2022.07.02.21
Files > Volume 7 > Vol 7 No 2 2022
Ruqaya M. Hamid Al-Sultan1, Ammar Abdulsalaam Al-Sultan2, Mohammed A. Hayawi3, Bilal J M Aldahham4, Mohanad Y. Saleh5,*, Hazim A. Mohammed6
1 Department of Pharmacy, Directorate of Health Nineveh, Ministry of Health.
2 Head & founder of the department of cardiac surgery Mosul Center for Cardiology and Cardiac surgery, Directorate of Health , Nineveh, Ministry of Health.
3 Department of Basic Science, College of Nursing , University of Mosul , Mosul, Iraq.
4 Department of Applied Chemistry, College of Applied Sciences-Hit, University Of Anbar, Hit 31007, Anbar, Iraq.
5 Department of Chemistry, College of Education for pure Science, University of Mosul, Mosul, Iraq.
6 Department of Biochemistry, College of Medicine, University of Mosul, Mosul, Iraq.
* Correspondence: [email protected]; Tel.: (009647738497703)
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.21
ABSTRACT
Thyroid hormones (THs) have a significant effect on the cardiovascular system. THs increase myocardium stretch, leading to the release of B-type Natriuretic Peptide (BNP), which is considered a diagnostic biomarker of heart failure (HF). Thyroid dysfunctions (subclinical hypothyroidism; SCH and subclinical hyperthyroidism; SCHyper) stimulate several changes in the heart by causing either diastolic or systolic left ventricular dysfunctions leading to HF. This study aims to measure the changes of B- type NP levels in cases of subclinical hypo and hyperthyroidism. The present study aims to measure the changes in B-type Natriuretic Peptide (BNP) levels in subclinical hypo and hyperthyroidism (SCH and SCHyper). A theoretical study was also conducted using a docking program to find the effectiveness of some drugs in inhibiting or promoting B-type Natriuretic Peptide (BNP).
A case study was conducted in a private clinic, Mosul- Iraq, from (April 1st – Sep 1) 2021, with 25 healthy participants with normal functioning thyroids as a control group (EU). A newly diagnosed 25 SCH and 17 SCHyper patients participated in this study, considering that none of them have thyroid dysfunctions taking medicine, hypertension, heart diseases, renal failure, and pregnant women. They all were checked for Thyroid Function Tests (TFTs), Free Triiodothyronine (FT3), Free Thyroxin (FT4) and Thyroid Stimulating Hormone (TSH). The plasma level of BNP was measured in all participants of the three groups. The results showed that the plasma level of BNP was higher in SCHyper patients (10.97 pg/ml) as compared to that of SCH patients (8.09 pg/ml) and EU subjects (8.27 pg/ml). Hereby, we could state that subclinical hyperthyroidism, SCHyper, triggers BNP release. Therefore, it should be kept in mind that any high BNP levels due to SCHyper should be considered a reliable diagnostic biomarker of heart failure (HF).
Keywords. Thyroid hormone(TH), Subclinical hypothyroidism(SCH), Subclinical hyperthyroidism(SCHyper), Chronic heart disease(CHD), Heart failure(HF), B-type natriuretic peptide(BNP), Docking Study.
INTRODUCTION
Thyroid hormones (THs) have essential effects on the cardiovascular system, including hemodynamic alterations; these effects are mediated on the cardiac myocytes through gene expression 1.2.
Subclinical hypothyroidism (SCH) is defined by high values of Thyroid Stimulating Hormones (TSH) along with normal serum levels of THs (T3 and T4)3-6. SCH is present in 15% of women over 60 years old, and etiologically most cases of SCH cases might be regarded as a temporary transition into overt thyroid disease usually caused by autoimmune thyroiditis, but this transition time might vary considerably. It is thought that most of these cases progress to clinical hypothyroidism1,7,8.
SCH is associated with an increased risk of Chronic Heart Disease (CHD) related events, including mortality and heart failure (HF), especially when TSH levels ≥ 10.0 mIU/L 9-12. In patients with or without underlying heart disease, persistent SCH can be associated with HF development 13. Through various mechanisms, the abnormalities of THs in SCH may lead to the development of HF complications such as systolic and diastolic dysfunction, blood pressure alterations, and endothelial and vascular dysfunction14-17 since SCH increases systemic vascular resistance(SVR) and arterial stiffness by impairing vascular smooth muscle cells relaxation18 a reduction of Nitric Oxide( NO) availability19 leading to decrease in stroke volume and cardiac index20,21. SCH is usually correlated with left ventricular diastolic dysfunction due to impaired ventricular filling and relaxation22-26; thus, poor exercise tolerance is attributed to both diastolic and systolic dysfunction in SCH27.
The symptoms and signs of SCH are not pathognomonic; therefore, the diagnosis and treatment monitoring depends fundamentally on the measurement of plasma THs and TSH28.
Subclinical hyperthyroidism (SCHyper) is defined as a subnormal serum level TSH level and serum-free T4 and T3 concentrations within the normal reference ranges29-31. As overt hyperthyroidism (OHyper), SCHyper can be caused by exogenous (secondary to excessive THs replacement therapy)32 or endogenous (thyroid disease causing thyroid over-activity) factors32-33.
SCHyper showed a higher heart rate and greater left ventricular mass than EU individuals34-35 and impaired diastolic function compared to OHyper20,36. SCHyper has a potentially arrhythmogenic effect with an increased risk of developing atrial fibrillation, especially in people over 65 years old37. This may explain why cardiac dysfunction has been found in patients with SCHyper rather than OHyper. Thus, SCHyper is associated with increased cardiovascular mortality38-39.
Progression and development of HF40-41 and cardiovascular mortality have been associated with persistently untreated SCHyper38.
The risk and severity of HF were associated with both higher and lower TSH levels significantly when TSH ˃10 µIu / ml and for TSH˂0.01 µIu / ml42, BNP level rises proportionately to systolic, diastolic dysfunctions and the severity of HF43.
In 1988, a peptide was purified from the porcine brain; it was named "Brain Natriuretic Peptide" (BNP)44. Later, this peptide was known as a" B-type natriuretic peptide" and synthesized primarily in the myocardium45-46. BNP is synthesized as a 134 amino-acid 'pre-proBNP' which is cleaved to 'proBNP'. Further processing gives rise to the inactive N-terminal pro-BNP (NT-proBNP) (76-residues) and the biologically active C-terminal BNP (32-residues)47.
BNP belongs to the natriuretic peptide family with different physiological effects, including a diuretic, natriuretic, and vasorelaxant actions48-49. Excessive stretching is the primary stimulus triggering BNP secretion by the ventricular myocytes of the heart50 rather than the trans-mural pressure load51-56. It was noted that FT3 has a direct stimulus for the BNP secretion from myocardial cells by increasing the gene expression. Thus, THs in a direct action increase myocardial BNP gene expression57,58.
It acts as a blood pressure regulatory hormone that is physiologically opposed and suppresses the renin-angiotensin-aldosterone system, endothelin-1, and the sympathetic nervous system59. Thus, it is considered a cardioprotective peptide60. An increase in heart rate, total blood volume, left ventricular end-diastolic volume and cardiac output in hyperthyroidism exerts a "stress" effect on the cardiac wall, BNP secreted from the myocardial ventricle is a result of a stretch of the myocardial wall. A possible stimulus for BNP secretion could subsequently increase plasma BNP levels61. Plasma BNP level has been recommended as a diagnostic and prognostic marker for patients with HF62-65.
RESULTS
The participant's clinical and biochemical characteristics with EU, SCHyper and SCH are summarized in table (1), which shows no significant difference between the mean values of age, body mass index (BMI), and systolic BP diastolic BP among the three groups.
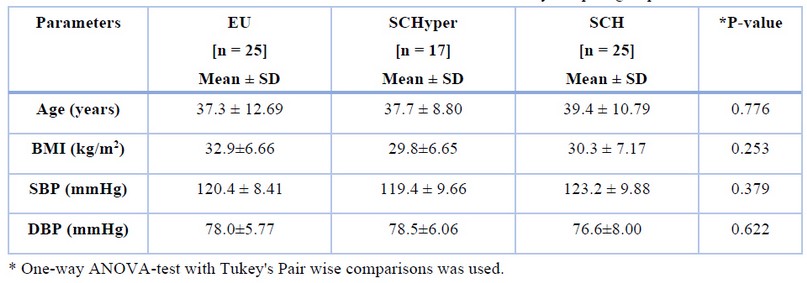
Table 1. Personal characteristics of the study sampled groups.
The mean FT3, FT4, TSH and BNP distribution values showed significant differences among the three groups. Table (2)
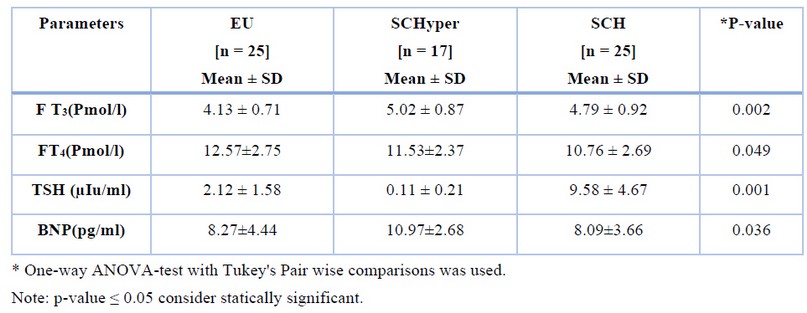
Table 2. Comparison of TFTs among the three study sampled groups.
Figure 1 shows the distribution of BNP among the three groups. A high value of BNP (10.97 pg/ml) for SCHyper group.
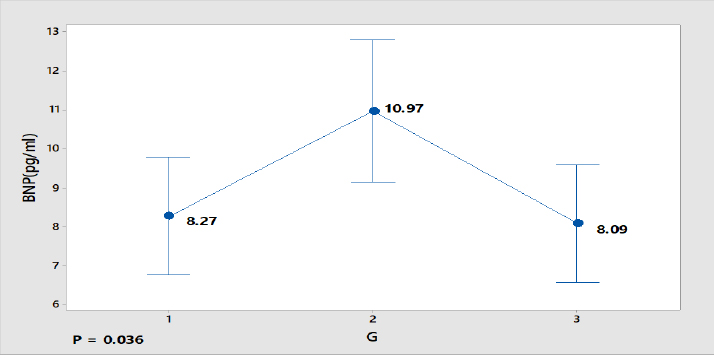
Figure 1. BNP distribution among the three groups.
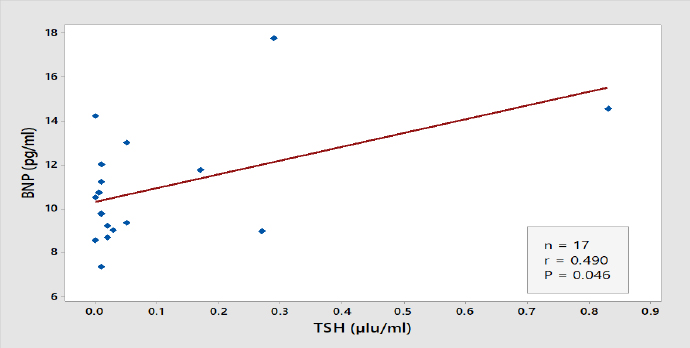
Figure 2. Correlation between TSH and BNP Level
Results of docking studies
Comparing the binding concerning to docking score from the Patchdock server is the main idea of the theoretical part in this study. Figure 3 and Figure 4 illustrate the 2D binding of T3 with NP receptor and T3 with its specific receptor, respectively.
It is evident from figures and Tables 3 and 4 that the binding T3 is stronger to the NP receptor than its specific receptor, which may lead to an increase in the production of NPB, because the values of Global energy have a slight difference. When data of docking scores in Tables 3 and 4 are compared, it can be noticed that the binding of T3 to the receptor of NP is possible because the active site of binding is similar to the thyroid hormone receptors. The highest docking score was obtained from binding with of T3 with NP receptor (5430), while the highest docking score was obtained from T3 with thyroid hormone receptor (4710). The interface area of T3 binding to NP receptor is (649.90) compared to the interface area of T3 with its receptor (613.80). The results indicate that T3 may bind strongly and affect the response of increasing the concentration of NP. The docking scores and interface area can theoretically explain the relationship between THs and NP. Three-dimensional pictures showing the interaction of T3 to NP receptor and to thyroid hormone receptor have been implemented in figure 5 and figure 6, respectively.
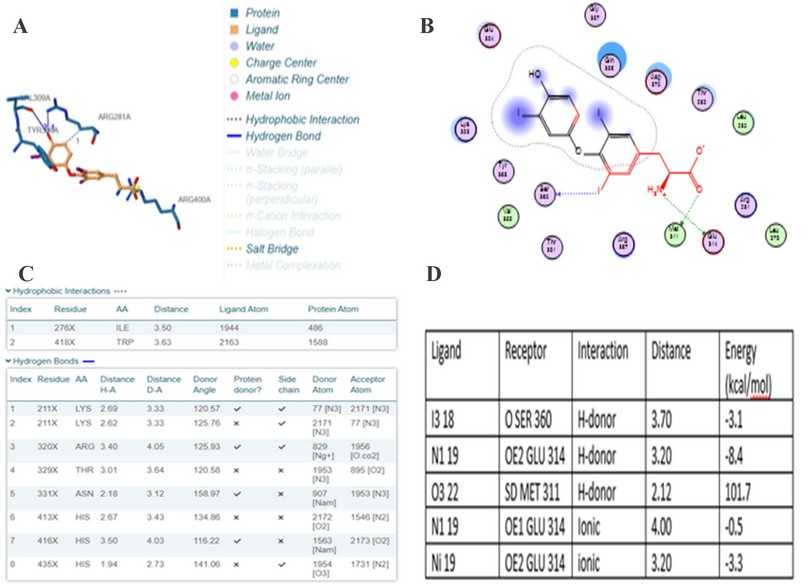
Figure 3. two-dimension docking visualization of T3 with NP receptor using PLIP (a), 2D visualizing docking using MOE (b), types of interaction and distance according to PLIP (c), and interaction distance and energy according to MOE74.
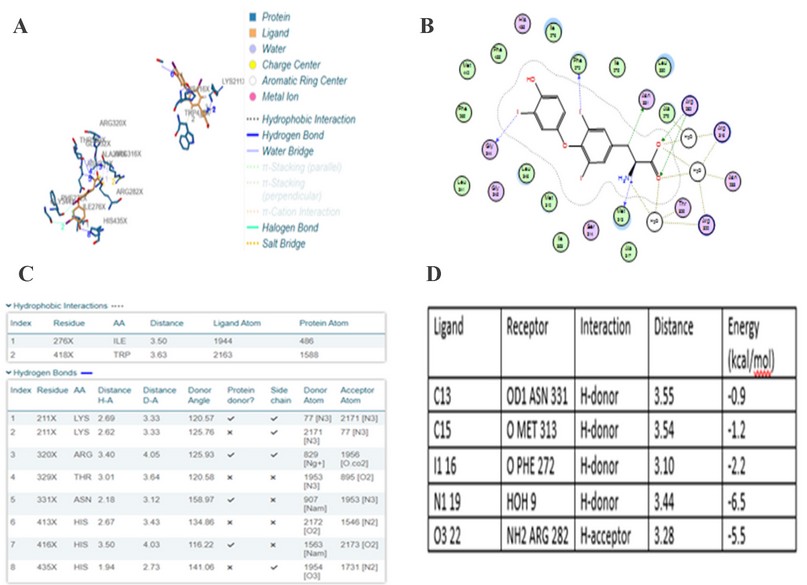
Figure 4. two-dimension docking visualization of T3 with thyroid hormone receptor using PLIP (a), 2D visualizing docking using MOE (b), types of interaction and distance according to PLIP (c), and interaction distance and energy according to MOE74.
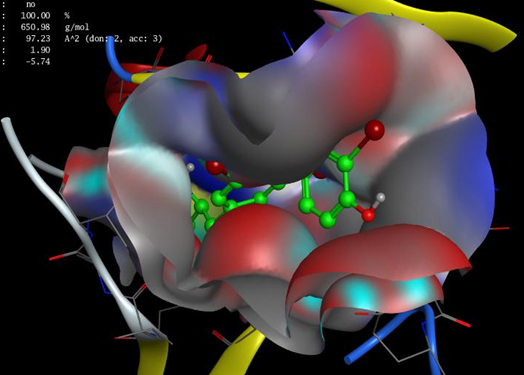
Figure 5. 3D of T3 interaction to NP receptor using MOE.
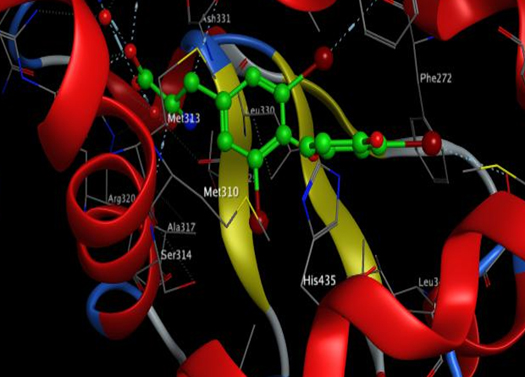
Figure 6. 3D of T3 interaction to thyroid hormone receptor using MOE.
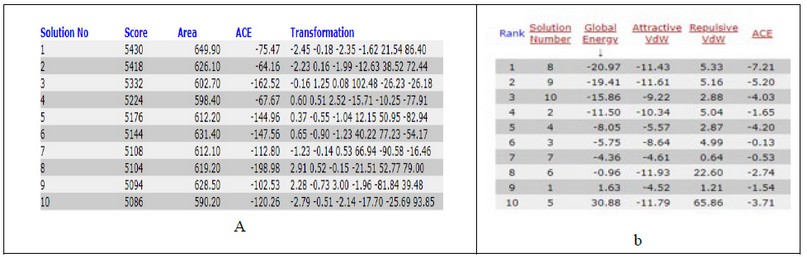
Table 3. docking score from patchdock server of T3 with NP receptor (a) and Fast Interaction Refinement in Molecular Docking "FIRE DOCK" (b).
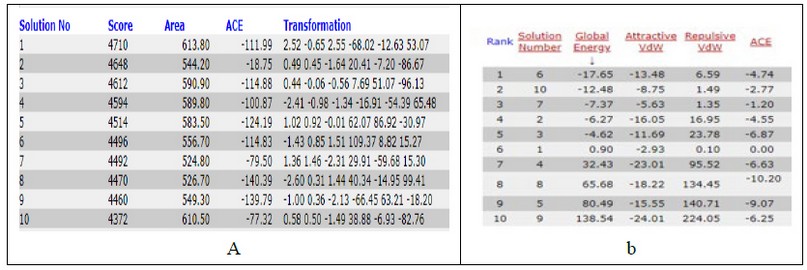
Table 4. docking score from patchdock server of T3 with thyroid hormone receptor (a) and Fast Interaction Refinement in Molecular Docking "FIRE DOCK" (b).
Patients and methods
A case-control study has been done in a private surgical clinic in Mosul, Iraq, from Apr 1 to Sep 1. 2021. Permission has been asked from all involved subjects after explaining the outlines of the study to them.
A questionnaire has been designed and fulfilled by all patients, including the information of name, age, height, weight, BMI, blood pressure (systolic and diastolic), presence of any diseases (diabetes mellitus, heart disease, hypertension, thyroid disease). The participants were selected according to the exclusion criteria (heart disease, hypertension, renal failure and pregnancy). Twenty-five subjects with SCH, seventeen subjects with SCHyper, and twenty-five healthy subjects were regarded as a control group, all the subjects in the three groups were females with ages between 20 - 67 years.
The patients were segregated based on thyroid function tests (TFTs), THs (free Tri-iodothyronine ;FT3 and Free thyroxin ;FT4) and TSH. The FT3 reference range(RR) (4.14-6.09 pmol/L), FT4 RR (8.4-14.42pmol/L) and TSH RR(0.38 -5.33µIU/L).66 SCH was defined by elevated TSH levels in the serum above 5.33 µIU/L in the presence of THs levels within normal range, while SCHyper defined by level of TSH lower than 0.38 µIU/L with normal levels of THs. A plasma level of BNP hormone was measured in all patients with RR (≤ 26.5 pg /ml).67
A blood sample (5 ml ) was taken from all patients, 2 ml were put in EDTA tube, then shaking for half minute and centrifugation. Plasma samples were needed to measure BNP by Tosoh AlA- 360, Japan through immune Enzymatic Assay system67. Another 3ml of blood was put in gel tube, serum was obtained by centrifugation, and TFTs estimation was done through Access 2 (Beckman coulter), NHANES, USA68-70. An electrochemiluminescence assay was performed for TFTs estimation.
Approval has been obtained from the Committee of Ethics at Nursing college, Mosul University, Iraq.
Docking studies
Two docking studies have been carried out using patchdock server trying to prove the practical data. tri-iodothyrionine (T3) was selected as the ligand to investigate its binding to the natriuretic peptide receptor and compared the results with T3 binding to the specific receptor (thyroid hormone receptor). Docking was carried out using PatchDock71,72 server (http://bioinfo3d.cs.tau.ac.il/ PatchDock/). The proteins in this study were receptors of T3 hormone with PDB ID (3gws) and NP receptor with PDB ID (1ky1), while the structure of T3 was designed and energy minimized using MOE. The protein-small ligand was chosen as the complex type, with a clustering RMSD of 1.5 Å. Protein ligand interaction profiler (PLIP)73 was used to visualize the 2D and 3D structures as well as MOE v14.
Statistical analysis
Descriptive statistical methods were used to tabulate and summarize data. One-way ANOVA-test with Tukey's Pairwise comparisons was used. The Data was expressed as a mean with standard deviation (SD), P-values ≤0.05 were considered statistically significant. Pearson correlation test was used to assess the strength and direction of the relation between BNP and FT3, FT4 and TSH levels in each sample group. All statistical procedures were performed using Minitab version 18 software statistical program.
DISCUSSION
THs have the leading role in the homeostasis of the cardiovascular system. Several processes, including the maintenance of heart structure, function, and cardiac contractility, are all regulated by THs75. These lead to many changes, such as hemodynamic changes, including myocardial contractility, cardiac output, and SVR changes76. Arises in THs cause stretching of the myocardium, through genomic and non-genomic pathways77,78. Therefore, thyroid dysfunctions lead to the pathogenesis of HF79.
Our study shows that the value of BNP in patients with SCHyper is higher than that of EU subjects and SCH patients and is statistically significant. Ohba et al. (2020)80 measured the BNP levels in patients with SCHyper, SCH and EU subjects; they reported that BNP levels were higher in SCHyper patients than that of SCH and EU subjects and statically different. Pakula et al. (2011)81 showed a significantly higher mean of NT-proBNP in SCHyper patients than in SCH patients and EU subjects; these results are similar to the results of this study. The results reported in this study agreed with that of Ertrugul et al.(2008)54. They found an increase in BNP levels for SCHyper patients compared to that of SCH patients and EU subjects and statically differences among the three groups.
The preferred binding orientation of a ligand into a receptor is predicted through docking82 and docking scores can be used just for classifying active ligands from in-actives; but furthermore, we should consider binding assays. Docking gives us the protein-ligand complex, where ligands get bound in the same active site as predicted experimentally or any predicted active site in case of another protein or homology modeled protein. It is the first time studying the association of T3 with NP receptors, which gives good indications and may explain the results of T3 and T4 with NP in our case study.
CONCLUSIONS
This study, from the results, shows that SCHyper has a significant increase in plasma levels of BNP more than SCH influence. Since SCHyper induces significant hemodynamic changes in the cardiovascular system, thyroid function affects the BNP level, and THs stimulate BNP release. Therefore, SCHyper should be considered in any patient presented with mildly elevated BNP levels. Also, we should consider any elevation of BNP levels in the presence of thyroid dysfunction that could lead to HF.
Acknowledgments
My best regards to Assistant Prof. Humam Ghanim lbrahim Zubeer, PhD of Community Medicine and Biostatistics, Department of Family and Community Medicine, College of Medicine /University of Mosul, who helped me computerize the data of this study for statistical analysis.
Conflict of interests
The author declares no conflict of interest.
REFERENCES
1. Perel C, Echin M. Insuficiencia cardíaca y tiroides. Daño miocárdico en el hipotiroidismo. Insuficiencia cardíaca. 2006;1(1):43-51.
2. Vale C, Neves JS, von Hafe M, Borges-Canha M, Leite-Moreira A. The role of thyroid hormones in heart failure. Cardiovascular drugs and therapy. 2019 Apr;33(2):179-88.
3. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. Jama. 2004 Jan 14;291(2):228-38.
4. Ripoli A, Pingitore A, Favilli B, Bottoni A, Turchi S, Osman NF, De Marchi D, Lombardi M, L'Abbate A, Iervasi G. Erratum: Does subclinical hypothyroidism affect cardiac pump performance? Evidence from a magnetic resonance imaging study (Journal of the American College of Cardiology (2005) 45 (439-445)). Journal of the American College of Cardiology. 2005 Mar 15;45(6):968.
5. Redford C, Vaidya B. Subclinical hypothyroidism: Should we treat?. Post reproductive health. 2017 Jun;23(2):55-62.
6. Cojić M, Cvejanov-Kezunović L. Subclinical Hypothyroidism–Whether and When To Start Treatment? Open Access Maced J Med Sci. 2017 Dec 15; 5 (7): 1042-1046.
7. Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Archives of internal medicine. 2005 Nov 28;165(21):2467-72.
8. Hak AE, Pols HA, Visser TJ, Drexhage HA, Hofman A, Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Annals of internal medicine. 2000 Feb 15;132(4):270-8.
9. Gencer B, Collet TH, Virgini V, Auer R, Rodondi N. Subclinical thyroid dysfunction and cardiovascular outcomes among prospective cohort studies. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 2013 Mar 1;13(1):4-12.
10. Hayashi T, Hasegawa T, Kanzaki H, Funada A, Amaki M, Takahama H, Ohara T, Sugano Y, Yasuda S, Ogawa H, Anzai T. Subclinical hypothyroidism is an independent predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure. ESC heart failure. 2016 Sep;3(3):168-76.
11. Duntas LH, Chiovato L. Cardiovascular risk in patients with subclinical hypothyroidism. European endocrinology. 2014 Aug;10(2):157.
12. Rodondi N, Bauer DC, Cappola AR, Cornuz J, Robbins J, Fried LP, Ladenson PW, Vittinghoff E, Gottdiener JS, Newman AB. Subclinical thyroid dysfunction, cardiac function, and the risk of heart failure: the Cardiovascular Health Study. Journal of the American College of Cardiology. 2008 Sep 30;52(14):1152-9.
13. Bielecka-Dabrowa A, Godoy B, Suzuki T, Banach M, von Haehling S. Subclinical hypothyroidism and the development of heart failure: an overview of risk and effects on cardiac function. Clinical Research in Cardiology. 2019 Mar;108(3):225-33.
14. Allam G, Abdel-Moneim A, Gaber AM. The pleiotropic role of interleukin-17 in atherosclerosis. Biomedicine & Pharmacotherapy. 2018 Oct 1;106:1412-8.
15. Pasqualetti G, Niccolai F, Calsolaro V, Bini G, Caraccio N, Monzani F. Relationship between thyroid dysfunction and heart failure in older people. Journal of Gerontology And Geriatrics. 2017 Sep 15;65(03 Special):184-91.
16. Taddei S, Caraccio N, Virdis A, Dardano A, Versari D, Ghiadoni L, Ferrannini E, Salvetti A, Monzani F. Low-grade systemic inflammation causes endothelial dysfunction in patients with Hashimoto's thyroiditis. The Journal of Clinical Endocrinology & Metabolism. 2006 Dec 1;91(12):5076-82.
17. Türemen EE, Çetinarslan B, Sahin T, Cantürk Z, Tarkun I. Endothelial dysfunction and low grade chronic inflammation in subclinical hypothyroidism due to autoimmune thyroiditis. Endocrine journal. 2011:1103090544-.
18. Cooper DS, Biondi B. Subclinical thyroid disease. The Lancet. 2012 Mar 24;379(9821):1142-54.
19. Owen PJ, Sabit R, Lazarus JH. Thyroid disease and vascular function. Thyroid. 2007 Jun 1;17(6):519-24.
20. Gencer B, Collet TH, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012 Aug 28;126(9):1040-9.
21. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. Jama. 2006 Mar 1;295(9):1033-41.
22. Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, Currò D. Thyroid hormones, oxidative stress, and inflammation. Mediators of inflammation. 2016 Oct;2016.
23. Biondi B, Fazio S, Palmieri EA, Carella C, Panza N, Cittadini A, Bonè F, Lombardi G, Saccà L. Left ventricular diastolic dysfunction in patients with subclinical hypothyroidism. The Journal of Clinical Endocrinology & Metabolism. 1999 Jun 1;84(6):2064-7.
24. Monzani F, Di Bello V, Caraccio N, Bertini A, Giorgi D, Giusti C, Ferrannini E. Effect of levothyroxine on cardiac function and structure in subclinical hypothyroidism: a double blind, placebo-controlled study. The Journal of Clinical Endocrinology & Metabolism. 2001 Mar 1;86(3):1110-5.
25. Ripoli A, Pingitore A, Favilli B, Bottoni A, Turchi S, Osman NF, De Marchi D, Lombardi M, L'Abbate A, Iervasi G. Erratum: Does subclinical hypothyroidism affect cardiac pump performance? Evidence from a magnetic resonance imaging study (Journal of the American College of Cardiology (2005) 45 (439-445)). Journal of the American College of Cardiology. 2005 Mar 15;45(6):968.
26. Duntas LH. Thyroid disease and lipids. Thyroid. 2002 Apr 1;12(4):287-93.
27. Pasqualetti G, Tognini S, Polini A, Caraccio N, Monzani F. Subclinical hypothyroidism and heart failure risk in older people. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 2013 Mar 1;13(1):13-21.
28. Gunturiz ML. Thyroid Hormones, Subclinical Hypothyroidism and Its Importance in Cardiovascular Disease. Ar Clin Cardiol Res 2: 106. DOI: 10.29011. ACCR-106/100006; 2019.
29. American Thyroid Association and American Association of Clinical Endocrinologists Taskforce on Hyperthyroidism and Other Causes of Thyrotoxicosis, Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011 Jun 1;21(6):593-646.
30. Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, Franklyn JA, Hershman JM, Burman KD, Denke MA, Gorman C. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. Jama. 2004 Jan 14;291(2):228-38.
31. Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT. Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and the Endocrine Society. Thyroid. 2005 Jan 1;15(1):24-8.
32. Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, Hamilton W, Okosieme O, Panicker V, Thomas SL, Dayan C. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks: evidence from a large community-based study. JAMA internal medicine. 2014 Jan 1;174(1):32-9.
33. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Archives of internal medicine. 2000 Feb 28;160(4):526-34.
34. Biondi B, Palmieri EA, Fazio S, Cosco C, Nocera M, Saccà L, Filetti S, Lombardi G, Perticone F. Endogenous subclinical hyperthyroidism affects quality of life and cardiac morphology and function in young and middle-aged patients. The Journal of Clinical Endocrinology & Metabolism. 2000 Dec 1;85(12):4701-5.
35. Sgarbi JA, Villaca FG, Garbeline B, Villar HE, Romaldini JH. The effects of early antithyroid therapy for endogenous subclinical hyperthyroidism in clinical and heart abnormalities. The Journal of Clinical Endocrinology & Metabolism. 2003 Apr 1;88(4):1672-7.
36. Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Annals of internal medicine. 2002 Dec 3;137(11):904-14.
37. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocrine reviews. 2008 Feb 1;29(1):76-131.
38. Ertek S, Cicero AF. Hyperthyroidism and cardiovascular complications: a narrative review on the basis of pathophysiology. Archives of medical science: AMS. 2013 Oct 31;9(5):944.
39. Barbesino G. Thyroid function changes in the elderly and their relationship to cardiovascular health: a mini-review. Gerontology. 2019;65(1):1-8.
40. Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016 Oct 1;26(10):1343-421.
41. Pasqualetti G, Niccolai F, Calsolaro V, Bini G, Caraccio N, Monzani F. Relationship between thyroid dysfunction and heart failure in older people. Journal of Gerontology And Geriatrics. 2017 Sep 15;65(03 Special):184-91.
42. Roos A, Links TP, Wolffenbuttel BH. Subclinical thyroid disease and heart failure. Eur J Heart Fail. 2014 Feb 1;16(2):119-21.
43. Osca J, Quesada A, Arnau MA, Osa A, Hervás I, Almenar L, Palencia M, Mateo A, Algarra F. Brain natriuretic peptide. Diagnostic value in heart failure. Revista espanola de cardiologia. 2002 Jan 1;55(1):7-15.
44. Sudoh, T., Kangawa, K., Minamino, N., & Matsuo, H. (1988). A new natriuretic peptide in porcine brain. Nature, 332(6159), 78-81.
45. Minamino, N., Aburaya, M., Ueda, S., Kangawa, K., & Matsuo, H. (1988). The presence of brain natriuretic peptide of 12,000 daltons in porcine heart. Biochemical and biophysical research communications, 155(2), 740-746.
46. Hosoda, K., Nakao, K., Mukoyama, M., Saito, Y., Jougasaki, M., Shirakami, G., ... & Imura, H. (1991). Expression of brain natriuretic peptide gene in human heart. Production in the ventricle. Hypertension, 17(6_pt_2), 1152-1155.
47. Gaggin, H. K., Chen-Tournoux, A. A., Christenson, R. H., Doros, G., Hollander, J. E., Levy, P. D., ... & ICON-RELOADED Investigators. (2017). Rationale and design of the ICON-RELOADED study: international collaborative of N-terminal pro–B-type natriuretic peptide re-evaluation of acute diagnostic cut-offs in the emergency department. American heart journal, 192, 26-37.
48. LaPointe, M. C. (2005). Molecular regulation of the brain natriuretic peptide gene. Peptides, 26(6), 944-956.
49. Chopra, S., Cherian, D., Verghese, P. P., & Jacob, J. J. (2013). Physiology and clinical significance of natriuretic hormones. Indian journal of endocrinology and metabolism, 17(1), 83.
50. Nielsen, L. S., Svanegaard, J., Klitgaard, N. A., & Egeblad, H. (2004). N‐terminal pro‐brain natriuretic peptide for discriminating between cardiac and non‐cardiac dyspnoea. European journal of heart failure, 6(1), 63-70.
51. Mair, J. (2008). Biochemistry of B-type natriuretic peptide–where are we now?. Clinical chemistry and laboratory medicine, 46(11), 1507-1514.
52. Pandit, K., Mukhopadhyay, P., Ghosh, S., & Chowdhury, S. (2011). Natriuretic peptides: Diagnostic and therapeutic use. Indian journal of endocrinology and metabolism, 15(Suppl4), S345.
53. Arikan, S., Tuzcu, A., Gokalp, D., Bahceci, M., & Danis, R. (2007). Hyperthyroidism may affect serum N‐terminal pro‐B‐type natriuretic peptide levels independently of cardiac dysfunction. Clinical endocrinology, 67(2), 202-207.
54. Ertugrul, D. T., Gursoy, A., Sahin, M., Unal, A. D., Pamuk, B., Berberoglu, Z., ... & Demirag, N. G. (2008). Evaluation of brain natriuretic peptide levels in hyperthyroidism and hypothyroidism. Journal of the National Medical Association, 100(4), 401-405.
55. Yoo, B. S. (2014). Clinical significance of B-type Natriuretic peptide in heart failure. Journal of lifestyle medicine, 4(1), 34.
56. Chowdhury, P., Kehl, D., Choudhary, R., & Maisel, A. (2013). The use of biomarkers in the patient with heart failure. Current cardiology reports, 15(6), 372.
57. Kishida, C., Naito, R., Kasuya, H., Kaneko, T., Yabe, K., Kakihara, M., ... & Nakazato, Y. (2018). A Case of Heart Failure with Hyperthyroidism Demonstrating Discrepancy between the Clinical Course and B-type Natriuretic Peptide Levels. Internal Medicine, 0118-17.
58. Liang F, Webb P, Marimuthu A, Zhang S, Gardner DG. Triiodothyronine increases brain natriuretic peptide (BNP) gene transcription and amplifies endothelin-dependent BNP gene transcription and hypertrophy in neonatal rat ventricular myocytes. Journal of Biological Chemistry. 2003 Apr 25;278(17):15073-83.
59. Silver, M. A., Horton, D. P., Ghali, J. K., & Elkayam, U. (2002). Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. Journal of the American College of Cardiology, 39(5), 798-803.
60. Kuwahara, K., & Nakao, K. (2010). Regulation and significance of atrial and brain natriuretic peptides as cardiac hormones. Endocrine journal, 57(7), 555-565.
61. Ertugrul, D. T., Yavuz, B., Ata, N., Yalcin, A. A., Kucukazman, M., Algul, B., ... & Tutal, E. (2009). Decreasing brain natriuretic peptide levels after treatment for hyperthyroidism. Endocrine journal, 56(9), 1043-1048.
62. Clerico, A., Zaninotto, M., Prontera, C., Giovannini, S., Ndreu, R., Franzini, M., ... & Study Group on Cardiovascular Risk Biomarkers of the Italian Society of Clinical Biochemistry (SIBIOC. (2012). State of the art of BNP and NT-proBNP immunoassays: the CardioOrmoCheck study. Clinica Chimica Acta, 414, 112-119.
63. Januzzi, J. L., Chen-Tournoux, A. A., Christenson, R. H., Doros, G., Hollander, J. E., Levy, P. D., ... & ICON-RELOADED Investigators. (2018). N-terminal pro–B-type natriuretic peptide in the emergency department: the ICON-RELOADED study. Journal of the American College of Cardiology, 71(11), 1191-1200.
64. Bui, A. L., Horwich, T. B., & Fonarow, G. C. (2011). Epidemiology and risk profile of heart failure. Nature Reviews Cardiology, 8(1), 30.
65. Xu-Cai, Y. O., & Wu, Q. (2010). Molecular forms of natriuretic peptides in heart failure and their implications. Heart, 96(6), 419-424.
66. Ruzhanskaya, A., Ichihara, K., Evgina, S., Skibo, I., Vybornova, N., Vasiliev, A., ... & Emanuel, V. (2021). Sources of variation and establishment of Russian reference intervals for major hormones and tumor markers. PloS one, 16(1), e0234284.
67. https://www.diagnostics.eu.tosohbioscience.com.
68. CDC. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES) 2009-2010 Laboratory Methods, Collaborative Laboratory Services, Laboratory Procedure Manual, Free T3 in Serum [Internet]. 2011 Sep. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/THYROD_F_met_Free_T3_CLS.
69. CDC. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES) 2009-2010 Laboratory Methods, Collaborative Laboratory Services, Laboratory Procedure Manual, Free T4 in Serum [Internet]. 2011 Sep. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/THYROD_F_met_Free_T4_access_CLS.
70. CDC. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey (NHANES) 2009-2010 Laboratory Methods, Collaborative Laboratory Services, Laboratory Procedure Manual, Thyroid Stimulating Hormone in Serum [Internet]. 2011 Sep. Available from: https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/THYROD_F_met_TSH_CLS.
71. Dedov NV, Tran VH, Duke CC, Connor M, Christie MJ, Mandadi S, Roufogalis BD (2002) Gingerols: A novel class of vanilloid receptor (VR1) agonists. British Journal of Pharmacology 137 (6), 793-798
72. Schneidman-Duhovny D, Inbar Y, Polak V, Shatsky M, Halperin I, Benyamini H, Barzilai A, Dror O, Haspel N, Nussinov R, Wolfson HJ (2003) Taking geometry to its edge: Fast unbound rigid (and hinge-bent) docking. Proteins 52 (1), 107-112
73. https://plip-tool.biotec.tu-dresden.de/plip-web/plip/index
74. Aldahham, B. J., Al-Khafaji, K., Saleh, M. Y., Abdelhakem, A. M., Alanazi, A. M., & Islam, M. A. (2020). Identification of naphthyridine and quinoline derivatives as potential Nsp16-Nsp10 inhibitors: a pharmacoinformatics study. Journal of Biomolecular Structure and Dynamics, 1-8.
75. Dorr M, Wolff B, Robinson DM, John U, Ludemann J, Meng W, Felix SB, Volzke H. The association of thyroid function with cardiac mass and left ventricular hypertrophy. The Journal of Clinical Endocrinology & Metabolism. 2005 Feb 1;90(2):673-7.
76. Delitala AP, Fanciulli G, Maioli M, Delitala G. Subclinical hypothyroidism, lipid metabolism and cardiovascular disease. European journal of internal medicine. 2017 Mar 1;38:17-24.
77. Khan R, Sikanderkhel S, Gui J, Adeniyi AR, O'Dell K, Erickson M, Malpartida J, Mufti Z, Khan T, Mufti H, Al-Adwan SA. Thyroid and cardiovascular disease: a focused review on the impact of hyperthyroidism in heart failure. Cardiology research. 2020 Apr;11(2):68.
78. Sabatino L, Iervasi G, Pingitore A. Thyroid hormone and heart failure: from myocardial protection to systemic regulation. Expert review of cardiovascular therapy. 2014 Oct 1;12(10):1227-36.
79. Nielsen LS, Svanegaard J, Klitgaard NA, Egeblad H. N‐terminal pro‐brain natriuretic peptide for discriminating between cardiac and non‐cardiac dyspnoea. European journal of heart failure. 2004 Jan;6(1):63-70.
80. Ohba K, Okada E, Goto Y, Suzuki S, Machii M, Nonaka D, Matsushita A, Sasaki S, Suda T, Oki Y, Takase H. Influence of thyroid dysfunction on brain natriuretic peptide level in health examination participants. Endocrine journal. 2020:EJ19-0380.
81. Pakuła D, Marek B, Kajdaniuk D, Krysiak R, Kos-Kudła B, Pakuła P, Gatnar A, Borgiel-Marek H, Nowak M, Siemińska L, Głogowska-Szeląg J. Plasma levels of NT-pro-brain natriuretic peptide in patients with overt and subclinical hyperthyroidism and hypothyroidism. Endokrynologia Polska. 2011;62(6):523-8.
82. RAMÍREZ, David; CABALLERO, Julio. Is it reliable to take the molecular docking top scoring position as the best solution without considering available structural data?. Molecules, 2018, 23.5: 1038.
Received: 5 December 2021 / Accepted: 10 January 2022 / Published:15 May 2022
Citation: Ruqaya M. Hamid Al-Sultan, Ammar Abdulsalaam Al-Sultan, Mohammed A. Hayawi, Bilal J M Aldahham, Mohanad Y. Saleh, Hazim A. Mohammed. The effect of subclinical thyroid dysfunction on B- type natriuretic peptide level. Revis Bionatura 2022;7(2) 21. http://dx.doi.org/10.21931/RB/2022.07.02.21
