2023.08.04.60
Files > Volume 8 > Vol 8 no 4 2023
Biological, serological and molecular characterization of Potato virus Y strains isolated from potato (Solanum tuberosum L.)
Dena Zuheer1, Hameed Ali 2,*
1 Departement of Plant Protection / College of Agriculture and Forestry/ University of Mosul/ Iraq. [email protected]
2 Departement of Plant Protection/ College of Agriculture and Forestry/ University of Mosul/ Iraq [email protected].
* Correspondence: [email protected]; Tel.: +9647740871120
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.60
ABSTRACT
Viral diseases are among the most critical determinants of potato production in many parts of the world, and this is reflected in the importance of developing early diagnosis methods to detect these diseases in the fields. The study showed that potato fields in Nineveh Province are infected with several strains of Potato Y virus, depending on the symptoms of potato or indicator plants and serological tests. For the first time locally in Nineveh Province and at the level of Iraq, using a single molecular test and one step, it was possible to distinguish between the ancient parental strains (PVYº and PVYN), as well as between the PVYNTNT hybrid strain resulting from recombination between the genome of the parental strains when using the pairs of primers N-868-7-F + N -9236-R, which yielded a genome segment of DNA with a length of 441 bp. The emergence of such a new strain in the tested samples in the potato fields in Nineveh province infected with PVY may have arisen from mixed infections.
Keywords: PVY strains; PVYN; RT-PCR; Solanum tuberosum L.
INTRODUCTION
The potato crop is one of the strategic crops, an important economic source of income in the world and is ranked fourth in the world in terms of nutritional importance after wheat, corn and rice, and tops the tuber crops1. Potato Solanum tuberosum L. is exposed to several pests and diseases that reduce productivity and quality. Viral diseases are among the most essential determinants of potato production in many parts of the world, and this is reflected in the importance of developing early diagnosis methods to detect these diseases in the fields. The spring crop is generally considered the primary season for producing potato seeds. The source of these seeds varies in the cultivated fields, as the most significant quantity comes from importing from European countries. Many farmers usually rely on keeping or selling part of their crop as local seeds for the next season, allowing the insertion, replication and hybrid evolution of locally new or old viruses, isolates or strains2. Potatoes are infected with more than 40 viruses, which causes significant losses in the crop3. The potato virus Y is one of this crop's most prevalent and harmful pathogens, as it poses a significant threat and danger to the crop and is one of the most important reasons for the low production in different regions around the world 4, 5, 6. PVY spreads in most countries worldwide, in addition to its spread in the field on different crops such as tomatoes and peppers and industrial crops such as tobacco, wild herbs, and ornamental plants 7. It is a highly genetically heterogeneous virus that exists in several strains that differ from each other in the symptoms they cause on the plants they infect and serological reactions, depending on the type of strain and the sensitivity of the affected varieties 5. These strains are widely distributed globally and locally 3. Potato virus Y strains cannot accurately be diagnosed by relying only on serological methods such as DAS-ELISA. However, they must resort to a more accurate method of characterizing strains, such as TAS-ELISA, using monoclonal antibodies and biological and molecular methods to reach a more accurate diagnosis. Since the potato virus, Y, is one of the most widespread viruses on the potato crop in the Arab and regional regions, it has been characterized in many countries 7,8. In Iraq, the virus has been recorded through many studies 9,10,11, but these studies did not address any information about the strains of this viral type, so we decided to conduct this study. To find out the pathological reality of this pathogen.
MATERIALS AND METHODS
Collection of potato samples: Several samples were collected from potato plants showing viral symptoms similar to those caused by the PVYN strain of the potato virus, and they were collected during field visits to potato fields planted with that crop in Nineveh province. These visits took place during the spring and autumn seasons for the years 2021-2022, which included several areas, namely the district of Mosul (Kubba, Rashidiya, Hawi al-Kanesa), Nimrud sub-district of Hamdaniya district and Sheikhan district (Al-Nyuran village).
Serological and biological diagnosis: The leaf samples of potato plants with suspected symptoms of the virus under study were crushed in a phosphate buffer (0.01 M, pH 7.0) at a ratio of 1:3 g/ml in a ceramic mortar. The extract was used for biodiagnostic purposes by inoculation of potato plants Solanum tubersum, Nicotiana tabacum and Nicotiana glutinosa, and DAS and TAS-ELISA serological assay, using monoclonal and polyclonal antisera of Potato Y virus and PVYN strain of Potato Y virus. They were equipped by the Swiss company BIOREBA, according to the attached protocol of the supplying company. The results of the ELISA test were evaluated chromatically and spectroscopically by examining the calibration wells of the ELISA dish containing the samples using a Spectrophotometer UV-9200 at 405 nm. Cut-off = (X – 3Sd + 10%) on the deterministic equation for the affected samples.
Isolation of Viral RNA: Total viral RNA was isolated from leaves of plants (potato and tobacco which gave positive results in the presence of the virus by ELISA tests, which depended on the extraction kit supplied by Real Total RNA From Tissue and Cell using Spin Column method.
Measurement of viral RNA purity: The purity of viral RNA was measured using a Nanodrop-spectrometer device to detect and measure nucleic acid concentration. The purity of RNA was measured by reading the absorption at a wavelength of 260/280 nm.
Complementary RNA preparation: For cDNA preparation, the RT-Pre-Mix kit was used, following the protocol of the Korean manufacturer (add bio), where the extracted template RNA was mixed with the reverse initiator, 20 picomoles concentrated in a 0.2 ml Eppindorf tube, and the mixture was heated at a temperature of 70 °C. The tube was then placed on ice for five minutes. The mixture was transferred to an RT Pre-Mix tube containing all the requirements for the reaction and the volume to 20 µL with sterile distilled water. All contents were mixed using an electric vibrator for 10 seconds. The centrifugation process was performed at 8000 rpm for another 10 seconds to bring the contents down to the bottom of the tube, and then the tube was placed in the polymerase chain (PCR) machine at 42 °C for one hour, then at 95 °C for 5 minutes. Five µL of the cDNA was taken and later used in PCR.
PCR polymerase chain reaction: PCR technique was applied using nucleic acids extracted from infected plants after cDNA synthesis, depending on (forward and reverse) primers, designated for detecting PVY virus and PVYN strain, as shown in Table (1).
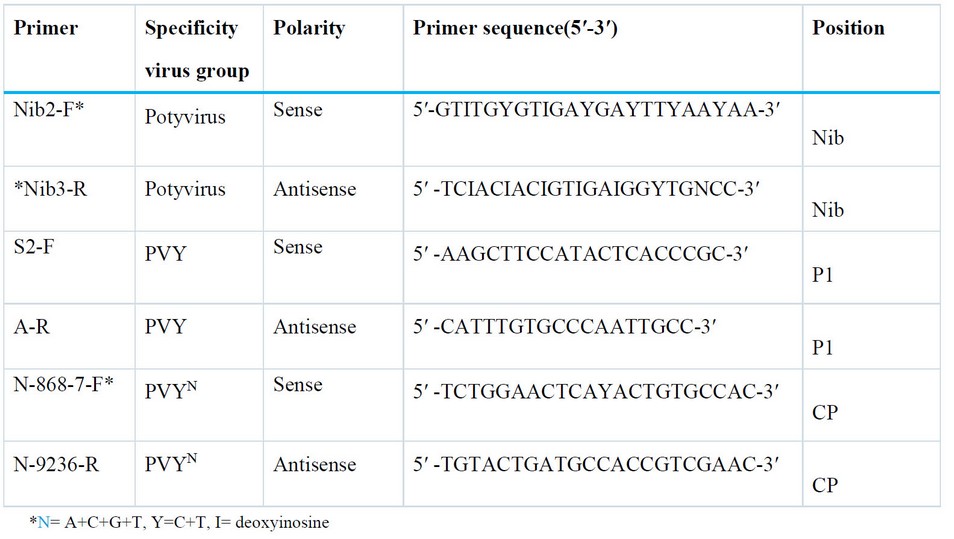
Table 1. Primers were designed for the detection of PVY and PVYN strains.
The Master-Mix was prepared for PCR amplification using the Korean kit Genet Bio, catalog No. KM4100, according to the sizes needed for the reaction components as shown in Table (2)
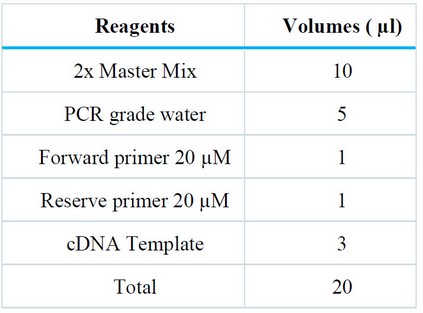
Table 2. Components of the master mix for RT-PCR.
After preparing the Eppendorf tubes for the Master-Mix, they were placed in the Thermocycler T 100TM, Bio-Rad, USA, and the programs for PCR amplification were used to suit each initiator as shown In Table (3), then the PCR-amplified products were separated on a 1.4% agarose gel stained with Red safe Nucleic acid (Intron Korea). After completing the electrophoresis process, the gel was examined using the EL Gel Documentation System (Bio-Rad USA), and images were taken.
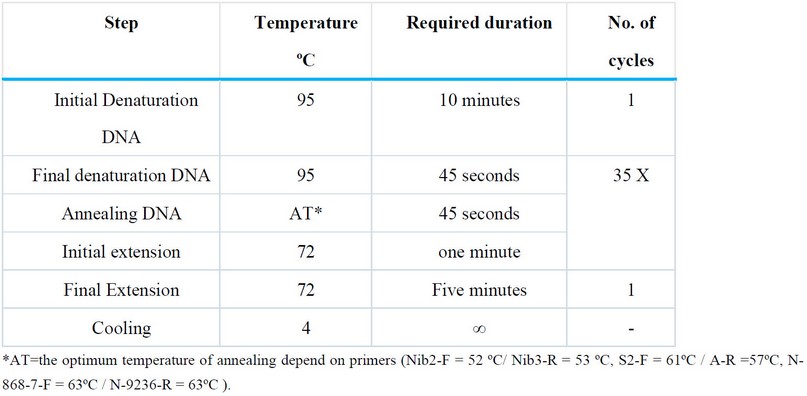
Table 3. PCR conditions were used in this study.
RESULTS
Symptoms on the potato plant: On the leaves of the infected potato plants in the different sampling areas in the field, slight mosaic symptoms were evident in the sensitive varieties Sifera (Figure 1); these symptoms were accompanied by the appearance of necrosis on the veins, on the main surface of the lower surface, this necrosis extended to include the secondary veins and the leaf holder, forming a network of blackish lines.

Figure 1. Symptoms of Potato virus Y strain N (PVYN) on Sifera cultivar (a whole plant, b- leaves)
DAS-ELISA. The DAS- ELISA test showed a positive reaction only in the wells of the ELISA dish containing extracts from infected potato samples when using polyclonal antibodies to the common potato Y virus strain PVYº. The highest concentration of the virus under study appeared in the asymptomatic samples of the potato variety (Siefra), as the absorbance value at 405 nm reached 0.606 nm compared to the positive sample, which was 0.790 nm.
TAS-ELISA. The triple ELISA test showed a positive reaction in the wells plate containing extracts from infected potato samples containing necrosis symptoms. In contrast, the reaction was adverse in the wells containing healthy potato samples when monoclonal antibodies of the PVYN strain were used.
The symptoms induced by the indicator plants
- Nicotiana glutinosa: The fresh apical leaves of the plant showed apparent mosaic symptoms after 12 dpi (days post-inoculation). The leaves with an extract of the leaves were infected, and these symptoms were accompanied by deformations that were less visible on the large lower leaves. (figure 2-a).
- Nicotiana Samsun: About 22 days after infection of the lower leaves of this plant with an extract containing the virus, small distinct yellow spots appeared on the fresh apical leaves after 30 days of infection (figure 2-b)

Figure 2. Symptoms of N. glutinosa (A) and N. tabacum (B), inoculated with extract of potato plants infected with PVYN.
Molecular diagnosis of potato virus Y strains using (RT-PCR): The RT-PCR test that was carried out on potato and tobacco samples gave positive results in the ELISA tests in the presence of the standard strain PVYº and PVYN, and this test was able to multiply the DNA of these isolates when using a pair of specialized Nib2-F + Nib3-R primers that double target regions of the viral genome responsible for encoding part of the (Nib) protein of the potato Y virus. All infected samples were given a piece of DNA with a length of 295 bp, characteristic of the presence of the potato Y virus (figure3-a). After confirming the presence of the virus in the tested samples, they were retested by RT-PCR reaction, but using more specialized primers and working to the gene region of the every day strains PVYº and PVYN of the PVY, where it gave samples in which the pair of primers S2- F + A-R duplication of the segment of the genome responsible for encoding a region operating on the gene (P1) of the PVYº strain with a size of 856 bp. While samples in which the primer pair N-868-7-F + N-9236-R was used, which encoded the cap protein (CP) segment of the genome of the PVYN strain, gave 549 bp (figure 3-b,c). Some samples also gave pieces of DNA of different lengths: 441, 250 and 175 bp, and this site may be characteristic of a different group of strains of the potato virus Y, including the PVYNTN strain, which may be the result of recombination between the parental strains of the potato Y virus (figure 3-d). For the first time locally in Nineveh province and at the level of Iraq, using a single molecular test and one step, it was possible to distinguish between the ancient parental strains (PVYº and PVYN), as well as between the PVYNTNT hybrid strain resulting from recombination between the genome of the parental strains when using the pairs of primers N-868-7-F + N -9236-R, which yielded a genome segment of DNA with a length of 441 bp.
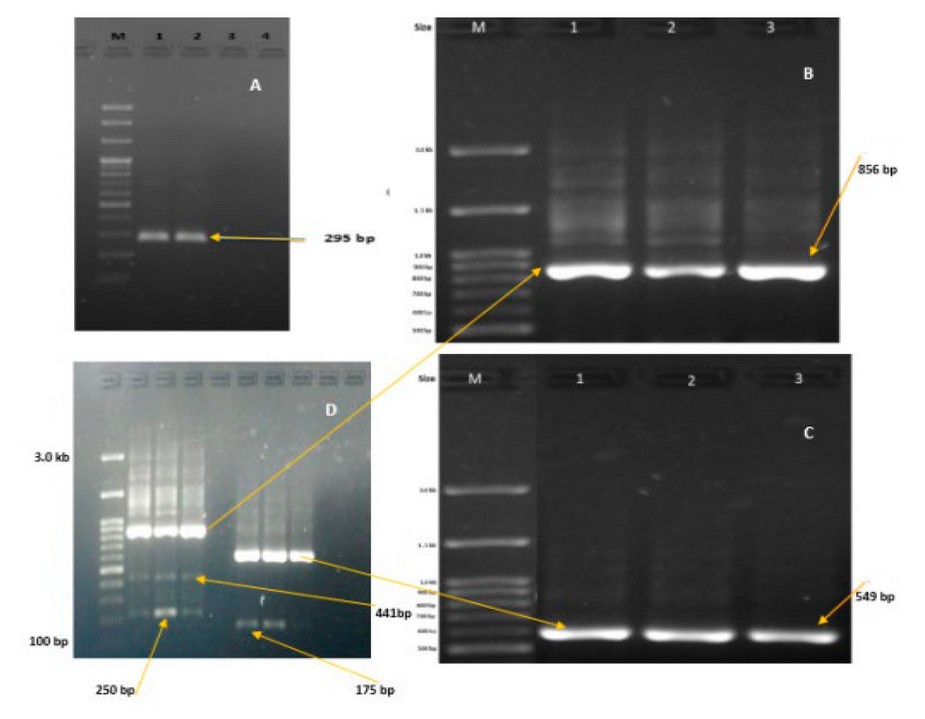
Figure 3. RT-PCR products of target gene segments Nib, P1 and CP, for detection of Potyviruses, potato samples infected with PVY strains with yields 295, 856, 549, 441, 250 and 175 bp (A, B, C, D), respectively.
DISCUSSION
The symptoms of potato plants agree with 12, 13, 14. This result does not agree with what 15 mentioned, as no necrosis symptoms were observed on potato plants infected with this strain in Finland. The ELISA test confirmed the presence of a potato virus Y in those samples. Many researchers consider the ELISA test one of the most efficient tests in detecting the potato virus, primarily because this virus is characterized as one of the viruses with a high immune capacity that is easy to purify 16. The ELISA test is one of the efficient serological tests for the detection of plant viruses, which provides high sensitivity in detecting low viral concentrations that range from 1-10 ng/ml in plant tissue; if 17 mentioned that a virus was detected Cucumber mosaic CMV at a concentration of 2.5 ng/ml by DAS-ELISA assay. At the same time, the TAS-ELISA test confirmed the possibility that the previous symptoms were caused by infection with a tobacco necrosis strain PVYN. The effectiveness of this test in detecting this strain has been reported by several researchers 14, 18. As a result of the powerful serological kinship between the strains of the Y potato virus, it is not possible to accurately determine and diagnose infection based on serological methods based on polyclonal antibodies only, as it is necessary to resort to more than one method for detecting the virus 19. The RT-PCR is an efficient method to detect many plant viruses from different viral groups, such as Potyvirus, Carlavirus, Begomovirus and Torradovirus 20,21 22. For the first time locally in Ninawa province and at the level of Iraq, using a single molecular test and one step, it was possible to distinguish between the ancient parental strains (PVYº and PVYN), as well as between the PVYNTNT hybrid strain resulting from recombination between the genome of the parental strains when using the pairs of primers N-868-7-F + N -9236-R, which yielded a genome segment of DNA with a length of 441 bp. The old parental isolates, especially the new strains or isolates resulting from recombination, have a high antagonistic ability, that is, more aggressive than the parental isolates 23. It was previously found that the most frequent isolates of PVY in Egypt belong to the PVYNTN group 24. Also, the PVYNTN and PVYNW strains dominate most potato-growing areas in Poland 25. The emergence of these hybrid strains, PVYNTN and PVYNW, resulting from the recombination of the ancient classic strains PVYº and PVYN, was followed by a sudden increase in their presence and spread within a relatively short time in the fields, which dominated most of the potato production areas in the world 26, 19, 27.
CONCLUSIONS
The emergence of such a new strain in the tested samples in the potato fields in Nineveh province infected with PVY may have arisen from mixed infections, and this is consistent with many recent studies that confirm the prevalence and spread of such strains compared to other strains. This reflects the evolutionary ability of the virus, which affects the mechanism of the methods used in its control, and also increases the importance of conducting more survey studies periodically to detect the components of the community of this virus in the different potato production areas to see the effectiveness of the control programs used.
Funding: "This research received no external funding. "
Acknowledgments: This study was supported by the College of Agriculture and Forestry /Mosul University-Iraq.
Conflicts of Interest: "The authors declare no conflict of interest."
REFERENCES
1. Singh, R. P., McLaren, D. L., Nie, X., & Singh, M. Possible escape of a recombinant isolate of Potato virus Y by serological indexing and methods of its detection. Plant Disease, .2003. 87(6), 679-685. https://doi.org/10.1094/PDIS.2003.87.6.679
2. Chikh Ali, M., Maoka, T., & Natsuaki, K. T. A point mutation changes the serotype of a Potato virus Y isolate; genomic determination of the serotype of PVY strains. Virus Genes, .2007. 35(2), 359-367.
3. Kerlan, C., Tribodet, M., Glais, L., & Guillet, M. Variability of potato virus Y in potato crops in France. Journal of Phytopathology, 1999.147(11‐12), 643-651. https://doi.org/10.1046/j.1439-0434.1999.00441.x
4. Seyller, T. Electronic properties of SiC surfaces and interfaces: fundamental and technological aspects. Applied Physics A, .2006 .85(4), 371-385. DOI:10.1007/S00339-006-3690-1.
5. Alaaraji, S.F.T., Awad, M.M., Ismail, M.A. Study the association of asprosin and dickkopf-3 with KIM-1, NTpro-BNP, GDF-15 and CPP among male iraqi with chronic kidney disease (2020) Systematic Reviews in Pharmacy, 11 (5), pp. 10-17.
6. Karasev, A. V., & Gray, S. M. Continuous and emerging challenges of Potato virus Y in potato. Annu. Rev. Phytopathol., .2013. 51, 571-586. https://doi.org/10.1146/annurev-phyto-082712-102332
7. Sahib, A. A.; Hussein, K. O.; Hameed, D. M. .; Salih, E.; Kareem, H. . Diffuse Anxiety Syndrome In Those With Stomach Ulcers In Samawa City's. JLSAR 2022, 3, 6-11
8. Al-Shahwan, I. M., Abdalla, O. A., & Al‐Saleh, M. A. Viruses in the northern potato‐producing regions of Saudi Arabia. Plant Pathol., 1997. 46(1), 91-94. https://doi.org/10.1046/j.1365-3059.1997.d01-203.x
9. Abou-Jawdah, Y., Saad, A., & Sobh, H. Incidence of potato virus diseases and their significance for a seed certification program in Lebanon. Incidence of potato virus diseases and their significance for a seed certification program in Lebanon, .2001. 1000-1006.
10. Al-Issawi, Osama Nazim Kazem. Diagnosis and resistance of potato Y virus (a strain of tobacco vein necrosis) on potato crop in Iraq. Master Thesis. College of Agriculture, University of Baghdad. 1999. 185 p (In Arabic).
11. Al-Maadidi, Muthanna Okaidi, Muasser Majeed Gerges and Raqib Akef Al-Ani.. Croup bush, bedstraw Fruited, safflower Wild and spontaneously grown potatoes, secondary hosts of Potato Y virus. Iraqi J. Agric. Sci. .2000. 5(1):21-26 (In Arabic).
12. Qassem, Nabil Aziz and Imad Qassem Muhammad. A survey and diagnostic study of Potato Y virus in Nineveh Governorate. Iraqi J. Agric. Sci. .2002. 3:110-115. (In Arabic).
13. Delgado, S., & Grogan, R. (1970). Potato virus Y. CMI/AAB. Descriptions of plant viruses, (37).
14. DeBokx, J. A. and H. Huttinga. Potato Virus Y. Descriptions of plant Viruses No. 242. Commonwealth Mycological Institute Association of Applied Biologists news . 1981. Surrey . England
15. Khudai M Y, Abdulateef S M, Mouhammed T Th, Alamili H S. Use of modern geometric design of fish ponds to increase welfare and blood parameters. Revis Bionatura 2023;8 (2) 82. http://dx.doi.org/10.21931/RB/2023.08.02.82
16. Kurppa, A. Potato viruses in Finland and their identification. Potato viruses in Finland and their identification. 1983., 55(3), 189-301.
17. Gerges, Muasser Majeed. Using the ELISA test for rapid detection of Potato Y virus in potato fields in Iraq. J of Arab Plant Prot. 2000., 19:46-50. (In Arabic).
18. Torrance L, Cowan GH, McLean K, MacFarlane S, Al-Abedy AN, Armstrong M, Lim TY, Hein I, Bryan GJ. Natural resistance to Potato virus Y in Solanum tuberosum Group Phureja. Theoretical and Applied Genetics. 2020 Mar;133:967-80.
19. McDonald, J. G., & Singh, R. P. Response of potato cultivars to North American isolates of PVYNTN. Am. J. Potato Res.1996., 73(7), 317-323. DOI: 10.1007/BF02855212
20. Nie, Zhihong, and Eugenia Kumacheva. "Patterning surfaces with functional polymers." Nature Materials 7.4 .2008.: 277-290.
21. Zheng, V., Cao, B., Zheng, Y., Xie, X., & Yang, Q. Collaborative filtering meets mobile recommendation: A user-centered approach. In Proceedings of the AAAI Conference on Artificial Intelligence .2010. (Vol. 24, No. 1).
22. Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol., .2018.35(6), 1547. DOI: 10.1093/molbev/msy096
23. Nagy, P. D. Recombination in plant RNA viruses. In Plant virus evolution 2008. (pp. 133-156). Springer, Berlin, Heidelberg.
24. El-Absawy, E. A., Mahmoud, A., Hemeida, A. A., & Helmy, M. Molecular variation of Potato virus Y isolated from Egypt. Int. J. Virol, 2012.8(1), 81-89. DOI: 10.3923/ijv.2012.81.89
25. Yin, Z., Chrzanowska, M., Michalak, K., Zagórska, H., & Zimnoch- Guzowska, E.. Recombinants of PVY strains predominate among isolates from potato crop in Poland. J. Plant Prot. Res. .2012. http://dx.doi.org/10.2478/v10045-012-0033-4
26. H. Naji, S., F. Hussein, F. Health Effect Of Using Alkaline Diet In The Prevention And Treatment Of Diabetic Ketoacidosis And Disruption Of Serum Electrolytes Of Experiment Rats. Anbar Journal Of Agricultural Sciences, 2023; 21(1): 188-204. doi: 10.32649/ajas.2023.179729
27. Schubert, J., Fomitcheva, V., & Sztangret-Wiśniewska, J. Differentiation of Potato virus Y strains using improved sets of diagnostic PCR-primers. J. Virol. Methods, .2007. 140(1-2), 66-74. https://doi.org/10.1016/j.jviromet.2006.10.017
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Zuheer D., Ali H.. Biological, serological and molecular characterization of Potato virus Y strains isolated from potato (Solanum tuberosum L.). Revis Bionatura 2023;8 (4) 60. http://dx.doi.org/10.21931/RB/2023.08.04.60
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. Submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
