2023.08.02.56
Files > Volume 8 > Vol 8 No 2 2023
Copper-to-Zinc Ratio as an Inflammatory Marker in Serum of Iraqi Patients with Axial Spondyloarthritis
Mohammed S. Al-Hindawi 1,*, Abdulnasser M. Al-Gebori 1, and Mohammed Hadi Munshed Alosami 2
1 Applied Sciences Department, Applied Chemists Division, University of Technology, Baghdad – Iraq.
2 Department of Medicine, Rheumatology Unit, Colleges of Medicine, University of Baghdad, Baghdad – Iraq.
* Correspondence: Mohammed S. Al-Hindawi, Applied Sciences Department, Applied Chemists Division, University of Technology, Baghdad – Iraq; [email protected].
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.56
ABSTRACT
Axial spondyloarthritis (axSpA) is a chronic rheumatic inflammatory disease affecting mainly the spine and sacroiliac joints. Since the copper-to-zinc ratio (Cu/Zn) indicates an inflammatory response, the change in ratio is expected to correlate with axSpA. This study compared levels of Cu/Zn in the serum of axSpA patients. Serum samples were obtained from 53 patients with axSpA divided according to biological treatment into cohorts A and B, and 28 healthy control as cohort C. Serum levels of Cu and Zn were determined first by a fully automated chemistry analyzer TC-Matrix Plus, then the ratio was obtained. The elevated serum Cu concentration means of cohort B (189.32 ± 13.808 µg/dL) compared to cohort A (168.85 ± 7.244 µg/dL) and cohort C (155.68 ± 3.707 µg/dL) with 0.029 p-values. Reduced Zn concentration means of cohort B (79.74 ± 4.344 µg/dL) compared to cohort A (91.26 ± 4.159 µg/dL) and cohort C (100.93 ± 6.161 µg/dL) with 0.031 p-values. The Cu/Zn mean of cohort B was (2.54 ± 0.25) compared to the Cu/Zn mean of cohort A (1.968 ± 0.125) and cohort C (1.679 ± 0.104) with 0.002 p-values. Due to the results suggesting that the differences between cohorts were associated with inflammatory responses since there was a similar change in ESR levels; however, the differences between cohorts A and B are due to the anti-inflammatory therapy (TNF inhibitor) that cohort A was treated with.
Keywords: Axial spondyloarthritis; copper-to-zinc ratio; copper; zinc; inflammation.
INTRODUCTION
Axial spondyloarthritis (axSpA) is a chronic rheumatic inflammatory disease with many symptoms affecting mainly the spine and sacroiliac joints (SIJ)1. The axSpA refers to both patients who have structural damage that can be seen on radiographs that knows as radiographic axial spondyloarthritis or ankylosing spondylitis (AS) and patients who do not appear a structural damage on radiographs that knows as non-radiographic axial spondyloarthritis (nr-axSpA)2. The disorder of axSpA has been linked to extra-articular manifestations (EAMs) like inflammatory bowel disease (IBD), psoriasis, and anterior uveitis3.
Copper (Cu) is an essential trace element that serves as a biocatalyst in human metabolism. It catalyzes the oxidation of ascorbic acid and oils and affects protein synthesis, tissue regeneration, bone formation, ATP production, hemoglobin synthesis, and nerve and muscle impulse transmission4,5. Zinc (Zn) is an essential dietary component for the body linked to cancer risk. It requires the production and activation of several enzymes, catalyzes their actions, and has a role in preserving a healthy immune system, DNA synthesis, protein synthesis, and cell division6,7. In addition to calcium, Zn is one of two metals that bind to a leukocyte protein called calprotectin, the main biomarker of IBD that can develop in axSpA patients and is associated with disease activity8,9.
The serum copper-to-zinc ratio (Cu/Zn) level is one of the characteristics linked to a lower ability to maintain or reestablish homeostasis following a destabilizing event. In older people, an increase in this ratio above 2.0 generally indicates an inflammatory response or a reduction in nutritional Zn status10.
This study will focus on the changes in Cu, Zn, and Cu/Zn effects and their association with an inflammatory response in patients with axSpA. Also, the correlation with some biochemical and inflammatory parameters.
MATERIALS AND METHODS
Patients and healthy control groups
The study comprised patients with axSpA, and about eighty-one serum samples were collected at Baghdad Teaching Hospital between October 24, 2021, and January 24, 2022, and divided into three cohorts. The trial's entry criteria for cohort A were that patients were previously diagnosed with axSpA according to the Assessment of Spondyloarthritis International Society (ASAS) and had been treated with a tumor necrosis factor (TNF) inhibitor. Thirty-four patients, with an age mean of 37.09 ± 1.381 years, were in this cohort. Cohort B trial entry criteria involved patients with axSpA who had not yet been treated with a TNF inhibitor; nineteen patients, with an age mean of 36.11 ± 1.597 years, were in this cohort. In cohort C, there were twenty-eight healthy control individuals with an age mean of 36.11 ± 1.138 years. The demographic information from cohorts, including gender, age, body mass index (BMI), and smoking, is in Table 1.
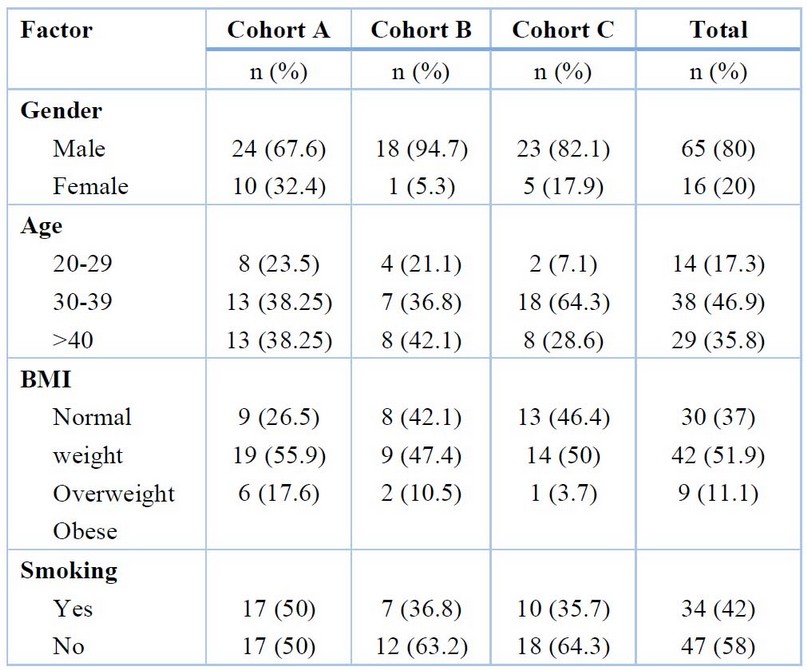
Table 1. Demographic information from cohorts.
Patients' assessment
Patients had a clinical examination at the hospital. A complete blood count, renal function tests (blood urea nitrogen and serum creatinine), liver function tests (serum alanine transaminase and aspartate transaminase), and erythrocyte sedimentation rate (ESR) were among the tests performed at the lab. Also, the genetic risk factor for axSpA, human leukocyte antigen B27 (HLA-B27), was assessed for all samples. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) was used to measure disease activity and functional status. The TC-Matrix Plus (Teco Diagnostics company, USA), an automated chemical analyzer, was used for Zn and Cu concentration measurements.
Statistical analysis
The Statistical Package for Social Sciences version 26 (SPSS-v26) program evaluated the results. Parametric data are supplied as means ± standard error mean (SEM), while Non-parametric data are presented as median using two independent samples tests, and the values are in median (IQR). Statistical analysis of serum Zn and Cu levels between axSpA patients and the healthy control group were compared using the independent samples T-test. Statistical analysis of serum Zn and Cu levels and the other tests that changed among cohorts was compared using the one-way ANOVA and Scheffe tests for post hoc multiple comparisons. Pearson's coefficients were used to perform correlation analysis, and it was based on variable distribution. At a p-value <0.05, the differences were deemed statistically significant.
RESULTS
Patient characteristics
Fifty-three patients with axSpA, thirty-four patients were on TNF inhibitor treatment (cohort A, 10 females, mean age = 37.09 ± 1.381 years, mean BMI = 27.673 ± 0.556 kg/m2), nineteen patients that had no treated with TNF inhibitor (cohort B, one female, mean age = 36.11 ± 1.597 years, mean BMI = 26.822 ± 0.793 kg/m2) and twenty-eight healthy control (cohort C, 5 females, mean age = 36.11 ± 1.138 years, mean BMI = 25.604 ± 0.509 kg/m2) were a part of the research. Twenty-six patients (49%) had BASDAI of less than four, and the mean of cohorts A and B was (3.191 ± 0.355) and (5.674 ± 0.414) respectively, with a p-value <0.001. All cohorts A and B patients had positive HLA-B27 (100%). The patient population's baseline features are shown in Table 2.
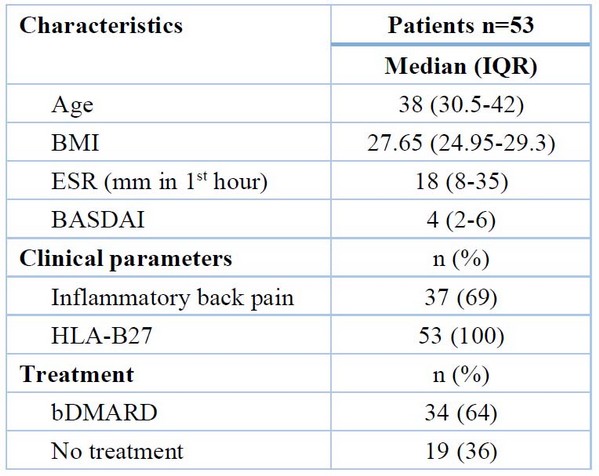
Table 2. The baseline characteristics of the patients
General laboratory tests
Cohort A: ESR mean = 18.03 ± 2.642 mm/hour, hemoglobin (Hb) mean = 13.183 ± 0.294 g/dL, glutamate oxaloacetate aminotransferase (GOT) mean = 21.946 ± 1.7 U/L, glutamate pyruvate aminotransferase (GPT) mean = 27.742 ± 2.645 U/L, urea mean = 26.037 ± 1.285 mg/dL, creatinine mean = 1.275 ± 0.508 mg/dL.
Cohort B: ESR mean = 34.37 ± 5.552 mm/hour, Hb mean = 13.451 ± 0.345 g/dL, GOT mean = 26.174 ± 3.2 U/L, GPT mean = 28.368 ± 5.619 U/L, urea mean = 27.053 ± 1.448 mg/dL, creatinine mean = 0.796 ± 0.025 mg/dL.
Cohort C: ESR mean = 11.46 ± 0.94 mm/hour, Hb mean = 13.943 ± 0.165 g/dL, GOT mean = 21.429 ± 1.222 U/L, GPT mean = 24.571 ± 0.987 U/L, urea mean = 25.893 ± 1.28 mg/dL, creatinine mean = 0.757 ± 0.03 mg/dL.
The mean difference among the cohorts and the p-value from the one-way ANOVA test are shown in Table 3.
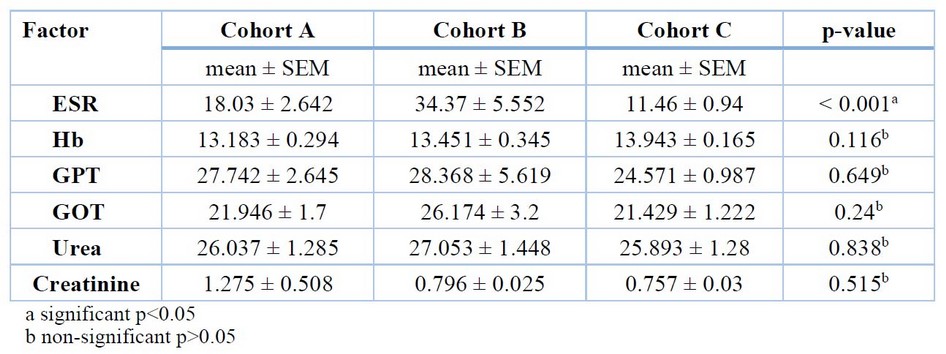
Table 3. The cohorts' mean deference and p-value.
The multiple comparisons between cohorts of the ESR test show a highly significant increase between cohort B and the other cohorts shown in Table 4.
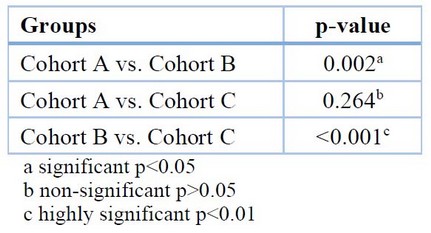
Table 4: Multiple comparisons for ESR among the different groups (Scheffe-test)
Serum copper and zinc levels in patients with axSpA
The results show that there is an increase in Cu levels in axSpA patients [176.19 ± 6.847 µg/dL] compared to the healthy control group [155.68 ± 3.707 µg/dL, p=0.04], and there is a decrease in Zn levels in axSpA patients [87.13 ± 3.158 µg/dL] compared to the healthy control group [100.93 ± 6.161 µg/dL, p=0.03].
Serum copper and zinc levels change with therapy.
The results show that there is an increase in Cu levels in cohort B patients that did not have bio treatment [189.32 ± 13.808 µg/dL] compared to cohort A patients that had bio treatment [168.85 ± 7.244 µg/dL]. The cohort C healthy control group [155.68 ± 3.707 µg/dL, p=0.029] and there is a decrease in Zn levels in cohort B [79.74 ± 4.344 µg/dL] compared to cohort A [91.26 ± 4.159 µg/dL] and cohort C [100.93 ± 6.161 µg/dL, p=0.031].
The differences in means among the cohorts in serum Cu and Zn concentration are shown in Figures 1 and 2, respectively.
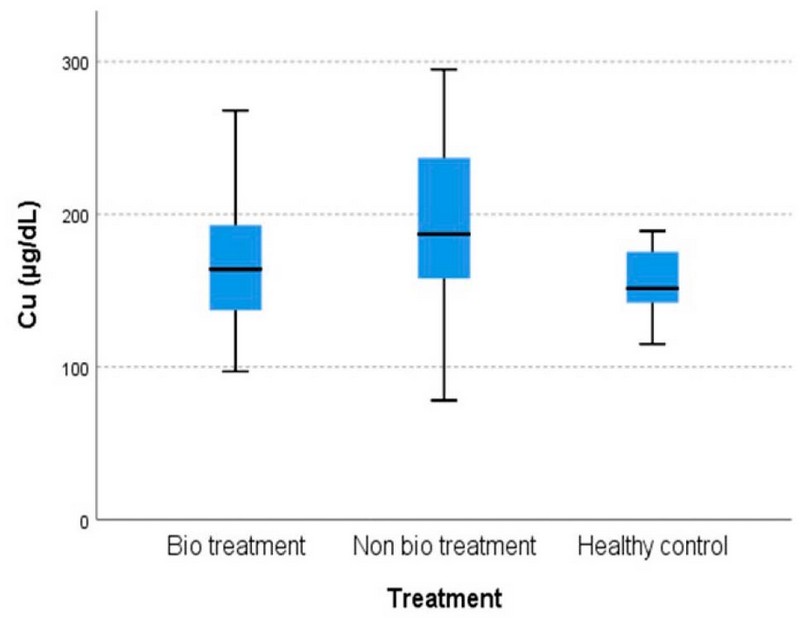
Figure 1. The comparison of serum Cu levels among cohorts.
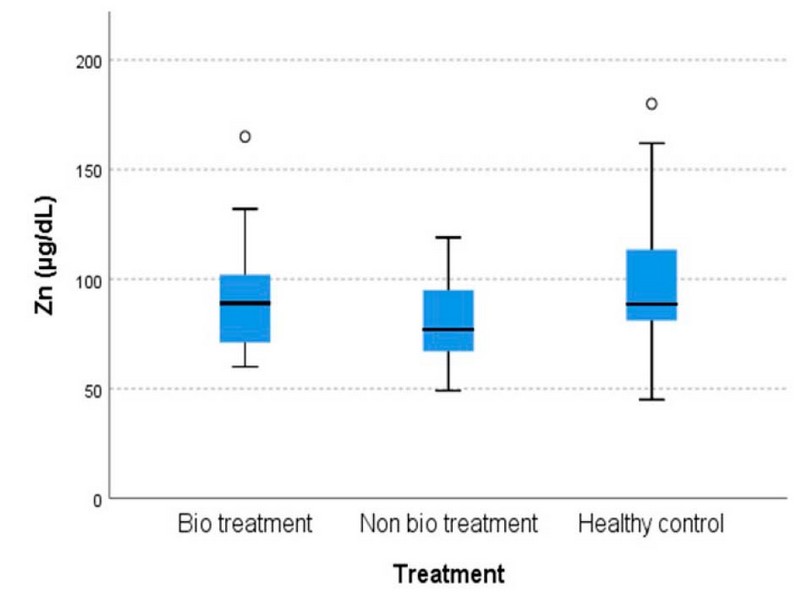
Figure 2. The comparison of serum Zn levels among cohorts
The multiple comparisons between cohorts of the Cu and Zn test show a significant increase in Cu levels and a significant decrease in Zn levels between cohort B and the other cohorts shown in Table 5.
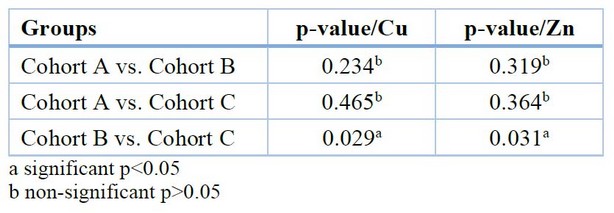
Table 5. Multiple comparisons for Cu and Zn between the different groups (Scheffe-test)
Serum copper to zinc ratio in patients with axSpA
The Cu/Zn shows an increase in the ratio mean of patients [2.173 ± 0.125] compared to the ratio mean of the healthy control group [1.679 ± 0.104, p=0.007].
Serum copper-to-zinc ratio changes with therapy.
The Cu/Zn shows that there is an increase in the ratio mean of cohort B [2.54 ± 0.25] compared to the ratio mean of cohort A [1.968 ± 0.125] and the ratio mean of cohort C [1.679 ± 0.104, p=0.002].
Correlation of serum copper to zinc ratio
Table 6 shows a positive correlation between Cu/Zn and Cu with ESR. In addition, a significant negative correlation of Cu/Zn and a positive correlation of Zn were observed with Hb. However, a non-significant negative correlation between Cu and Zn has been shown, Zn has a non-significant negative correlation with ESR, and Cu has a non-significant positive correlation with Hb.
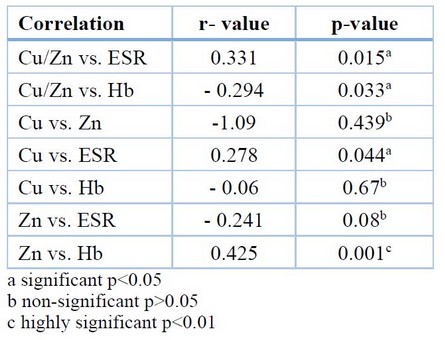
Table 6. Correlation of Cu/Zn with ESR and Hb in axSpA patients.
DISCUSSION
Compared to the healthy control group, serum Cu concentrations were higher in axSpA patients, and serum Zn concentrations were lower in axSpA patients, specifically in cohort B patients that did not have biotreatment. This, therefore, led to increased Cu/Zn.
The newness or novelty of this research is represented by the determining levels of these trace elements and their ratio in this disease, which considers observation that leads to a new knowledge discovery in the relationship between these trace elements and their ratio in this disease for the first time in Iraqi patients. However, many limitations can be identified. The involvement of females in the study sample was shallow because axSpA is a male sexual disorder, with males having nine times the frequency of women; therefore, we could not compare with gender. Also, there were no patients with negative HLA-B27, so we could not make a comparison on this parameter.
As far as we know, there have been very few studies on the changes of Cu and Zn in axSpA patients, so the discussion of the results will be focused on the roles of these parameters in inflammation, as axSpA is an inflammatory disorder, and will be compared to another rheumatic disease like rheumatoid arthritis (RA).
Inflammation causes Cu metabolism to be altered, increasing serum Cu levels, as has been concluded in previous studies11-13. Cu is required for immunological response, including the creation of interleukin-2 (IL-2) by activated lymphocytic cells, and it enhances cellular and humoral immunity by influencing T lymphocytes14. More than 70% of serum Cu is linked to glycoprotein ceruloplasmin (Cp) that is upregulated by pro-inflammatory cytokines TNF in the liver14,15. The increased serum Cu concentration is most likely due to a Cp elevation13. Cu pro-inflammatory effect is due to its ability to cause oxidative stress by catalyzing the production of reactive oxygen species (ROS) via many reactions or by lowering glutathione levels, a powerful cellular antioxidant15. The pathophysiology of AS is complicated by oxidative stress components induced by neutrophil activation16. The serum Cu concentration results of the study agreed with a study that performed an analysis on AS and RA that confirmed the presence of an elevated serum Cu level in this disorders17.
The results showed that they agreed with a previous study on RA patients, which found that the increased production of acute reactive phase proteins may cause higher Cu concentrations in RA18. Cu increases are thought to be anti-inflammatory in RA and other joint diseases, and they can also be used as inflammatory indicators19.
Zn deficiency assimilation can cause an increase in the inflammatory state, immune system changes, extracellular matrix breakdown, a decrease in polymorphonuclear cell phagocytic activity, and Zn-mediated disruption of the TH1/TH2 balance with an increase in TH1720. That leads to increased IL-17 production, which has been reported as one of the elevated parameters in axSpA patients21,22. Inflammation can be induced by altered cytokine synthesis during Zn deficiency, such as IL-1 release23. Furthermore, a comparison of circulating cytokines and Zn status revealed that lower Zn levels may have been associated with higher levels of IL-6, IL-8, and TNF24,25.
In inflammatory diseases, some processes decrease serum Zn concentrations and raise serum Cu concentrations. A typical symptom of these disorders is raised Cu/Zn10. The Cu/Zn ratio results agree with a study confirming that the Cu/Zn appears clinically significant as an inflammatory-nutritional indicator. Increases in Cu/Zn may be related to age, and the link between Cu/Zn and mortality in the elderly might be due to the factors that cause Cu/Zn to rise, such as chronic low-level inflammation, poor nutritional status, and particular underlying conditions such as cardiovascular disease (CVD)26.
The parameters correlated with each other in axSpA patients. They showed that ESR was positively correlated with serum Cu/Zn (r= 0.331, p= 0.015), and these results agreed with the study of (Malavolta M)26.
CONCLUSIONS
A comparison between axSpA patients that had been treated or had not been treated with a TNF inhibitor showed a significant difference in arthritis disease activity as assessed by BASDAI towards the group that had not been treated with a TNF inhibitor. Patients with axSpA showed high levels of Cu and low levels of Zn, especially in the non-TNF inhibitor group, which led to an increase in Cu/Zn ratios, suggesting that the differences between cohorts were due to inflammatory responses since there was a similar change in ESR levels. However, cohorts A and B differ due to the anti-inflammatory therapy (TNF inhibitor) with which cohort A was treated.
REFERENCES
1. van der Heijde D, Ramiro S, Landewé R, et al. update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2016 ; 2017;76(6):978-991. doi:10.1136/annrheumdis-2016-210770.
2. Sieper J, Poddubnyy D. Axial spondyloarthritis. Lancet. 2017;390(10089):73-84. doi:10.1016/S0140-6736(16)31591-4.
3. Rondags A, Arends S, Wink FR, Horváth B, Spoorenberg A. High prevalence of hidradenitis suppurativa symptoms in axial spondyloarthritis patients: A possible new extra-articular manifestation. Semin Arthritis Rheum. 2019;48(4):611-617. doi:10.1016/j.semarthrit.2018.03.010.
4. Husamettin V, et al. Reference Ranges of Trace Elements (Selenium, Zinc, Copper) in Tokat Province. Int J Biochem Physiol 2019;4(4):000170. DOI: 10.23 880/ijbp-16000170.
5. Zainal I, AL-Gebori A. Estimation of serum anti-cyclic citrullinated peptide, glutathione, copper and zinc in patients with multiple sclerosis. Eng Tech J. 2012;30:2461–6.
6. Al-ansari R, Al-Gebori A, Sulaiman G. Serum levels of zinc, copper, selenium and glutathione peroxidase in the different groups of colorectal cancer patients. Caspian J Intern Med, 2020;11(4): 384-390. DOI: 10.22088/cjim.11.4.384.
7. Al-GEBORI AM, TARIK M, RAJAB A, Al-OSAMI MH, TURKI KM: Levels of magnesium, zinc, calcium and copper in serum of patients with fibromyalgia syndrome. The Iraqi Postgraduate Medical Journal, 2011;10:180-183.
8. Bahri R., Elfarssani F., Khayati S., Eddyb S., Saffour H., Boukhira A., Chellak S. Analytical characteristics of faecal and serum calprotectin or calprotectin assay: What clinical interest?. GSC Advanced Research and Reviews. 2021;6(03),156–163. DOI: 10.30574/gscarr.2021.6.3.0052.
9. Kang KY, Park SH, Hong YS. Relationship between faecal calprotectin and inflammation in peripheral joints and entheses in axial spondyloarthritis. Scand J Rheumatol. 2020;49(5):397-404. doi: 10.1080/03009742.2020.1748707.
10. Malavolta M, Piacenza F, Basso A, Giacconi R, Costarelli L, Mocchegiani E. Serum copper to zinc ratio: Relationship with aging and health status. Mech Ageing Dev. 2015;151:93-100. doi:10.1016/j.mad.2015.01.004.
11. Lewis AJ. The role of copper in inflammatory disorders. Agents Actions. 1984;15(5-6):513-519. doi:10.1007/BF01966765.
12. Beshgetoor D, Hambidge M. Clinical conditions altering copper metabolism in humans. The American journal of clinical nutrition. 1998 May;67(5):1017S-21S. doi:10.1093/ajcn/67.5.1017S.
13. Bo S, Durazzo M, Gambino R, Berutti C, Milanesio N, Caropreso A, Gentile L, Cassader M, Cavallo-Perin P, Pagano G. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. The Journal of nutrition. 2008 Feb 1;138(2):305-10.
14. Hordyjewska A, Popiołek Ł, Kocot J. The many "faces" of copper in medicine and treatment. Biometals. 2014 Aug;27(4):611-21. DOI 10.1007/s10534-014-9736-5.
15. Abdulateef, S.M. ,O.K. Atalla, MQ. Al-Ani, TH. T. Mohammed, F.M. Abdulateef, O.M. Abdualmajeed , K. Mahmod. The effect of the electric shock on embryonic development and neurophysiological traits in the chick's embryo . IOP Conference Series: Earth and Environmental Science, 2021; 761(1), 012090.Danaii S, Abolhasani R, Soltani-Zangbar MS, Zamani M, Mehdizadeh A, Amanifar B, Yousefi B, Nazari M, Pourlak T, Hajialiloo M, Yousefi M. Oxidative stress and immunological biomarkers in Ankylosing spondylitis patients. Gene Reports. 2020 March 1;18:100574. doi:10.1016/j.genrep.2019.100574.
16. Aiginger P, Kolarz G, Willvonseder R. Copper in ankylosing spondylitis and rheumatoid arthritis. Scandinavian Journal of Rheumatology. 1978;7(2):75-8.
17. Abedalhammed, H. S., Naser, A. S., Al-Maathedy, M. H., Mohammed, Th. T., Jaber, B. T. & Al-Asha'ab, M. H. 2020. The effect of vitamin e as an antioxidant with different levels of dried tomato pomace supplementation on diets of common carp (cyprinus carpio l) on blood indices. Biochemical and Cellular Archives, 20(2): 5173-5176. Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, Regtop HL, Davies NM. Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coordination Chemistry Reviews. 2002;232(1-2):95-126.
18. ALobaidy, B. .; Al-Falahi, M. N. .; Almarie, A. A. . Effect Of 6-Bap Growth Regulator On Seed Priming Of Several Bread Wheat Varieties Under Water Irrigation Salinity Stress. JLSAR 2021, 2, 21-26..
19. Taams LS, Steel KJ, Srenathan U, Burns LA, Kirkham BW. IL-17 in the immunopathogenesis of spondyloarthritis. Nature Reviews Rheumatology. 2018;14(8):453-66. Doi: 10.1038/s41584-018-0044-2.
20. Brown MA, Li Z, Cao KA. Biomarker development for axial spondyloarthritis. Nature Reviews Rheumatology. 2020;16(8):448-63. Doi: 10.1038/s41584-020-0450-0.
21. Summersgill H, England H, Lopez-Castejon G, Lawrence CB, Luheshi NM, Pahle J, Mendes P, Brough D. Zinc depletion regulates the processing and secretion of IL-1β. Cell death & disease. 2014;5(1):e1040-e1040. doi:10.1038/cddis.2013.547.
22. Mariani E, Cattini L, Neri S, Malavolta M, Mocchegiani E, Ravaglia G, Facchini A. Simultaneous evaluation of circulating chemokine and cytokine profiles in elderly subjects by multiplex technology: relationship with zinc status. Biogerontology. 2006;7(5):449-59. DOI: 10.1007/s10522-006-9060-8.
23. Maares M, Haase H. Zinc and immunity: An essential interrelation. Archives of biochemistry and biophysics. 2016;611:58-65. Doi: 10.1016/j.abb.2016.03.022.
24. Malavolta M, Giacconi R, Piacenza F, Santarelli L, Cipriano C, Costarelli L, Tesei S, Pierpaoli S, Basso A, Galeazzi R, Lattanzio F. Plasma copper/zinc ratio: an inflammatory/nutritional biomarker as a predictor of all-cause mortality in an elderly population. Biogerontology. 2010;11(3):309-19. DOI: 10.1007/s10522-009-9251-1.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Al-Hindawi, M.S.; Al-Gebori, A.M.; Alosami, M.H.M. Copper-to-Zinc Ratio as an Inflammatory Marker in Serum of Iraqi Patients with Axial Spondyloarthritis. Revis Bionatura 2023;8 (2) 56. http://dx.doi.org/10.21931/RB/2023.08.02.56
