challenge experiments involving 116 Porvac vaccinated and 38 unvaccinated animals were analyzed. All vaccinated animals remained free from CSF clinical signs and pathological lesions and were negative for viral isolation after the challenge.
In contrast, all unvaccinated pigs developed clinical and pathological signs of the disease and had to be euthanized eight days post-challenge. All vaccinated pigs exhibited high neutralizing antibody titers, with a geometric mean value of 1: 5153. The lower titer registered was 1: 800. A complete correspondence between neutralizing antibody titers and protection was demonstrated. These results support substituting the viral challenge test for the neutralizing peroxidase-linked assay in the release of Porvac® batches.
Keywords. Classical swine fever; virus; subunit vaccine; viral challenge; neutralizing antibodies
INTRODUCTION
Classical Swine Fever (CSF) is a highly contagious and devastating viral disease with a severe socio-economic impact on the pig industry worldwide 1. The etiological agent, CSF virus (CSFV), is icosahedral and enveloped positive-stranded RNA virus member of the Pestivirus genus of the Flaviviridae family 2.
In endemic areas, vaccination using modified live vaccines (MLV) is mainly directed to limit the effects of the disease or as a first step within a general program for the control and eradication of the virus 3. However, MLV can't discriminate between infected and vaccinated animals, require a cold distribution chain, and maternal-derived neutralizing antibodies compromise its immunogenicity in the piglets 4. These drawbacks have limited the application of MLV in disease-free regions. Several subunit vaccines have been developed to substitute MLV, but they show a late onset of protection compared to MLV and provide incomplete protection against vertical transmission 3,5-7.
Porvac® is a novel subunit marker vaccine against CSFV. It is based on a chimeric antigen (E2-CD154), comprising the E2 protein of CSFV and the molecular adjuvant CD154. This vaccine has been safe and capable of inducing an unusually rapid onset of protection against a challenge with a highly pathogenic CSFV strain, similar to the one described for MLV 8-10. The animals immunized with Porvac® develop high neutralizing antibodies (NAb) titers and cell-mediated immune responses. Furthermore, the administration of Porvac® to pregnant sows interferes with the transplacental transmission of the virus 11.
Porvac® has been registered in Cuba since 2017 (Sanitary Registry 763) and has currently been used in large state-owned pig production units, genetic units, and small private farms. A potency test, consisting in the challenge of the vaccinated animals with 105 PLD50 of a highly virulent strain, has been established to release the batches of Porvac®. This test has been previously used in the batch release process for MLV 12,13. However, it involves suffering for control animals, which would have to die to ensure vaccine quality. Furthermore, it requires the handling of large amounts of virus, and, therefore, special contention facilities are needed to avoid dissemination of the virus, increasing the costs of quality control.
As stated in the last edition of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 14, the efficacy of veterinary vaccines should be demonstrated through accurate statistical analysis of the host species. Nevertheless, challenge experiments in the final species may be unnecessary if data on the predictive value of serological tests are available. Wherever possible, applying procedures to replace, reduce and refine (the three R rule) the animals involved in such studies should be encouraged. The potency tests required for the approval of each vaccine batch must correlate with the vaccine's efficacy in the host species 15.
Previous work has established that neutralizing antibody (NAb) titers are a good correlate of protection against CSFV in swine. A NAb titer equal to or higher than 1:50 is considered protective 16-18. In line with these experimental data, the chapter of the OIE Terrestrial Manual about protein subunit vaccines based on the CSFV E2 protein recommends that the induction of specific anti-E2 antibodies in vaccinated pigs can be used to confirm the potency of each batch once the title has been correlated with the results of the efficacy test 14.
Therefore, this study aimed to demonstrate the correspondence between the NAb titers in the serum of Porvac-vaccinated animals and protection from lethal viral challenges. The final goal is replacing the viral challenge test for the neutralizing peroxidase-linked assay in the routine release of Porvac® batches.
Vaccine
Five batches of the Porvac® were evaluated (P51031, P61011-1, P61021-1, P71011-1, P81011-1). Each 2 mL dose contains 50 μg of E2-CD154 antigen (25 μg/mL) in MontanideTM ISA 50V2 oil adjuvant (SEPPIC, Paris). The vaccine was kept at 2-8 C until use. Vaccination and viral challenge were repeated every six months for some batches, which increased the number of animals evaluated in this analysis to a total of 116 vaccinated animals and 38 unvaccinated control animals.
Experimental animals
Nine weeks old Crossbred Duroc/Yorkshire swine (25-30 Kg) (CENPALAB, Cuba), belonging to an unvaccinated, CSF-free herd, were used. A total of 154 animals were used, 116 were vaccinated with Porvac®, and the other 38 were used as unvaccinated controls for the challenge. The animals were housed in separate experimental boxes, following the animal welfare regulations and standards established by the Animal Health Department of the Ministry of Agriculture in Cuba. Vaccination/challenge experiments were conducted under appropriate high containment facilities, following the animal welfare regulations and standards according to EU Directive 2010/63/EU and Good Clinical Practices 14. The Ethical and Animal Welfare Committee of CENPALAB, Cuba, approved and supervised the protocols.
Vaccination and challenge schedules
A two-dose vaccination schedule was conducted, with 21 days interval between injections. A volume of 2 ml was administered intramuscularly in the neck. The animals were challenged between 8 and 10 days post-vaccination (dpv) with 105 PLD50 of the highly virulent CSFV "Margarita" strain, belonging to subgenotype 1.4 19,20, by intramuscular injection in the neck.
The clinical signs compatible with CSFV infection in the animals after viral challenge were scored from 0 to 18, according to Mittelholzer et al. 21, with some modifications. The changes consisted of eliminating three parameters (body tension, body shape, and breathing), and adding the rectal temperature. The pigs were visually inspected for at least 15 minutes per day before and during the feeding and cleaning of the boxes. A dairy registry was filled for each animal with all the relevant clinical signs (rectal temperature, dullness, anorexia, prostration, constipation, diarrhea, cutaneous hyperemia, posterior paralysis and paresis, conjunctivitis, unsteady gait, convulsions, lack of response to stimulus, and presence of cutaneous or mucosal lesions. For ethical reasons, control animals were euthanized eight days post-challenge (dpc) when they reached clinical scores higher than 10. Vaccinated animals were evaluated until 21 dpc. The macroscopic anatomopathological analysis was conducted immediately after the sacrifice. Samples from the spleen and tonsils were collected in some cases. Some of these samples were processed for virus detection through the viral isolation technique (VI).
The challenge test for Porvac® includes several acceptance criteria, which are listed below:
● All control animals must get the disease severely or die after the challenge.
● More than 80 % of the animals vaccinated with the full schedule must show clinical protection.
● No vaccinated animal must develop a fever (temperature above 40 ºC) for 3 or more consecutive days.
● After the viral challenge, vaccinated animals should be free from CSF-compatible macroscopic lesions.
Blood samples
Blood samples were taken in the presence or absence of an anticoagulant. Whole blood and serum samples were stored at -70 °C or -20 °C, respectively, until their use for virus isolation or antibody detection. The samples were taken on day 0 (before vaccination), day 21 (after one immunization), at 28-30 days (7-10 days after the second administration), and 7 and 21 days after the challenge.
Cell line
The PK-15 cell line (ATCC CCL-33TM) was used for the Neutralizing Peroxidase Linked Assay (NPLA) 22 and viral isolation 23. A cell culture-adapted variant of the highly virulent CSFV strain Margarita, gently provided by the Centro Nacional de Sanidad Agropecuaria, Mayabeque Cuba, was used in NPLA.
CSFV NAb detection (NPLA test)
NAb titers in the serum were determined by the Neutralizing Peroxidase Linked Assay (NPLA) 22. Titers were expressed as the reciprocal of the higher dilution of serum that neutralized 100 TCID50 of Margarita strain in 50% of the culture replicates. The virus was detected by incubation with the anti-E2 Mab CBSSE2.3 (CIGB-SS, Cuba) conjugated to horseradish peroxidase, followed by 3-amino-9-ethyl carbazole substrate and hydrogen peroxide.
Viral isolation
For viral isolation, organs of vaccinated and control animals were taken at sacrifice, and blood samples at different post-challenge moments. One cubic centimeter of each organ was macerated in a mortar with 1 mL of DMEM medium. The macerated material was suspended in 4 mL of DMEM medium and allowed to stand for one hour at room temperature, then centrifuged for 15 min at 1200 g. The supernatant was collected and stored at -70 °C. Two successive passages of this supernatant were made in the PK15 cell line (ATTC CCL-33). After the last incubation, the cells were fixed with heat. The presence of CSFV antigens in the cells was detected by incubation with the anti-E2 Mab CBSSE2.3 (CIGB-SS, Cuba) conjugated to horseradish peroxidase, followed by 3-amino-9-ethyl carbazole substrate and hydrogen peroxide.
Statistical analysis
The software GraphPad Prism v6.0 (GraphPad Software, Inc., La Jolla, USA) was used for statistical analysis. The geometric mean (GM) of the NAb titers and the confidence intervals of the GM were calculated for each vaccine batch and all vaccinated animals after one or two administrations of the vaccine. The Kolmogorov-Smirnov test and variance homogeneity determined normality by the Levene test. Mann-Whitney test was used to compare the NPLA titers between two experimental groups and the Kruskal-Wallis test, followed by the Dunn test, was applied to compare the GM of the NAb titers among several experimental groups.
RESULTS AND DISCUSSION
NAb response in Porvac® vaccinated animals
The 116 Porvac-vaccinated pigs seroconverted after the first administration of the vaccine (Figure 1). The GM of the NAb at day 21 after the first immunization was 1: 358.6, and the GM's lower and upper 95% confidence intervals (CI) were 302.3 and 425.4, respectively. The lowest NAb titer observed was 1:10 (in only one pig), and the highest was 1:1600.
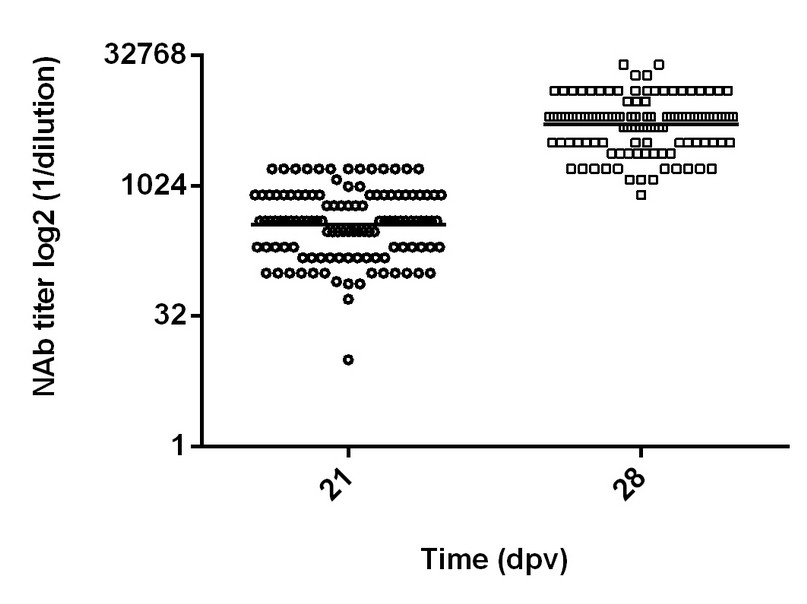
Figure 1. NAb titers in Porvac® vaccinated pigs. The X-axis scale is log2. Dpv: days post-vaccination. NAb was measured at 21 dpv (1 immunization) and 28 dpv (2 immunizations). n = 116. Horizontal lines represent the geometric mean of each group. Significant statistical differences were found between the two groups (Mann-Whitney, p = < 0.0001).
A strong anamnestic response was observed after the second immunization (Figure 1). The GM of the NAb titers increased to 1: 5217, with CI of 4571 and 5954. Those NAb titers are significantly higher than those observed after day 21 after the first immunization (Mann-Whitney, p = 0.0001). The lowest individual NAb titer was 1:800, and the highest was 1:25600. Moreover, 78 % of the NAb titers were more increased than 1:3200.
The frequency distribution of the NAb titers at 21 and 28 dpv is presented in Figure 2. The marked differences between one and two doses of Porvac® are evident. From these results, and based on the published data that indicate that a NAb titer of 1:50 is sufficient to protect pigs from CSFV challenge 16-18, it is possible to predict that all animals should be protected after the challenge experiments.
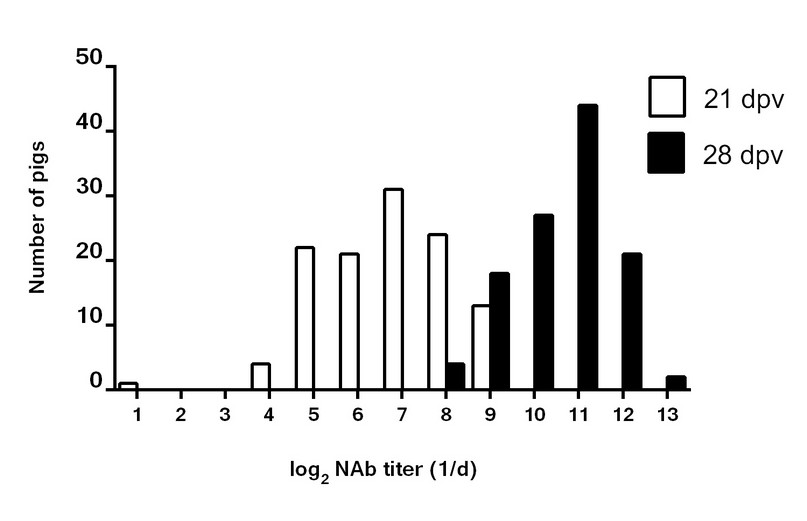
Figure 2. Frequency distribution of the NAb titers in pigs with 1 or 2 immunizations with Porvac®. Dpv: days post vaccination, 21 dpv (1 dose), 28 dpv (2 doses), n = 116.
Even after a single immunization, only one animal out of 116 showed NAb titers below the protective threshold. Therefore, it is very likely that the protective status of the animals can be achieved after only one dose in the 99.14% of vaccinated animals. This is an advantageous property for a vaccine that is intended to be applied in an endemic region where a rapid onset of immunity is needed. Other recombinant subunit vaccines have conferred only incomplete protection after one administration 18,24,25.
The five batches of Porvac® studied induced similar levels of NAb
Figure 3 shows the NAb titers induced by the five batches of Porvac looked at 28 dpv. No statistically significant differences were observed among the five batches (Kruskal-Wallis, p = 0.4207). These results demonstrate the robustness and stability of the Porvac® production process.
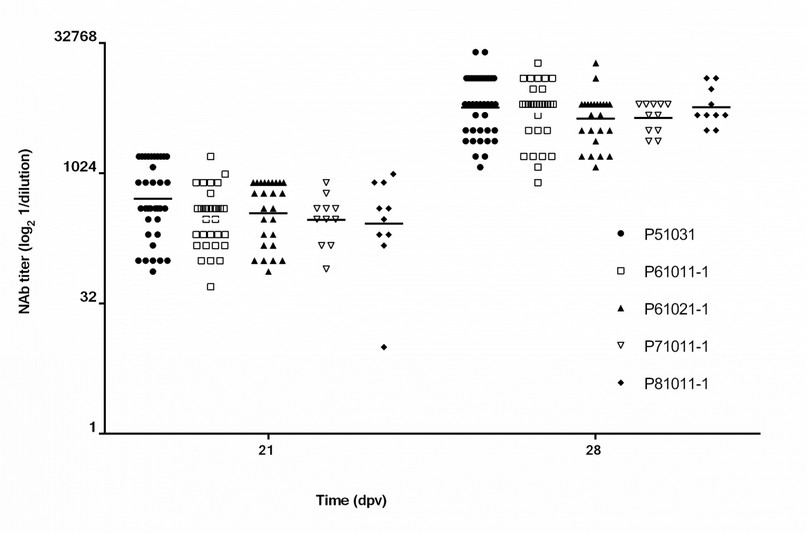
Figure 3. NAb titers induced by five production batches of Porvac. NAb titers were measured at 21 dpv (1 dose) and 28 dpv (2 doses). Horizontal lines represent the geometric mean of each group. Batches: P51031, n=37; P610111-1, n=32; P61021-1, n=26; P71011-1, n =11; P81011-1, n=10). No significant differences were found among the different batches after one or two immunizations (Kruskal-Wallis p>0.05).
All Porvac vaccinated pigs were protected against the CSFV challenge
All 22 independent challenge assays fulfilled the success criteria established for the test. All 116 vaccinated animals were protected from the viral challenge. They remain with good health status and appetite and without clinical signs of the disease. All pigs gain weight during the post-challenge period. Even the pig with the lowest NAb titer on the day of the challenge (1:800) was fully protected. The macroscopic examination of the main target organs conducted after the sacrifice at 21 dpc showed no pathological lesions in the vaccinated animals.
95 % of the vaccinated animals show typical temperature values after the challenge. The average temperature of the animals during the 21 dpc, did not exceed 39.6 ºC, within the physiological values for species 26 (Figure 4). Temperature values above 40 ºC were observed in 6 out of 116 animals in the study (5%) in punctual days after the challenge. However, the vaccines passed the test since the acceptance criterion established that temperature must be above 40 ºC for 3 consecutive days to be considered as relevant fever. Therefore, these transient temperature increases were supposed to be related to other punctual health issues in the animals.
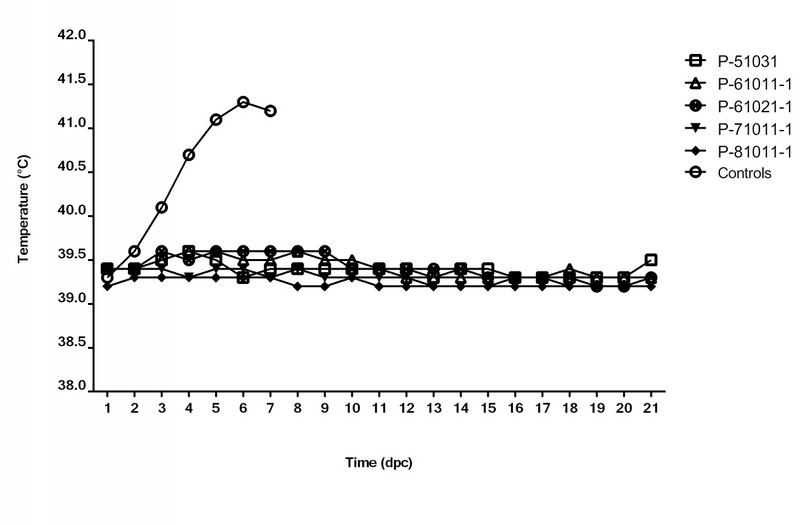
Figure 4. Time course of the average body temperature after the challenge. The average temperatures in the five groups of animals vaccinated with different batches of Porvac® and in control animals are represented. Vaccinated pigs were monitored during 21 dpc. All control pigs were euthanized at 8 dpc. dpc, days post challenge.
Moreover, all samples from vaccinated animals analyzed in these studies were negative for viral isolation. These results correspond with o the animals' good health after the viral challenge and the examination of the target organs after the necropsy.
In contrast, control animals experience a rapid rise in the rectal temperature (Figure 4) and CSF-compatible clinical signs from 3 dpc. In this group, the temperature raised sustainably above 40 ºC up to values above 41.4 ºC. From six dpc, a marked deterioration in the animal health was observed (>10 points in the clinical score), therefore, all control animals were sacrificed at 8 dpc to avoid unnecessary suffering. The pigs suffered diarrhea, conjunctivitis, respiratory disorders, anorexia, ataxia, and prostration due to severe nervous symptoms. Figure 5 shows the time course of the clinical score in vaccinated and control animals after the viral challenge.
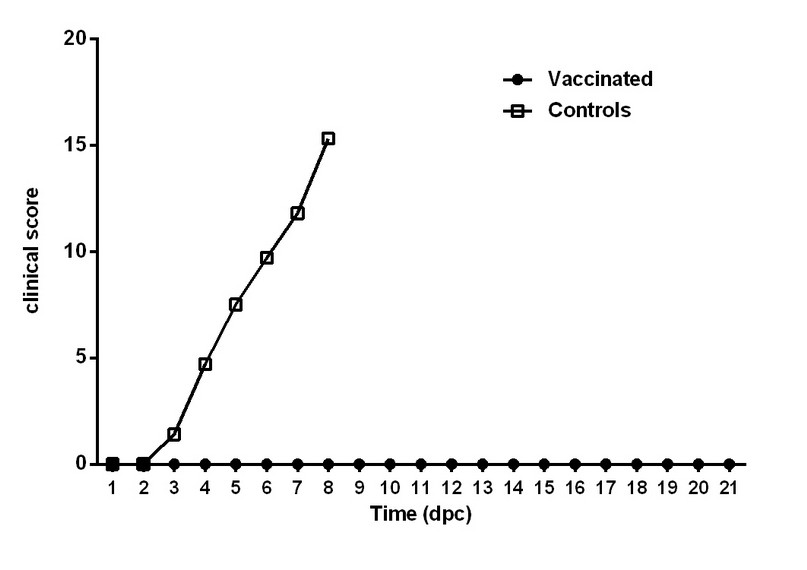
Figure 5. Time course of the clinical score after the challenge. The average clinical scores in vaccinated and control animals after the challenge are represented. Vaccinated pigs were monitored for 21 dpc. All control pigs were euthanized at 8 dpc. dpc, days post challenge
Moreover, CSF-compatible macroscopic lesions were also observed in the target organs. Histological examination of the immune system in unvaccinated animals exposed to the virus shows peripheral and local depression (lymphopenia in the spleen, mesenteric lymph nodes, and Peyer's patches in the ileum). An inflammatory reaction (diffuse and perivascular) in the central nervous system with the predominance of lymphocytes at the cortex level and meninges was observed in the control animals evaluated. These lesions corresponded with this group's clinical score and the disease's development.
Finally, and in agreement with the lesions found in the target organs, 100 % of blood samples and organs analyzed from control pigs were positive for viral isolation.
These findings confirm that the viral challenge was effective in all the independent experiments conducted and, therefore, the high NAb titers developed in Porvac® vaccinated pigs conferred full protection against a lethal CSFV challenge. NAb are capable of sequestering the virus outside the target cells and arresting its multiplication.
Table 1 summarizes the results of the 22 challenge experiments conducted. There is an absolute correspondence between the presence of CSFV NAb in the serum of Porvac® vaccinated animals and protection against the viral challenge. Taking together, these results demonstrate for the first time that NAb elicited by immunization with Porvac® is a good correlate of protection against CSFV infection, as reported by other authors for MLV or other subunit vaccines in vaccinated/challenge animals.
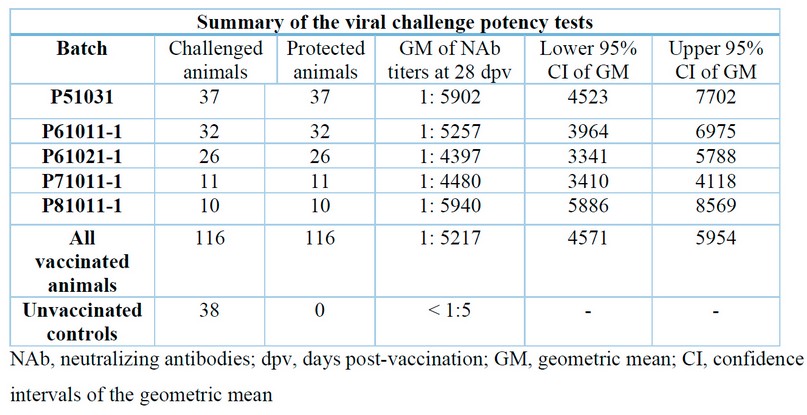
Table 1. Summary of the challenge experiments with Porvac® vaccinated pigs
Therefore, a potency test consisting of vaccinating pigs with Porvac® and measuring the NAb titers with the NPLA test can be proposed as a surrogate for the viral challenge experiment.
A NAb titer of 1:400 could be established as a cutoff value for approval of the production batches. This value is well above the 1:50 titer proposed by others as the cutoff for protection, implying a safety margin for a vaccine intended to be used in CSFV-endemic regions.
The following acceptance criteria would be considered for each production batch:
● All animals must develop NAb titers equal to or higher than 1: 400 after completing the vaccination schedule (21-day interval between the two doses)
● The geometric mean of the NAb titers of all vaccinated animals must be greater than 1: 1000.
By eliminating the viral challenge, the suffering of the animals will be reduced, as well as the risk of dissemination of the high-virulence strain used. The duration of the test and the costs will also be reduced.
CONCLUSIONS
In summary, a complete correspondence between the neutralizing antibody titer in the sera of Porvac© vaccinated pigs and protection from CSFV challenge have been demonstrated in a large set of experimental data. These results support substituting the viral challenge test for the neutralizing peroxidase-linked assay in the release of Porvac© batches. By eliminating the viral challenge, the suffering of the animals will be alleviated, as well as the risk of dissemination of the high-virulence strain used in those experiments. The duration of the test and the associated costs will also be reduced.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the contribution of all researchers and technicians from the National Center for the Production of Laboratory Animals (CENPALAB), and the Central Laboratory Unit for Agricultural Health (ULCSA), for their unconditional support in the handling and care of the animals.
ETHICS
The Center for Genetic Engineering and Biotechnology ethics committee approved the study design
CONFLICT OF INTERESTS
The authors declare no conflicts of interest regarding the results presented in this article.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
1. Moennig V, Greiser-Wilke I. Classical swine fever virus. In: Mahy B, Van Regenmortel M, eds. Encyclopedia of Virology Third Edition ed: Oxford: Academic Press; 2008: 525-32.
2. Blome S, Staubach C, Henke J, Carlson J, Beer M. Classical swine fever—an updated review. Viruses 2017;9:86.
3. Van Oirschot J. Vaccinology of classical swine fever: from lab to field. Veterinary microbiology 2003;96:367-84.
4. Coronado L, Perera CL, Rios L, Frías MT, Pérez LJ. A Critical Review about Different Vaccines against Classical Swine Fever Virus and Their Repercussions in Endemic Regions. Vaccines 2021;9:154.
5. Dong XN, Chen YH. Marker vaccine strategies and candidate CSFV marker vaccines. Vaccine 2007;25:205-30.
6. Bouma A, De Smit A, De Jong M, De Kluijver E, Moormann R. Determination of the onset of the herd-immunity induced by the E2 sub-unit vaccine against classical swine fever virus. Vaccine 2000;18:1374-81.
7. Uttenthal A, Le Potier MF, Romero L, De Mia GM, Floegel-Niesmann G. Classical swine fever (CSF) marker vaccine. Trial I. Challenge studies in weaner pigs. Vet Microbiol 2001;83:85-106.
8. Sordo-Puga Y, Suárez-Pedroso M, Naranjo-Valdéz P, et al. Porvac® Subunit Vaccine E2-CD154 Induces Remarkable Rapid Protection against Classical Swine Fever Virus. Vaccines 2021;9:167.
9. Suárez M, Sordo Y, Prieto Y, et al. A single dose of the novel chimeric subunit vaccine E2-CD154 confers early full protection against classical swine fever virus. Vaccine 2017.
10. Suárez-Pedroso M, Sordo-Puga Y, Sosa-Teste I, et al. Novel chimeric E2CD154 subunit vaccine is safe and confers long lasting protection against classical swine fever virus. Veterinary Immunology and Immunopathology 2021;234:110222.
11. Muñoz-González S, Sordo Y, Pérez-Simó M, et al. Efficacy of E2 glycoprotein fused to porcine CD154 as a novel chimeric subunit vaccine to prevent classical swine fever virus vertical transmission in pregnant sows. Veterinary microbiology 2017;205:110-6.
12. OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 5th Edition, Chapter 2.8.3. 2018;1.
13. Blome S, Meindl-Bohmer A, Loeffen W, Thuer B, Moennig V. Assessment of classical swine fever diagnostics and vaccine performance. Revue Scientifique et Technique-Office International des Epizooties 2006;25:1025-38.
14. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 3.8.3. Classical swine fever (hog cholera) infection with classical swine fever virus. OIE, 2019. (Accessed 3/4/2020, 2020, at http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.08.03_CSF.pdf; .)
15. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, Chapter 1.1.8. Principles of Veterinary Vaccine. 2019. (Accessed 3/7/2020, 2020, at https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/1.01.08_VACCINE_PRODUCTION.pdf.)
16. Biront P, Leunen J, Vandeputte J. Inhibition of virus replication in the tonsils of pigs previously vaccinated with a Chinese strain vaccine and challenged oronasally with a virulent strain of classical swine fever virus. Veterinary microbiology 1987;14:105-13.
17. Terpstra C, Wensvoort G. The protective value of vaccine-induced neutralising antibody titres in swine fever. Vet Microbiol 1988;16:123-8.
18. Bouma A, de Smit AJ, de Kluijver EP, Terpstra C, Moormann RJ. Efficacy and stability of a subunit vaccine based on glycoprotein E2 of classical swine fever virus. Vet Microbiol 1999;66:101-14.
19. Ganges L, Barrera M, Díaz de Arce H, et al. Antigenic, biological and molecular characterization of the Cuban CSFV isolate "Margarita". Rev Salud Anim Vol 2007;29:182-92.
20. Postel A, Schmeiser S, Perera CL, Rodríguez LJP, Frias-Lepoureau MT, Becher P. Classical swine fever virus isolates from Cuba form a new subgenotype 1.4. Veterinary microbiology 2013;161:334-8.
21. Mittelholzer C, Moser C, Tratschin JD, Hofmann MA. Analysis of classical swine fever virus replication kinetics allows differentiation of highly virulent from avirulent strains. Vet Microbiol 2000;74:293-308.
22. Terpstra C, Bloemraad M, Gielkens AL. The neutralizing peroxidase-linked assay for detection of antibody against swine fever virus. Vet Microbiol 1984;9:113-20.
23. De Smit A, Terpstra C, Wensvoort G. Comparison of virus isolation methods from whole blood or blood components for early diagnosis of CSF. Report on Meeting of the EU National Swine Fever Laboratories, Brussels; 1994. p. 22-4.
24. De Smit A, Bourne A, De Kluijver E, Terpstra C, Moormann R. Prevention of transplacental transmission of moderatevirulent classical swine fever virus after single or double vaccination with an E2 subunit vaccine. Veterinary Quarterly 2000;22:150-3.
25. Ziegler U, Kaden V. Vaccination of weaner pigs against classical swine fever with the subunit vaccine" Porcilis Pesti": influence of different immunization plans on excretion and transmission of challenge virus. Berliner und Munchener tierarztliche Wochenschrift 2002;115:267-73.
26. Zhang Z, Zhang H, Liu T. Study on body temperature detection of pig based on infrared technology: A review. Artificial Intelligence in Agriculture 2019;1:14-26.
Received: December 23, 2022 / Accepted: January 30, 2023 / Published:15 February 2023
Citation: Suárez-Pedroso M, Sordo-Puga Y, Rodríguez-Moltó M P, Naranjo-Valdés P, Pérez-Pérez D, Sosa-Teste I, Montero-Espinosa C, Fuentes-Rodríguez Y, Sardina-González T, Santana-Rodríguez E, Vargas-Hernández M, Oliva-Cárdenas A, González-Fernández N, Bover-Fuentes E, Duarte C A., Estrada-García M P. Neutralizing antibodies as a correlate of protection against classical swine fever in Porvac® vaccinated pigs. Revis Bionatura 2023;8 (1) 49. http://dx.doi.org/10.21931/RB/2023.08.01.49
