2022.07.04.3
Files > Volume 7 > Vol 7 No 4 2022
The Babylon River's common carp (Cyprinus carpio) gills were used for the histopathological examination and PCR detection of Koi herpesvirus disease (KHVD).
1 College of veterinary medicine / Al-Qasim Green University/ department of pathology and poultry diseases; [email protected].
2 Ministry of Agriculture, the veterinary education hospital, Babylon, Iraq; [email protected].
* Correspondence: [email protected]; Tel.: (009647722106661)
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.3
ABSTRACT
The first outbreak of koi herpesvirus in Iraq occurred in Babylon Province in the floating cages in the Euphrates River from the Musayyib thermal electric power station to the Al-Hindiya Dam. Fish were suffering from gills rot that did not respond to treatment and lesions and ulcers on the fish's body. The fatalities reached 80%. was water temperature 24°C, pH 6.85, Salinity 760 ppt. So the present study aims to highlight the pathological changes in gills after the Koi herpes virus infection in Babylon Province. The study started after the detection of (KHV) by polymerase chain reaction ( PCR) from the suspected samples (30 ) for the detection of virus nucleic acid. Also, study the water characterization during the periods of outbreaks. The gills specimen was collected from the positive cases for gross and histopathological examination. The result showed that ten samples were positive for (KHV) infection. The gross and histopathological examination results showed severe congestion with necrotic foci and sloughing of secondary lamella with increased mucus secretion. In addition, necrosis in the primary lamellae, Edam and inflammatory cells infiltration with hyperplasia of the secondary lamellae with the presence of intranuclear inclusion body in all exanimated slides.
Keywords: Koi Herpes; Cyprinus Carpio; Euphrates River; KHV; secondary lamellae; floating cages
INTRODUCTION
Koi herpesvirus disease (KHVD) is a rare virus that can infect koi and carp quickly and widely1. The first report of the koi herpes disease outbreak in Iraq occurred in Babylon Province (north of the Province) in the floating cages in the Euphrates River in the area extending from the Musayyib thermal electric power station to the Al-Hindiya Dam. The first death report was on 10/28/2018, and the death continued until 27/11/2018. Affected fish lost their appetite and exhibited abnormal swimming patterns before they died. Discoloration, elevated respiratory rate2, swollen gills, and grayish and patchy skin lesions are the most constant signs of the sickness3. The koi herpes virus is very contagious and causes massive koi (Cyprinus carpio koi) and common carp mortality4. For at least four hours, the virus is contagious in water. Which explains why it is so contagious in lakes5. It's unclear if The virus enters the fish's body through its gills. Then, it multiplies and causes necrosis and mucosal sloughing or enters the intestine5,6. During overt infection, KHV is most prominent in the gill, kidney, and spleen7. Gill injury is most likely a major factor in fish mortality. The virus replicates in the infected gills before being released into the water and transmitted to the kidney by white blood cells. The virus causes severe interstitial nephritis in the kidneys. This idea fits with the contagious disease's rapid spread and tends to be similar to respiratory viruses in mammals 8
MATERIALS AND METHODS
Samples collection
Thirty live fish suspected samples were collected randomly from fish breeding cages in the Hilla River in October 2021. The project consists of ten floating cages with a capacity of ten thousand fish. The fish were suffering from gills rot that did not respond to treatment and lesions and ulcers on the fish's body. The fatalities were more than 80%. The samples were sent directly to the central laboratory of the Veterinary Department for PCR detection.
PCR detection
The real-time PCR detection of the KHV nucleic acid was done in the laboratory of the Veterinary Directorate by using Microboss Hightech GmbH /Germany) kit and the following primers:
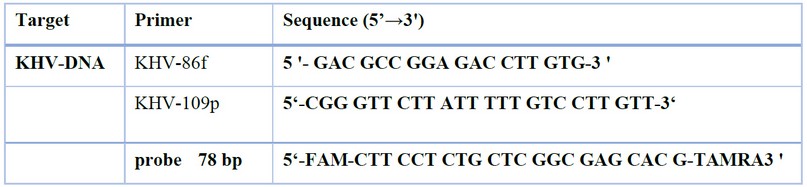
Table 1. Oligonucleotides used in PCR
Water characteristics
Some characteristics of water were measured over five days from sample collection, such as Salinity, Ph and Temperature.
Gross and Histopathological examination
All collected samples were examined grossly for recording the gross pathological changes and for taking the specimen for histopathological section; the gills were fixed in 10 % formaldehyde processed routinely by histokinette, embedded in paraffin, sectioned for 5µm stained by hematoxylin and eosin stain as described by8.
RESULTS
PCR Detections
The Real-time PCR detection of KHV nucleic acid showed positive results (ct 30.35) according to the manufacturing kit (up to 30-40 Ct should be taken positive results ) as shown in figure (1)
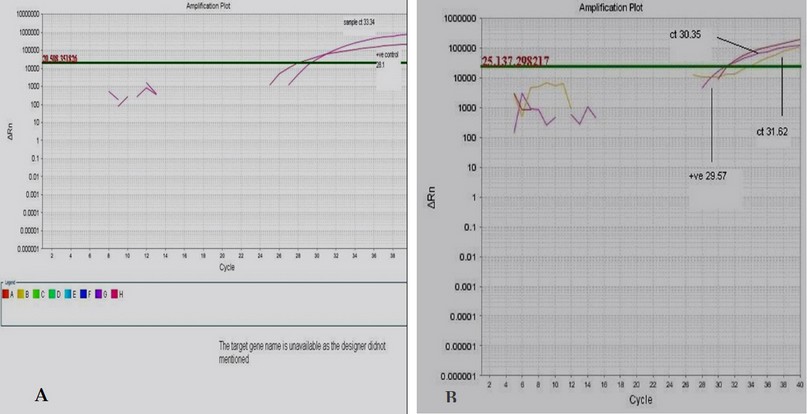
Figure 1.Amplification plot for Real time detection of KHV (A)for control(Ct33.34) and( B) for collected samples(Ct 30.35).
Water characteristics
The mismeasurement of some water characteristics during the study is illustrated in the table(1). The mean result of temperature was (24 ˚c ), Ph (6.85), and salinity (760).
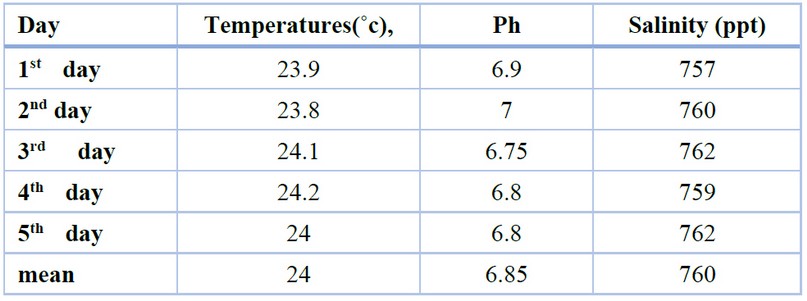
Table 2. Measurement of the Temperatures (˚c), Ph, and Salinity (ppt) during the sample collection period.
Gross and histopathological examination
The grossest observation in most cases was severe congestion with necrotic foci and sloughing of secondary lamella with an increase in mucus secretion (figure2). However, the primary histopathological lesion in most examination sections was necrosis in the primary lamellae, with degenerative changes in the epithelial cells lining the secondary lamellae, with the presence of an intranuclear inclusion body (figure 3). The presence of viral inclusion was shown in most sections characterized by chromatin migration with vacuolation in the lamellar epithelium (figure 4). Edam inflammatory cell infiltration and hyperplasia of the secondary lamellae are also seen (figure 5). Many examined sections showed congestion of the central venous sinus with fusion and desquamation of secondary lamellae ( figure 6,7).
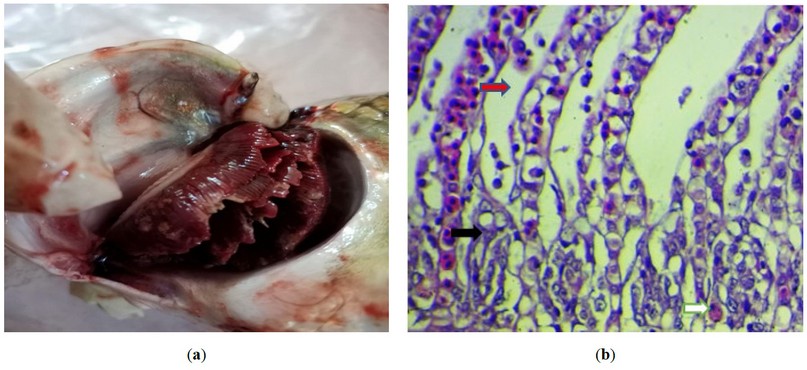
Figure 2. (a) Gross photograph of the fish gills infected with (KHV) shows severe congestion with necrotic foci and sloughing of secondary lamella with the increase in mucus secretion.; (b) Histopathological section of the fish gills shows necrosis in the primary lamellae (black arrow), with degenerative changes in the epithelial cells lining the secondary lamellae (red arrow), with the presence of intranuclear inclusion body (white arrow). H&E stain, 400X).
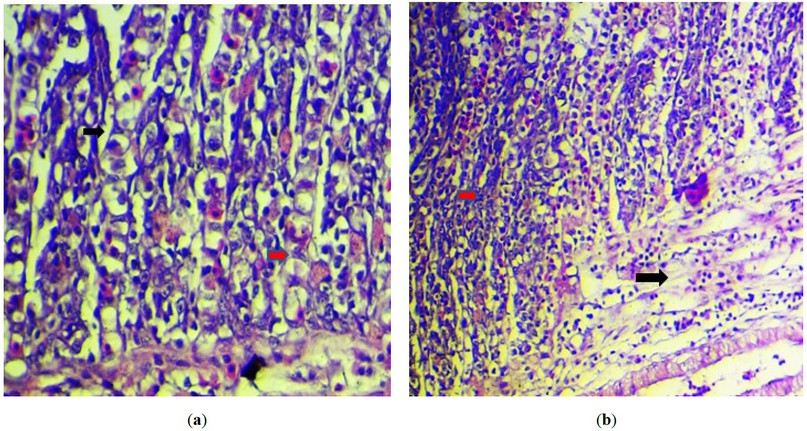
Figure 3. (a) Histopathological section of the fish gills showing intranuclear inclusion body with chromatin migration (black arrow ) with vacuolation in the lamellar epithelium(red arrow). H&E stain, 400X).; (b) Histopathological section of the fish gills showed edam inflammatory cells infiltration (black arrow ) and hyperplasia of the secondary lamellae (red arrow ). (H&E stain, 200X).
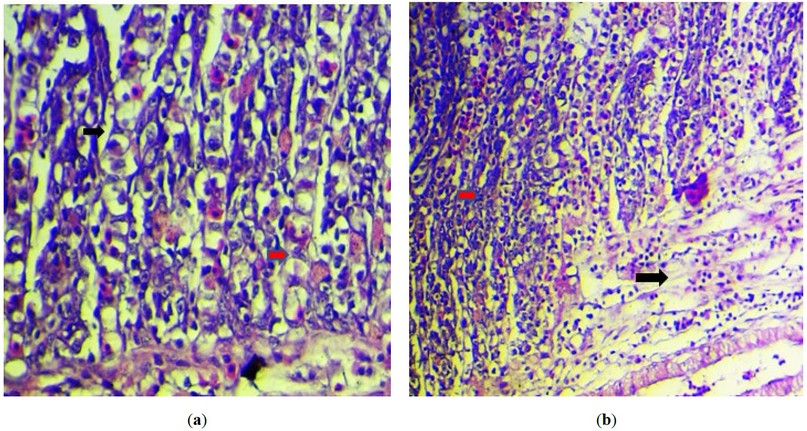
Figure 4.:(a) Histopathological section of the fish gills shows fusion of the secondary lamellae (black arrow ) with sloughing of the others (red arrow). (H&E stain, 400X).; (b) Histopathological section of the fish gills shows severe congestion of the central venous sinus (black arrow) with fusion and desquamation of secondary lamellae (red arrow). (H&E stain, 200X).
DISCUSSION
In poikilothermic species, temperature dramatically influences how the disease progresses3. Water temperature has been found to influence the initiation and severity of viral infection by influencing virus multiplication and indirectly by improving the efficacy of the host immune response9. Temperatures in the water have a direct impact on cellular and humeral immunity. KHV outbreaks in koi and common carp are influenced by water temperature. Viral load in the environment is not as crucial for infection outbreaks when the temperature of infected fish shifts from lower to warmer temperatures (e.g., 23°C); a death occurs quickly (7–12 days from onset to death). This finding matched that of 6, who discovered that water temperature, virus pathogenicity, fish age and condition, high population, and stress conditions all influence sickness patterns (e.g., transportation, spawning, poor water quality). Other research has suggested that the infection is temperature dependant, occurring between 16 and 25 ° C. 5,6,10,11. Gilad et al.; Ilouze et al. 7,12 found that the disease caused high mortality under experimental conditions at 28°C but not at 29°C or 30°C, nor at 13°C.
However, viral DNA was identified in the fish by PCR at 13°C, suggesting that virus reservoirs may exist among infected fish surviving at low temperatures7.
The primary histological abnormalities in gill filaments were confirmed by13. He discovered that most of the gill lamellae's respiratory epithelial cells were enlarged or vacuolated, with nuclear degeneration. Among the nuclear alterations seen were pale coloration, karyorrhexis, and the formation of an intranuclear inclusion body (IIB), defined as basophilic material within the nucleus with marginal hyperchromatic generated by heterochromatin deposition on the inner nuclear membrane. The injured respiratory epithelial cells seemed to have combined with cells from nearby lamellae, causing lamellar fusion and gill filament clubbing. According to to5–7, the primary target cells in the per-gill infection were respiratory epithelial cells of the gill lamellae, where nuclear degradation and IIB were indicators of infection. The formation of IIB was shown to be caused by an increase in filamentous nucleoproteins and the construction of multiple viral capsids and nucleocapsids.
CONSLUSION
We concluded that the disease eruptions occurred during seasonal and sudden temperature changes and caused a severe mortality rate of fish of various weights, especially in marketing weight. Also, KHVD can cause severe pathological changes in fish gills with the presence of an intranuclear inclusion body in histological exanimated sections.
Author Contributions: Conceptualization, Sadeq. Al-Haider. Ghusoon .Alneamah And Samer Alshkarchy.; methodology, Ghusoon A. A. Alneamah; software, Samer .Alshkarchy; validation, Samer.Alshkarchy, Sadeq. Al-Haider. and Ghusoon .Alneamah; formal analysis, Sadeq. Al-Haider. Ghusoon .Alneamah And Sa-mer Alshkarchy; investigation, Ahmed Farhood.; resources, Ahmed Farhood.; data curation, Samer Alshkarchy.; writing—original draft preparation, Ghusoon .Alneamah.; writing—review and editing, Ghusoon .Alneamah. Samer.Alshkarchy; All authors have read and agreed to the published version of the manuscript."
Funding: "This research received no external funding"
Institutional Review Board Statement: "Not applicable."
Informed Consent Statement "Not applicable."
Acknowledgments: We also want to thank Babylon Veterinary Hospital for their assistance with the live fish care during the trial.
Conflicts of Interest: "The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results".
REFERENCES
1. Ilouze M, Dishon A, Kahan T, Kotler M. Cyprinid herpes virus-3 (CyHV-3) bears genes of genetically distant large DNA viruses. FEBS Lett. 2006;580(18):4473-4478.
2. Gray WL, Mullis L, LaPatra SE, Groff JM, Goodwin A. Detection of koi herpesvirus DNA in tissues of infected fish. J Fish Dis. 2002;25(3):171-178.
3. Oh MJ, Jung SJ, Choi TJ, et al. A viral disease occurring in cultured carp Cyprinus carpio in Korea. Fish Pathol. 2001;36(3):147-151.
4. Pokorova D, Vesely T, Piackova V, Reschova S, Hulova J. Current knowledge on koi herpesvirus (KHV): a review. Vet Med (Praha). 2005;50(4):139-148.
5. Farhan, S. M., Abdulateef, S. M. Al-Enzy, A. F. M, Mohammed, Th. T., Saeid, Z. J. M., Al-Khalani, F. M. H. & Abdulateef, F. M. Effect of heat stress on blood alkalinity of broiler chicks and its reflection in improving the productive performance. Indian Journal of Ecology. 2020, 47: 107-109.
6. Hedrick RP, Gilad O, Yun S, et al. A herpesvirus associated with mass mortality of juvenile and adult koi, a strain of common carp. J Aquat Anim Health. 2000;12(1):44-57.
7. Gilad O, Yun S, Zagmutt-Vergara FJ, Leutenegger CM, Bercovier H, Hedrick RP. Concentrations of a Koi herpesvirus (KHV) in tissues of experimentally-infected Cyprinus carpio koi as assessed by real-time TaqMan PCR. Dis Aquat Organ. 2004;60(3):179-187.
8. Pikarsky E, Ronen A, Abramowitz J, et al. Pathogenesis of acute viral disease induced in fish by carp interstitial nephritis and gill necrosis virus. J Virol. 2004;78(17):9544-9551.
9. Alcorn SW, Murray AL, Pascho RJ. Effects of rearing temperature on immune functions in sockeye salmon (Oncorhynchus nerka). Fish Shellfish Immunol. 2002;12(4):303-334.
10. Haenen OLM, Way K, Bergmann SM, Ariel E. The emergence of koi herpesvirus and its significance to European aquaculture. Bull Eur Assoc Fish Pathol. 2004;24(6):293-307.
11. Sano M, Ito T, Kurita J, et al. First detection of koi herpesvirus in cultured common carp Cyprinus carpio in Japan. Fish Pathol. 2004;39(3):165-167.
12. Ilouze M, Davidovich M, Diamant A, Kotler M, Dishon A. The outbreak of carp disease caused by CyHV-3 as a model for new emerging viral diseases in aquaculture: a review. Ecol Res. 2011;26(5):885-892.
13. Miyazaki T, Kuzuya Y, Yasumoto S, Yasuda M, Kobayashi T. Histopathological and ultrastructural features of Koi herpesvirus (KHV)-infected carp Cyprinus carpio, and the morphology and morphogenesis of KHV. Dis Aquat Organ. 2008;80(1):1-11.
Received: 20 July 2022 / Accepted: 15 October 2022 / Published:15 November 2022
Citation: Al-Haider S, Alneamah G, Alshkarchy S, Farhood A. The Babylon River's common carp (Cyprinus carpio) gills were used for the histopathological examination and PCR detection of Koi herpesvirus disease (KHVD). Revis Bionatura 2022;7(4) 3. http://dx.doi.org/10.21931/RB/2022.07.04.3
