2021.06.02.10
Files > Volume 6 > Vol 6 No 4 2021 > Vol 6 No 2 2021
INVESTIGATION / RESEARCH
Induction of apoptosis by Kola nut extract as a recent and promising treatment strategy for Leukemia.

Hamdah Alsaeedi1,2, Rowaid Qahwaji1, Talal Qadah1*
Available from: http://dx.doi.org/10.21931/RB/2021.06.02.10
ABSTRACT
Kola nut extracts have recently been reported to contain chemopreventive compounds providing several pharmacological benefits. This study investigated Kola nut extracts' anti-cancer activity on human immortalized myelogenous leukemia cell line K562 through apoptosis and cell cycle arrest. Fresh Kola nuts were prepared as powder and dissolved in DMSO. Different concentrations (50, 100, 150, 200, and 250 μg/ml) of working solutions were prepared. The K562 cells were treated with the different concentrations of Kola nut extract or vehicle control (10% DMSO) followed by incubation at 37°C for 24, 48, and 72 hours, respectively.
Treatment activity was investigated in K562 cells; by Resazurin, and FITC/Propidium Iodide and 7-AAD stained cells to evaluate apoptotic cells and the cell cycle's progression. Inhibition of leukemia cell proliferation was observed. The extract effectively induced cell death, early and late apoptosis by approximately 30% after 24 and 48 hours incubation, and an increase in the rate of dead cells by 50% was observed after 72 hours of incubation. Also, cell growth reduction was seen at high dose concentrations (150 and 200 µg/ml), as evident by cell count once treated with Kola nut extract. The total number of apoptotic cells increased from 5.8% of the control group to 27.4% at 250 µg/ml concentration.
Moreover, Kola nut extracts' effects on K562 cells increased gradually in a dose and time-dependent manner. It was observed that Kola nut extracts could arrest the cell cycle in the G2/M phase as an increase in the number of cells by 29.8% and 14.6 % were observed from 9.8% and 5.2% after 24 and 48 hours of incubation, respectively. This increase was detected in a dose and time-dependent manner. Kola nut extracts can be used as a novel anti-cancer agent in Leukemia treatment as it has shown significant therapeutic potential and therefore provides new insights in understanding the mechanisms of its action.
Keywords: Kola nut extracts, Leukemia, K562 cell line, Apoptosis, Cancer.
INTRODUCTION
Leukemia is defined as blood cancer as it originates from blood-forming tissues such as bone marrow; therefore, large quantities of abnormal blood cells are made before its circulation. Clinical symptoms vary between leukemia types based on its stage, genetic and environmental factors involved in the pathophysiological process1. Leukemia is divided into two main categories depending on the affected cell lines, including myeloid and lymphoid Leukemia. Further classification is done based on the onset of disease, including the acute or chronic stage. This all together characterizes Leukemia into four major types, which include Acute Myeloid Leukemia (AML), Chronic Myeloid Leukemia (CML), Acute Lymphocytic Leukemia (ALL), and Chronic Lymphocytic Leukemia (CLL)2. The CML is responsible for 15-20% of the total adult leukemia cases3. The Philadelphia chromosome's presence is a common hallmark linked to CML disorder that results from the fusion of two genes, i.e., Abelson murine leukemia (ABL1) gene located on chromosome 9 with breakpoint cluster region or BCR gene present on chromosome 22. The output product of this fusion is a protein termed BCR-ABL1 with oncogenic properties. Independent of a ligand's presence, this BCR-ABL1 tyrosine kinase remains constitutively active, causing stimulation of multiple downstream signaling pathways like RAS, JUN kinase, STAT, RAF, and MYC that enhance the growth and replication process4. The exact basis of most leukemia cases is yet to be discovered. However, environmental or inherited factors such as smoking, chemicals (i.e., benzene), ionizing radiation, previous exposure to chemotherapy, and Down-syndrome have been linked to it5. In addition to this, DNA mutations that activate oncogenes or inhibit the tumor suppressor gene can promote Leukemia by causing impaired cell death regulation, division, or differentiation6.
Multiple treatment options are available for Leukemia, such as chemotherapy, ionizing radiation, and hematopoietic stem cell transplantation7. Several side effects are associated with chemotherapy including cytotoxicity8, hair loss, coagulopathies, dry skin, brain abnormalities, and weekend immune system9-12. Another complication associated with chemotherapy is that patient develops resistance against chemotherapy through different pathways13. Hematopoietic stem cell transplantation is an alternative strategy for treating different diseases such as Leukemia and Lymphomas as these hematopoietic stem cells can differentiate into any mature blood cell types, and they also possess the ability of self-renewal14. However, it is quite expensive and associated with several complications like hemorrhagic cystitis, bacteremia, mucositis, and graft versus host disease15, 16. Ocular effects, post-transplant immunosuppression, organ toxicity, and congestive heart failure are some of the other complications commonly seen16. All these can result in cancer relapse after few months of transplantation16. Therefore, efforts have been made to find alternative treatment options with less toxicity and better prognosis.
One approach is to trigger the intrinsic apoptotic pathways, which will promote the cell death of leukemic cells17 as dysfunction in apoptotic pathway and lack of cell differentiation are among the hallmarks of leukemia18. Another therapeutic approach was to use interferon-alpha to induces immune cells of the host, including B lymphocytes, T lymphocytes, antigen-presenting dendritic cells, and natural killer cells to manage CML19, thereby INF α activates the immune response, modulates hematopoiesis and interleukin signaling to produce a cytogenetic response20. However, different side effects such as muscle aches, autoimmune hemolytic anemia (AIHA), immune-mediated renal disease, underactive thyroid function, chronic fatigue, neurotoxicity, and many others are associated with interferon therapy19. Nowadays, treatment with tyrosine kinase inhibitors (TKIs) is the norm. While selecting a particular TKI as a treatment option, various parameters such as patient's age, comorbidities, cost TKI resistance, and the toxicity profile of TKI in question need to be considered carefully and with proper monitoring4.
One treatment regimen includes medicinal plants as an anti-cancer agent as these plants produce a wide range of chemical compounds, although not useful for the plant yet having anti-cancer ability against various human cancer21. According to an estimate, about 60% of drugs commonly used as anti-cancer agents have been acquired from natural sources, among which plants are major contributors22. Around more than 3000 plants have been recorded to possess ant cancerous characteristics; therefore, these medicinal plants are used as an alternative treatment strategy in different countries. This alternative approach to prevent or delay cancer development is called chemoprevention, which utilizes medicinal herbs and food23-25. Among such medicinal plants include Kola nuts of the Sterculiaceae family of plants. These plants are native to African tropical rainforests and are a rich source of caffeine; therefore, these nuts are used in several beverage preparations. These nuts are also chewed by a person or in a different social gathering in several West African cultures. Commonly used Kola nuts species include Cola acuminate/Cola nitida and bitter Cola (Gracina Kola)26, 27. Although it has many benefits, excessive consumption can cause anxiety, hypertension, neuro stimulatory effect, and irritation of the gastrointestinal tract28-30, which may be due to caffeine's excessive content in these nuts31, 32. These Kola nuts possess antimicrobial activity in a range of 8-24 nm compared to Gentamicin and nystatin, and they have been used to treat fever, malaria, scabies, ringworm, gonorrhea, and dysentery33.
Apart from these, the presence of anti-androgenic and anti-estrogenic components of Kola nuts compelled the researchers to study Kola nut extract's pharmacological effect when applied on solid tumors such as breast and prostate cancer cell lines34, 35. The results reveal Kola nut extracts' selective toxicity against MDA-MB-468 and MCF-7 breast cancer cell lines, and DU145 and LNCaP prostate cancer cell line34, 35. This proved that Kola nuts have anti-cancer activity, and its extract can be used for inhibiting cancer cell growth. These results increased Endrini and his colleagues' interest in finding the apoptotic mechanism underlying the Kola nut extracts in MCF-7 breast cancer cell line36. The MCF-7 cell lines were treated with 60-80 µg/ml of Kola nut extracts for 24 hours, and analyzed the treated cells by flow cytometry. He observed an increased number of apoptotic cells in MCF-7 cell line. This means that the Kola nut extract influence the cell cycle (S and G2/M phases) and can promote MCF-7 cell lines apoptosis. The K562 cell line was initially established from patients with chronic myelogenous Leukemia (CML) and expressed as the typical hallmark of CML37. Further, the molecular level reveals that it is positive for the Philadelphia (Ph) chromosome made by Bcr/Abl fusion gene through a reciprocal translocation between chromosomes 9 and 2237. These cell lines were also selected because they were highly undistinguishable with a dynamic proliferative capacity and inhibition of apoptosis38.
Although treatment of CML cases with current chemical approaches is considered adequate, specific adverse reactions associated with this treatment option such as pleural effusion, vascular pathology, musculoskeletal and gastrointestinal symptoms have been reported39, 40. Therefore, this study aimed to investigate the potential anti-cancer effect of herbal products, namely Kola nut extract, on the human immortalized myelogenous leukemia cell line K562 through analyzing apoptotic activity and cell cycle arrest. It can be taken as an experimental trial to overcome adverse reactions associated with chemical treatment as well as to widen treatment options for patients suffering from Myelocytic Leukemia.
MATERIALS AND METHODS
Kola nut extract preparation. A stock of Kola Nut extracts powder was dissolved in sterile Dimethyl sulfoxide (DMSO) (10%) and aliquoted into an Eppendorf tube covered with aluminum foil in a final concentration of 2.8 mg/ml. The aliquots stocks were kept at −20°C for further use. Different working concentration points (50, 100, 150, 200, and 250 μg/ml) were adopted for the prepared stock solution's experimental procedure. Besides, the required volume of extract to obtain a final concentration was calculated.
Annexin V. For apoptosis detection, double-staining of the cells with Annexin V-Propidium iodide (PI) were used. 400 µL of 1X Annexin V binding buffer per sample was used. The initial 10X buffer was diluted with deionized distilled water in a 1:10 ratio and set aside on ice. For Annexin V incubation reagent, 100 µl reagent (10 µl of 10X binding buffer, 10 µl propidium iodide (PI), 1 µl Annexin V-FITC and 79 μl deionized distilled water) was utilized per sample with cells density of 1 x 106. Individual samples (seeding cells at 1 x 106) were then stained with 7-Amino- Actinomycin D (7-AAD) stain, which was prepared by incubated 7-Amino- Actinomycin D (7-AAD) (20 μg/ml) with RNase (20 μg/ml) at 37°C for 30 minutes.
Culturing and treatment of K562 cells
K562 cells were cultured in 6 wells plate at the desired density of 15 x 104 cells in 3ml /well, treated with the targeted concentrations of Kola nut extract (0, 50, 100, 150, and 200 μg/ml) or vehicle control (10% DMSO), and then incubated for 24, 48 and 72 hours, respectively at 37°C. After incubations, the cell suspension was gently mixed and carefully loaded into hemocytometer chambers to count the cells. Additionally, the average cell count was determined, and the procedure was repeated to ensure accuracy.
The proliferation of cytotoxic assays
Trypan blue assay:50 μl of aliquoted of cells suspension was gradually mixed with 50 μl of trypan blue stain in (1.5 ml) Eppendorf tube and kept at room temperature for 5 minutes. 20 μl of the stained cells were then picked and loaded onto chambers of hemocytometer, and then the live and dead cells were separately quantified in four corner squares using the L rule of both chambers. The average cell count was determined, and to ensure the accuracy of the method, the procedure was repeated. The findings represent as percentages of live or dead cells were then calculated.
Proliferation assay 96 wells plate: The cytotoxic potential of Kola nut extract against myeloid leukemia cell line (K562) was determined using Resazurin cell viability assay kit after the experimental treatments in triplicate wells and incubation period of 24, 48, and 72 hours at 37°C. Results of cell viability were carried out by the colorimetric method and calculated by Subtracted background absorbance (culture medium without cells at 600 nm from resolution and absorbance at 570 nm).
Flowcytometry of annexin V/PI stained cells for apoptotic assay
Treated cells with different concentrations and at different time intervals were pelleted and incubated in 100 μl prepared Annexin V incubation reagents in the dark for 15 minutes at room temperature and then immediately re-suspended in 400 μl of prepared 1X binding buffer. At the same time, apoptotic cells were detected on a BD FACSAriaIII Flow cytometer (Becton Dickinson) within one hour; to obtain the maximal signal. Additionally, results were analyzed using BD FACSDiva software, which sorted cells into intact or viable cells (Annexin negative/PI negative), early apoptotic cells (Annexin positive/PI negative), late apoptotic cells (Annexin positive /PI-positive), and necrotic cells (Annexin negative/PI-positive)
Cell cycle assay
Flow cytometry of 7-AAD stained cells was used to detect cell cycle progression. The cells were pelleted and fixed with ice-cold 80% ethanol by dropwise 2 ml from a Pasteur pipette immediately mixed on vortex and then stored at −20°C for a week. Following the fixative time, the preserved cells were pelleted and incubated with prepared 7-Amino- Actinomycin D (7-AAD) at 37°C for 15 minutes. Similarly, as the apoptotic assay, the cell cycle analysis was done on a BD FACSAria III Flow cytometer, and the results were analyzed using BD FACSDiva software through collected 250,000 events per sample in population 2.
Statistical evaluation
results were analyzed by excel software by formulated precise illustrative figures and tables to compare cellular proliferation and death rates for two groups of experimental leukemic cell lines; control and test.
RESULTS
Kola Nut Extract Inhibits Cell Proliferation of K562 Cell Line and Decreases the Cell Viability.
To study Kola nut extracts' effect on cell viability, we performed resazurin cell viability assay and live/dead trypan blue assays. Our results showed that Kola nut extract's anti-proliferative ability against the K562 leukemic cell line increases cell death rate. These effects were investigated at three incubation periods of 24, 48, and 72. (Figure 1). Positive anti-proliferative activities were visible in 48 and 72 hours of incubation, from the lowest concentration of the treatment (50 μg/ml) and steadily up to the highest concentration (200 μg/ml). Although the maximum inhibitory effect on K562 proliferation was not exceeding 30%, the rate of dead K562 cells was sharply raised to approximately 50% after 72 hours incubation, which is greater than that of 24 and 48 hours incubation period (Figure 2). The cell growth rate was also significantly reduced at concentrations of 150 and 200 μg/ml), results not shown. Therefore, from the results above, the inhibitory rate of K562 cell proliferation treated by Kola nut was increased in a dose and time-dependent manner. Moreover, Kola nut extract proved a high possible activity was inducing apoptosis and leukemia inhibition in K562 cell proliferation.
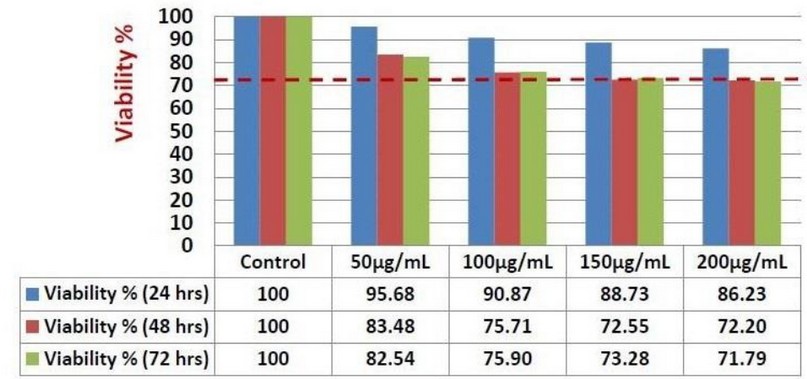
Figure 1. Anti-proliferative Kola nut extract activity against K562 cells after 24-, 48-, and 72-hours incubation. The resazurin cell viability assay was used to indicate an anti-proliferative effect on the seeding of 5 x 103 K562 cells. The control represents untreated K562 cells. Several treatment concentration points were used (50, 100, 150, and 200 μg/ml). The results are based on a single experiment, and RPMI/ DMSO media was used as a blank. The red line indicated the maximum inhibitory effect obtained by Kola nut treatment after 24-, 48-, and 72-hours incubation.
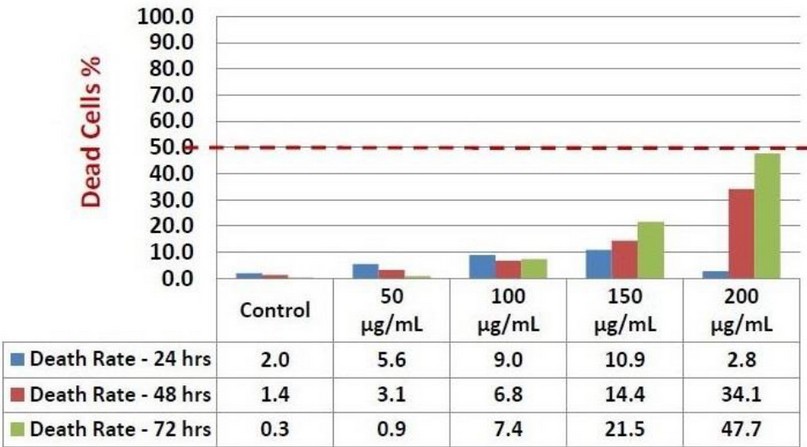
Figure 2. Death rate obtained by Kola nut extract against K562 cells after 24-, 48-, and 72-hours incubation. The trypan blue assay was used to investigate Kola nut's possibility to induce cell death on seeding of 15 x 104 K562 cells. The control represents untreated K562 cells. Several treatment concentration points were used (50, 100, 150, and 200 μg/ml). The results are based on a single experiment. The red line indicated the maximum cellular death rate obtained by Kola nut treatment after 24-, 48-, and 72-hours incubation.
Kola Nut Extract Induces Variable Forms of Cell Death in K562 Cell Lines.
By using flow cytometer technique with FITC annexin V and PI staining of K562 cell line treated with Kola nut extract with indicated doses (50,150, 250 µg/ml) and time intervals (24 and 48 hours), we observed that kola nuts successfully induced cell death; apoptosis (early and late) and necrosis; after 24 (figure 3), and 48 hours (figure 4) incubation period. The results revealed an increase in total apoptotic cells from 5.8% (control) to 27.4% (250 μg/ml) compared to the rate of necrosis (not higher than 7%). Also, 4-time increases in the percentage of late apoptotic cells were observed compared to early apoptotic cells (a maximum of 5% early apoptotic and 22.4% late apoptotic). The percentage of cells declined by approximately 23% (from 91% to 68% live cells population).
After 48 hours of incubation, the Kola nut extract's apparent effect was observed with all designed concentrations and quite a high efficiency compared with 24 hours, as seen in figure 4 and table 1. The K562 cells showed a relatively similar dose-dependent decrease in the population of live cells (from 93% to 71%) as after 24 hours, where the percentage of total apoptotic cells increased by about 22% compared with untreated control cells. In both 24 and 48 incubation periods, Kola nut's ability to induce necrosis was also observed; however, it was neglected compared to the apoptotic population.
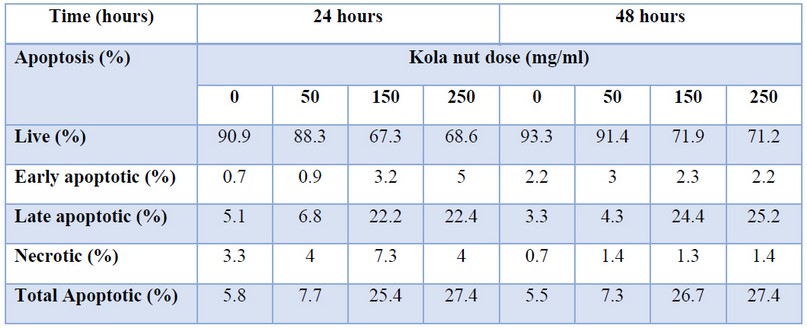
Table 1. Effect of Dose and Time Course –Dependence of Kola Nut Extract on Induction of Apoptosis and Necrosis in K562 Cells
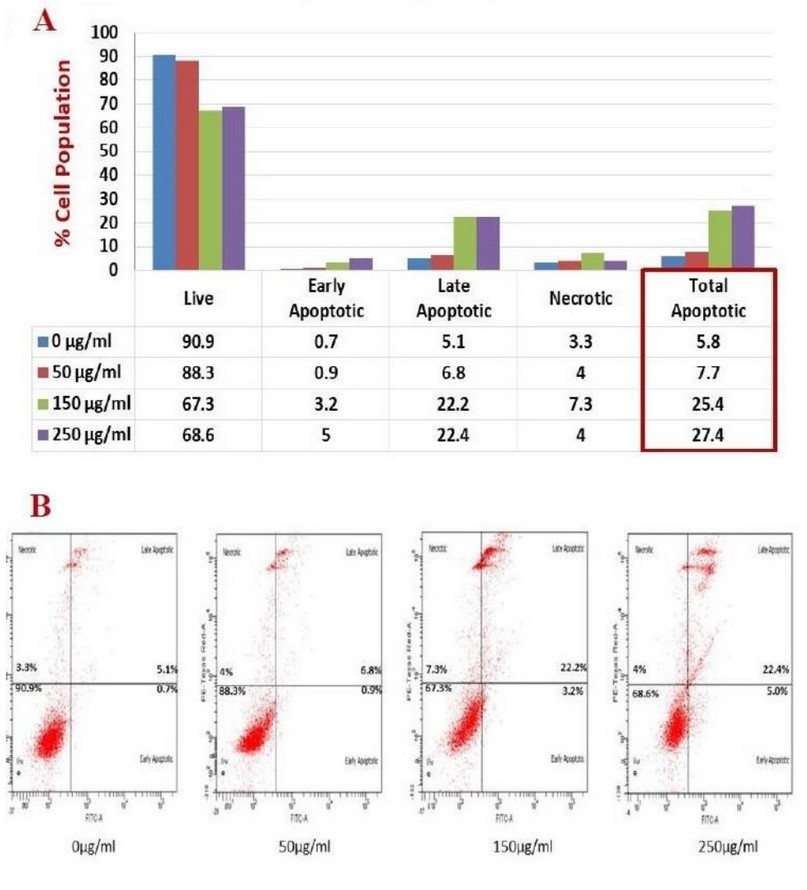
Figure 3. The illustrative chart (A) and flow cytometer scatter plot (B) showed the activity of Kola nut extract to induce apoptosis/necrosis on K562 cells that incubated for 24 hours, stained with Annexin V/PI, and analyzed by flow cytometer. Total apoptosis represents both early and late apoptotic forms.
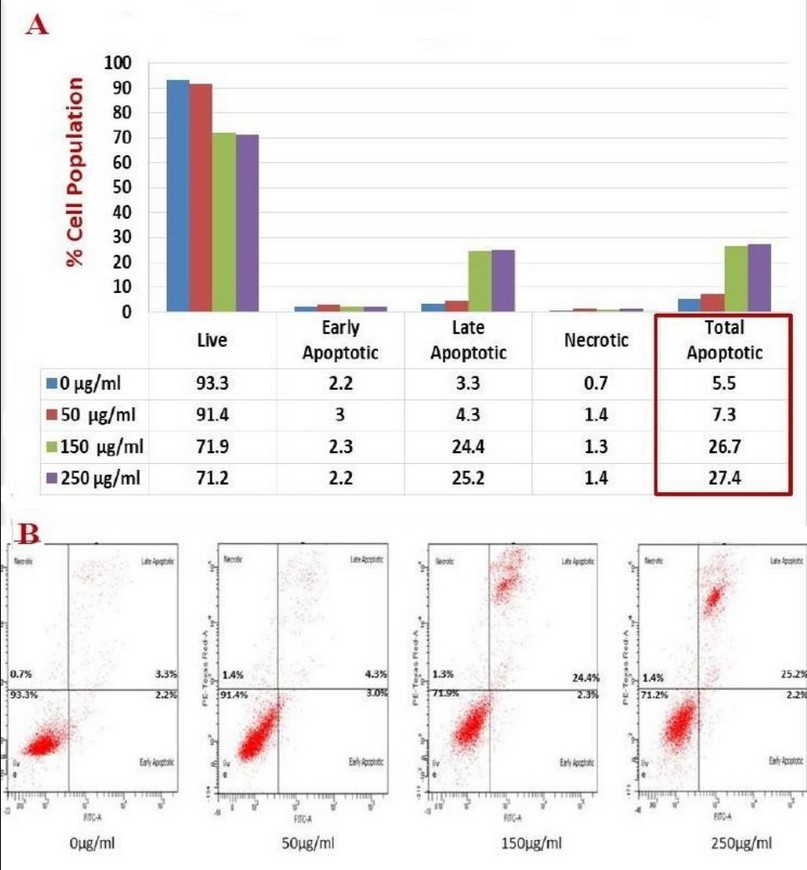
Figure 4. The illustrative chart (A) and flow cytometer scatter plot (B) showed the activity of Kola nut extract to induce apoptosis/necrosis on K562 cells that incubated for 48 hours, stained with Annexin V/PI stain, and analyzed by flow cytometer. Total apoptosis represents both early and late apoptotic forms.
Kola Nut Extracts Arrest the Cell Cycle in G2/M phase of the Cell Cycle.
While studying the impact of Kola nut extract on the distribution of cell cycle phases, different Kola nut extract (from 0 to 250 μg/ml) were applied following two incubation periods of 24 and 48 hours. The results are shown in figure 3 (After 24 hours incubation), and figure 4 (After 48 hours incubation) showed the dose-dependent impact of Kola nut extract on the distribution of cell cycle phases.
As seen in table 2, the cells treated with Kola nut extract showed rise in the G2/M phase percentage from approximately 9.8% to the maximum of 29.8% from 5.2% to 14.6% following 24 and 48 hours, respectively. Alternatively, a noticeable decline in the overall G0/G1 and S phases was observed by approximately 27% and 19%, respectively, following the treatment of indicated doses of Kola nut extract.

Table 2. K562 Cell Cycle Distribution (G0/G1, S and G2) After Followed Dose and Time Course Incubation with Kola Nut Extract Treatment.
DISCUSSION
Treating CML patients with TKI is accepted as a practical option with an increased survival rate in most patient populations; however, specific adverse effects are associated with this treatment option. Studies report toxicities and different adverse events such as pleural effusion, vascular events, and arterial hypertension associated with TKIs. The estimated treatment cost per patient in European countries is reported to be ~30 000-40 000 € per year39. Also, indefinite treatment with TKI might be needed for some patients even after attaining a deep molecular remission41. Due to these complications, efforts were made to find an alternative route more inclined toward naturally available options with minimal adverse effects and more cost-effective. For that reason, our study was designed to evaluate the response of the human immortalized myelogenous leukemia cell line K562 against Kola nut extract at different doses and incubation periods. As the work to study Kola nut extracts' pharmacological action on breast and prostate cancer cell lines has already been reported in the literature34-36. Our work proved that the Kola nut extract could inhibit the growth of human myelogenous Leukemia and reduce cell viability in a dose and time-dependent manner. Fontenot and his colleagues (2007) initially screened the cytotoxicity of several extracts (hexane, ether, acetone, methanol, and distal water) of Kola nut on the viability of the estrogen-responsive breast cancer cells (MCF-7) that showed diverse effects of each extract at 100 ppm after 24 hours; in proliferation assay, they detected that the acetone extract induced more significant than 50% of cell death. Contrarily, the other extracts showed a growth stimulator to MCF-7 cell line, especially ether extract, while in exclusion trypan blue assay, the affected extracts acetone and ether showed a reduction in the viable cells number as compared to control cells. These findings are consistent with the present study, which displayed a decline in the number of the K562 cells treated with Kola nut extract.
Additionally, Fontenot and his colleagues (2007) have found out that the ether extract of Kola nut can impede the growth of the androgen-dependent (LNCaP) prostate tumor cell model due to the strong affinity for the androgenic receptor. Those findings indicate that the different extractable bioactive compounds of Kola nut may have chemopreventive activities, which are selectively toxic to cancerous cells34-36. In consideration of this, the apoptotic assay was performed on K562 cell lines treated with Kola nut extracts, and the results revealed that Kola nut extract could stimulate different forms of cell death: early and late apoptosis and necrosis with the percentages of late apoptotic cells more noticeable than other forms of cell death. Late apoptotic cells or secondary necrotic cells arise from early apoptotic cells that develop due to their unrecognizable signals by the phagocytic cells42, 43. This is because the impairment of phagocytosis makes the early apoptotic cells accumulate and progress to late apoptotic cells or secondary necrotic cells42, 43. The late apoptotic cells also indicate that they carry a remaining early hallmark of apoptosis signal, Annexin-V binding protein, which is produced when the phosphatidylserine of cell membrane undergoes exposure to internal cells stimulus44. Besides, the stimulus of Kola nut extract may affect a time-dependent manner as several hours are needed for late apoptosis enhancement45.
Other than the possible type of cell death inducement, the mechanism of target action on cell cycle distribution has been studied. It is well-known that the impaired cell cycle is a hallmark of cancer cells18, 46. As evident from this study, Kola nut extract arrested the cell cycle in G2/M phase, with increased cells in this phase and reduced the number of cells in G0/G1 and S phases. Interestingly, the G2/M phase has a checkpoint that signifies as a potential target for cancer therapy, which prevents the cells that have damaged DNA in the late S or G2 phase to enter the mitotic phase, to re- repairing and thus, retarded the proliferation of cell through halting the cell cycle progression47. Thus, the promoted cell cycle arrest thought to provide evidence that the Kola nut extract could regulate the proliferation of the cells, although a previous study showed that Kola nut extract treatment caused dysregulation of cell cycle in breast cancer cell line MCF-7; after 24 hours at two indicted doses (80 and 60 μg/ml); however, their results revealed that the Kola nut extract has an obvious effect on both the S and G2/M phases by reducing the percentages of cells involved in each phase; while, the G2/M phase was increased in the present study36.
CONCLUSION
Kola nut extracts can be used as an anti-cancer agent against CML in vitro as it has shown a tremendous therapeutic potentiality and therefore providing new insights in understanding the mechanisms of its action against CML. However, an experimental plan for using the Kola nut extracts against a cell line of acute myeloblastic leukemia origin representing a study limitation in our current work may give a better picture and understanding of the anti-cancer activity of this promising therapeutic agent.
Acknowledgment:
King Abdulaziz City funded this work for Science and Technology (KACST) (Grant no. 1-17-00-009-0020). The authors, therefore, acknowledge with thanks KACST for financial support.
Conflict of Interest:
All authors declare that they have no conflict of interest associated with this publication
REFERENCES
1. Davis AS, Viera AJ, Mead MD. Leukemia: an overview for primary care. American family physician. 2014;89(9):731-8.
2. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-405.
3. Soverini S, De Benedittis C, Mancini M, Martinelli G. Best practices in chronic myeloid leukemia monitoring and management. The Oncologist. 2016;21(5):626.
4. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. American journal of hematology. 2018;93(3):442-59.
5. Hutter JJ. Childhood leukemia. Pediatr Rev. 2010;31(6):234-41.
6. Radivoyevitch T, Sachs R, Gale R, Molenaar R, Brenner D, Hill B, et al. Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation. Leukemia. 2016;30(2):285-94.
7. Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459(7246):508-9.
8. Corrie PG. Cytotoxic chemotherapy: clinical aspects. Medicine. 2008;36(1):24-8.
9. Staat K, Segatore M. The phenomenon of chemo brain. Clinical journal of oncology nursing. 2005;9(6):713.
10. Rodgers GM, Becker PS, Blinder M, Cella D, Chanan-Khan A, Cleeland C, et al. Cancer-and chemotherapy-induced anemia. Journal of the National Comprehensive Cancer Network. 2012;10(5):628-53.
11. Al-Mohanna H, Al-Khenaizan S. Permanent alopecia following cranial irradiation in a child. Journal of cutaneous medicine and surgery. 2010;14(3):141-3.
12. Elad S, Zadik Y, Hewson I, Hovan A, Correa MEP, Logan R, et al. A systematic review of viral infections associated with oral involvement in cancer patients: a spotlight on Herpesviridea. Supportive care in cancer. 2010;18(8):993-1006.
13. Chorawala M, Oza P, Shah G. Mechanisms of anti-cancer drugs resistance: an overview. Int J Pharm Sci Drug Res. 2012;4(1):1-9.
14. Hatzimichael E, Tuthill M. Hematopoietic stem cell transplantation. Stem cells and cloning: advances and applications. 2010;3:105.
15. Kasteng F, Sobocki P, Svedman C, Lundkvist J. Economic evaluations of Leukemia: a review of the literature. International journal of technology assessment in health care. 2007;23(1):43.
16. Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood cancer journal. 2011;1(4):e16-e.
17. Samudio I, Konopleva M, Carter B, Andreeff M. Apoptosis in leukemias: regulation and therapeutic targeting. Acute Myelogenous Leukemia: Springer; 2009. p. 197-217.
18. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. cell. 2011;144(5):646-74.
19. Talpaz M, Hehlmann R, Quintás-Cardama A, Mercer J, Cortes J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia. 2013;27(4):803-12.
20. Woessner DW, Lim CS, Deininger MW. Development of an effective therapy for chronic myelogenous Leukemia. Cancer J. 2011;17(6):477-86.
21. Kooti W, Servatyari K, Behzadifar M, Asadi-Samani M, Sadeghi F, Nouri B, et al. Effective medicinal plant in cancer treatment, part 2: review study. Journal of evidence-based complementary & alternative medicine. 2017;22(4):982-95.
22. Solowey E, Lichtenstein M, Sallon S, Paavilainen H, Solowey E, Lorberboum-Galski H. Evaluating medicinal plants for anti-cancer activity. The Scientific World Journal. 2014;2014.
23. Jo E-H, Hong H-D, Ahn N-C, Jung J-W, Yang S-R, Park J-S, et al. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. Journal of agricultural and food chemistry. 2004;52(6):1715-9.
24. Rech Franke SI, Guecheva TN, Henriques JAP, Prá D. Orange juice and cancer chemoprevention. Nutrition and cancer. 2013;65(7):943-53.
25. Steward W, Brown K. Cancer chemoprevention: a rapidly evolving field. British journal of cancer. 2013;109(1):1-7.
26. Dah-Nouvlessounon D, Adjanohoun A, Sina H, Noumavo PA, Diarrasouba N, Parkouda C, et al. Nutritional and anti-nutrient composition of three kola nuts (Cola nitida, Cola acuminata and Garcinia kola) produced in Benin. Food and Nutrition Sciences. 2015;6(15):1395.
27. Atawodi SE-o, Pfundstein B, Haubner R, Spiegelhalder B, Bartsch H, Owen RW. Content of polyphenolic compounds in the Nigerian stimulants Cola nitida ssp. alba, Cola nitida ssp. rubra A. Chev, and Cola acuminata Schott & Endl and their antioxidant capacity. Journal of agricultural and food chemistry. 2007;55(24):9824-8.
28. Ishidate Jr M, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M, et al. Primary mutagenicity screening of food additives currently used in Japan. Food and chemical toxicology. 1984;22(8):623-36.
29. Ibu J, Iyama A, Ijije C, Ishmael D, Ibeshim M, Nwokediuko S. The effect of Cola acuminata and Cola nitida on gastric acid secretion. Scandinavian Journal of Gastroenterology. 1986;21(sup124):39-45.
30. Tende J, Ezekiel I, Dare S, Okpanachi A, Kemuma S, Goji A. Study of the effect of aqueous extract of kolanut (Cola nitida) on gastric acid secretion and ulcer in white wistar rats. British Journal of Pharmacology and Toxicology. 2011;2(3):132-4.
31. Okoli B, Abdullahi K, Myina O, Iwu G. Caffeine content of three Nigerian cola. Journal of Emerging Trends in Engineering and Applied Sciences. 2012;3(5):830-3.
32. Gaspar S, Ramos F. Caffeine: consumption and health effects. Encyclopedia of Food and Health. 2016:573-8.
33. Adam SI, Yahya AA, Salih WM, Abdelgadir WS. Antimicrobial activity of the masticatory Cola acuminata Nut (Gooro). Current Research Journal of Biological Sciences. 2011;3(4):357-62.
34. Fontenot K, Naragoni S, Claville M, Gray W. Characterization of Bizzy Nut extracts in estrogen-responsive MCF-7 breast cancer cells. Toxicology and applied pharmacology. 2007;220(1):25-32.
35. Solipuram R, Koppula S, Hurst A, Harris K, Naragoni S, Fontenot K, et al. Molecular and biochemical effects of a kola nut extract on androgen receptor-mediated pathways. Journal of toxicology. 2009;2009.
36. Endrini S, Jaksa S, Marsiati H, Othman F, Rahmat A. Effects of cola nut (Cola nitida) on the apoptotic cell of human breast carcinoma cell lines. Journal of Medicinal Plants Research. 2011;5(11):2393-7.
37. Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. 1975.
38. Koeffler H, Golde D. Human myeloid leukemia cell lines: a review. 1980.
39. Saussele S, Richter J, Hochhaus A, Mahon F. The concept of treatment-free remission in chronic myeloid Leukemia. Leukemia. 2016;30(8):1638-47.
40. Francis J, Palaniappan M, Dubashi B, Pradhan SC, Chandrasekaran A. Adverse drug reactions of imatinib in patients with chronic myeloid Leukemia: A single-center surveillance study. J Pharmacol Pharmacother. 2015;6(1):30-3.
41. García-Gutiérrez V, Hernández-Boluda JC. Tyrosine kinase inhibitors available for chronic myeloid Leukemia: Efficacy and safety. Frontiers in Oncology. 2019;9:603.
42. Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nature Reviews Immunology. 2007;7(12):964-74.
43. Poon IKH, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death & Differentiation. 2010;17(3):381-97.
44. Smith BA, Smith BD. Biomarkers and molecular probes for cell death imaging and targeted therapeutics. Bioconjugate chemistry. 2012;23(10):1989-2006.
45. Ziegler U, Groscurth P. Morphological Features of Cell Death. Physiology. 2004;19(3):124-8.
46. Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nature Reviews Cancer. 2009;9(3):153-66.
47. Wang Y, Ji P, Liu J, Broaddus RR, Xue F, Zhang W. Centrosome-associated regulators of the G2/M checkpoint as targets for cancer therapy. Molecular Cancer. 2009;8(1):8.
Received: 15 November 2020
Accepted: 15 February 2021
Hamdah Alsaeedi1,2, Rowaid Qahwaji1, Talal Qadah1*
1 Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, King Abdulaziz University. Jeddah, Saudi Arabia
2 Clinical Laboratory Department, College of Applied Medical Sciences, Shaqra University. Shaqra, Saudi Arabia
* Corresponding Author:
Name: Talal Qadah, PhD
Email: [email protected]
Postal address:
Department of Medical Laboratory Technology
Faculty of Applied Medical Sciences
King Abdulaziz University
P.O.Box: 80324. Postcode: 21589
Jeddah, Saudi Arabia
Tel: +966 (12) 6400000 Ext. 51526
Fax: +966 (12) 6400000 Ext. 22289
Mobile: +966 (0) 55 554 0081
ORCID: https://orcid.org/0000-0002-8425-1119