2023.08.01.10
Files > Volume 8 > Vol 8 No 1 2023
Regeneration of cocoa (Theobroma cacao L.) via somatic embryogenesis: Key aspects in the in vitro conversion stage and in the ex vitro adaptation of plantlets.
Ana María Henao Ramírez 1*, Julián David Morales Muñoz 2, Diana Marcela Vanegas Villa 3 , Ruth Tatiana Hernández Hernández 4
, Ruth Tatiana Hernández Hernández 4 , Aura Inés Urrea-Trujillo 5
, Aura Inés Urrea-Trujillo 5
1 Center of Agrobiotechnological Development and Innovation – CEDAIT, Universidad de Antioquia, Km. 1.7 vía San Antonio de Pereira - Carmen de Viboral, A.A 054048, Colombia; https://orcid.org/0000-0002-8957-702X
2 Center of Agrobiotechnological Development and Innovation – CEDAIT, Universidad de Antioquia, Km. 1.7 vía San Antonio de Pereira - Carmen de Viboral, A.A 054048, Colombia.
3 Center of Agrobiotechnological Development and Innovation – CEDAIT, Universidad de Antioquia, Km. 1.7 vía San Antonio de Pereira - Carmen de Viboral, A.A 054048, Colombia;
4 Microbiology Institute, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, A. A 050010, Colombia;
5 Biology Institute, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, A. A 050010, Colombia;
* Correspondence: [email protected]; Tel.: +57 3013920674
http://dx.doi.org/10.21931/RB/2023.08.01.10
ABSTRACT
Adapting plantlets to ex vitro conditions is a decisive step in the micropropagation process via organogenesis or somatic embryogenesis (ES). The percentage of success in this stage determines the quality of the product, an example of which is found in cocoa plantlets regenerated by ES, which require specific conditions to overcome the stress of the new environment. Considering the quality of the in vitro plantlets largely determines the survival and growth in ex vitro conditions, the effect of two culture media between the embryo maturation stage and the initial stage of conversion to plantlet was evaluated (EM2 - MM6 and EM2 – MF medium), achieving with the latter greater stem height, root length and the number of true leaves. In the final stage of the conversion and growth of the plantlet, the effect of five culture media was evaluated (ENR6, MF, ENR8, EDL, PR), achieving better results in stem height, root length, and the number of true leaves on MF medium. In addition, it was found that the transition of the EM2-MF had a significant development in the presence of the desired pivoting root and fibrous roots. Under nursery conditions, the growth and development of the plantlets was tested through the inoculation of beneficial microorganisms to promote survival. The plantlets that met the minimum morphological parameters for acclimation were planted in a substrate of coconut palm and sand (3:1 v/v) previously selected in the laboratory (BS). The effect of Pseudomonas ACC deaminase (PAACd), Trichoderma asperellum (Ta) and arbuscular mycorrhiza forming fungus (AMF) and different concentrations of phosphorus (PC) (0%, 50% and 100%) in the Hoagland nutrient solution (1:10) was evaluated. First, for CCN5, 62.5% of survival was obtained with PAACd + AMF. Second, the largest leaf size and survival were obtained with PAACd + Ta for CNCh12 and CCN51; likewise, for CNCh13, the best result was obtained with PAACd.
Keywords: Cacao, Clonal propagation, Mycorrhiza, Pseudomonas, Trichoderma.
INTRODUCTION
Cocoa (Theobroma cacao L.) is an economically important crop and the most valuable agricultural product worldwide 1 . It has been grown in the lowlands of tropical regions, South and Central America, West Africa, and Southeast Asia, with social and economic importance. Cocoa powder and cocoa butter are the major cocoa seed products with several common usages, especially in high-demand food industries 2 . Cocoa seeds are rich in phenolic compounds and flavonoids and contain several bioactive compounds, such as procyanidins, anthocyanins, flavone and flavonol glycosides, epicatechin, gallocatechin, epigallocatechin, etc. 3,4 . Cocoa polyphenols have anti-inflammatory, anticarcinogenic, antimicrobial, antiulcer, and immune-modulating properties, and antioxidants with a protective effect against cardiovascular diseases 5–8 . Thus, producing high-quality and uniform cocoa planting materials is crucial to supply industrial demands. This could be achieved through plant tissue culture techniques such as somatic embryogenesis (SE) since conventional methods do not complete the quantities required by the market. In SE a single somatic cell obtained from leaf, flower, or stem explants undergoes several differentiation processes before developing into the whole plant after culture 9 . For cocoa, the protocols for somatic embryogenesis culture have been developed by several researchers 10–12. H owever, low acclimation percentages of around 8.3% to 54.57% with different genotypes were reported 13–15
The acclimation is a transitory phase between the laboratory and the field, whose objective is to take the plantlet from an in vitro culture to ex vitro conditions 16 . The ex vitro adaptation of Vitro plantlets requires time and conditions for the plantlets to acquire the necessary vigor to survive. They usually need several weeks under shade and gradually decreasing air humidity to acclimate to the new requirements and correct some changes in their anatomy and physiology induced by in vitro culture conditions. For plant survival, the most important changes include the development of cuticles, epicuticular waxes, and effective stomatal regulation of transpiration leading to stabilization of water status 17 . From Aguilar et al. (1992) and Figueira & Janick, (1995) 1 , to Jones et al. (2022) 20 and Manlé et al. (2021) 1 , the acclimation of cocoa vitro-plants is a difficult and crucial stage as it happens in most plantlets produced by in vitro tissue cultures 21 . Therefore, knowing the factors that affect the survival of vitroplantlets is essential when implementing the production of plant material on a large scale, whose main contribution is the development of an optimal propagation protocol.
On the other hand, it is necessary to have plantlets produced in laboratory commercials that ensure plant material is in good physiological condition to survive the definitive transplant without affecting their establishment and optimal development in the field. In this sense, crucial developmental phases such as the maturation of the somatic embryo, the conversion of the embryos to plantlet, and the acclimation of plants to nursery conditions are essential to ensure physiological vigor characteristics. This last step can be favored using microorganisms as nursery substrate improvers, which are recognized as promoters of root development in cocoa seedlings and their effect on the increase and nutrient absorption capacity 22–26 .
Many plants' growth-promoting microorganisms (PGPM) can help in rooting and shoot elongation and can be useful in the acclimation phase. They can protect against the biotic and abiotic stress that occurs in vitro propagation, mainly in the acclimation phase, a crucial step for the success of micropropagation 27 . Within these microorganisms, the Trichoderma fungus is considered a soil organism associated with plant roots and is commonly viewed for its potential to control plant diseases in what may be a close association with many typical aspects of endophytic associations with cocoa 28 , in addition to being able to behave as a plant growth stimulator 29,30 . Other beneficial microorganisms are mycorrhizal fungi, which are the mutualistic symbiosis between the fungi of the Glomeromycota phylum and the roots of most vascular plants, which are supposed to play a key role in the nutrient cycle of agroforestry systems 31 . In the case of crops with thick roots and few root hairs, such as cocoa, coffee, timber trees, and citrus, they tend to be highly dependent on mycorrhizae 32 . Specifically in cocoa, due to its mycorrhizal dependency, the fertilization practice, mainly nitrogenous and phosphate, must be fully evaluated, considering not only the yield of the crop and the availability of nutrients in the soil but also the composition and behavior of the biota, in order not to inhibit these biological processes or stimulate dependence on external inputs in these systems 33 .
Other microorganisms are the bacterias that produce the enzyme ACC (1-aminocyclopropane-1-carboxylate) deaminase, being a critical bacterial trait to facilitate plant growth. This enzyme is responsible for the cleavage the plant ethylene precursor, ACC, into ammonia and ketobutyrate. Ethylene is an essential plant hormone, also known as a stress hormone because the induction of a variety of biotic and abiotic stress accelerates its synthesis by lowering ACC levels in plants, ACC deaminase-producing organisms lower ethylene levels, which, when present in high concentrations, can lead to plant growth inhibition or even death 33 . These bacteria are relatively common in soil, having been found in a wide range of environments around the world 33 .
According to the above, the objective of this work was to evaluate from the stage of maturation and conversion to plantlet in vitro the effect of culture media composition and, in the acclimation phase, the effect of beneficial microorganisms such as Pseudomonas ACC deaminase, the fungus Trichoderma asperellum and mycorrhizal fungi on characteristics physiological vigour in plantlets to establish a complete process in the production of plant material on an industrial scale.
MATERIALS AND METHODS
Plant materials and culture conditions
For this research, the experiments included CCN51, CNCh12, and CNCh13 genotypes, the primary genotype used in the experiments was CCN51, a universal genotype from Ecuador 34 . CNCh12 and CNCh13 are Colombian genotypes from the certified clonal garden of the Colombian National Chocolate Company (CNCH). The immature closed flower buds were collected from La Nacional Farm field-grown plants (Támesis, Antioquia-Colombia) and Yariguíes farm (Barrancabermeja, Santander-Colombia). The flower bud's collection and disinfection process were performed following the methodology described by Henao et al. (2018) 3 . The explants consisted of staminodes (sterile stamens) for CCN51, CNCh13, and CNCh12. Staminodes were extracted from the basal portion of the flower bud using sterile scalpels and placed on the culture media on the Petri dish. For CNCh12 was used, the following culture media was according to the stages of the embryogenic process: PCG-SCG-ED-CM2-EM2-MM6, for CNCh13, was INDI-INDexp-CM2-EM2-MM6 and for CCN51 was INDI-INDexp-CM2-EM2-MM6 (Table 1).
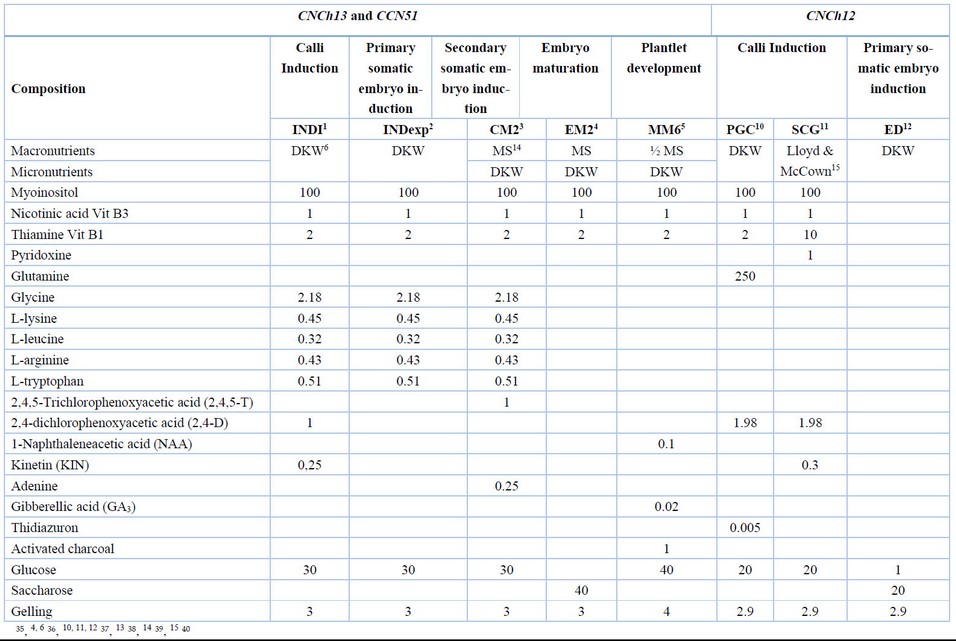
Table 1. Culture media for primary and secondary somatic embryogenesis with CCN51, CNCh13, and CNCh12 genotypes of cacao (T. cacao L.). 1,2,3,5
All the cultures were randomly placed in a growth chamber in continuous darkness for callogenesis, induction of primary embryo, and induction of secondary embryo stages, at an average temperature of 26˚C ± 2˚C and 70% relative humidity. The mature embryos were cultured in a Petri dish, and later plantlets were cultured in 500 ml vessels. The cultures were placed in a growth chamber under light with a 16-hour photoperiod and a photosynthetic photon flux density (PPFD) of 50 μmol per second, at an average temperature of 26˚C ± 2˚C and 70% relative humidity.
In vitro growth
Embryo maturation.
Once the secondary somatic embryos of CCN51 had been developed, they were transferred for maturating and growth. In this stage, the effect of two different culture media transitions between embryo maturation - plantlet development stage (EM2 – MF and EM2 – MM6) (Table 2) and culture time (30, 60, and 90 days) were evaluated on stem height (cm), root length (cm) and the number of true leaves per plantlet (n). The experiment was laid out in a Completely Randomized Design (CRD) with two factors: culture media (2 levels) and culture time (3 levels). Each treatment with at least 30 experimental units, for a total of n = 524.
Plantlet development.
For CCN51, the effect of five different plantlet development culture media (ENR6, MF, ENR8, EDL, PR) (Table 2) was evaluated on stem length (cm), root length (cm), number of true leaves (n) after 30 days from the plant out. The experiment was laid out in a CRD with one factor, culture media, with five levels. Each treatment with at least 10 experimental units, for a total of n = 43.
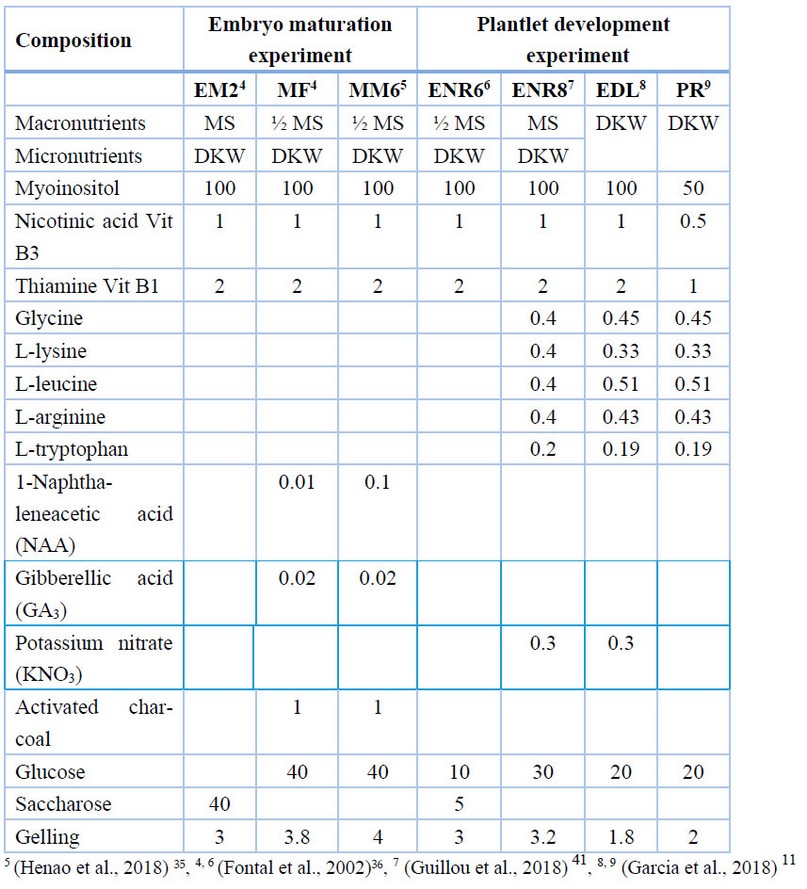
Table 2. Culture media for primary and secondary somatic embryogenesis with CCN51 genotype of cacao (T. cacao L.) in embryo maturation and plantlet development experiments.
Ex vitro adaptation
Plantlets of different genotypes with 60 days in MF culture medium were used for the different experiments; these plantlets had stem and root development. The plantlets were removed from culture vessels and washed with running water to eliminate excess gelled culture medium. The plantlets were subsequently transferred to 50 germination trays with 10 cm deep cavities, with a capacity between 67 - 70 g of basal substrate. The tray was covered with a transparent dome for one day, and holes are subsequently made to allow exchanges until 15 days were completed, keeping the substrate hydrated with water as required and a nutrient solution of Hoagland (1:10) every 15 days. After 50 days, the plants were transplanted into 45cm deep bags. Plantlets were kept in growth conditions at the mesh house, relative moisture major to 40%, temperature between 24 - 30°C at day and 16-18 °C at night, 16:8 natural sun photoperiod, and shade of 50%.
Basal substrate (BS).
The basal substrate consisted of a 1:3 (w/w) proportion of washed river sand and ground coconut fiber without enrichment. Once the substrate is prepared, the electrical conductivity of the soil (EC) must be verified since; for cocoa, it must be kept at an optimal level of <1 mS/cm. Likewise, the pH must be verified, which must be within the optimal range of 5.8 to 7.0 42,43 .
Minimum quality characteristics for in vitro plantlets.
Plantlets from in vitro conditions were classified according to quality characteristics. The minimum quality characteristics of plantlets were evaluated in terms of stem height (cm), root length (cm), number of true leaves (n), length leaf (cm), and width of the leaf (cm) on the survival of the plantlets in the BS.
Beneficial microorganisms' preparation.
Pseudomonas ACC desaminasa (PAACd) inoculum
To prepare the inoculum of PAACd, the strains of 5 bacteria preserved in the deep freezer were placed in the TSA (trypticase soy agar) culture medium. They were then grown under stirring for 72 hours in a 50% TSB (trypticase soy broth) liquid medium. After this time, the bacteria were mixed and centrifuged at 3,500 rpm for 50 minutes to eliminate the culture medium. The pellet was resuspended in a 0.03M magnesium sulfate solution with the help of a vortex. Subsequently, this solution was brought to an OD of 0.1-0.15 at 600 nm, which indicates an approximate concentration of 1x108 CFU/mL. 10mL of the bacterial inoculum near the plantlet was added to each well with 69 - 70g.
Mycorrhizal fungi (AMF) inoculum.
For the preparation of the inoculum of AMF, the commercial product Mycorfos® (contained a minimum concentration of 230 spores/gram with Glomus sp., Acaulospora sp., Scutellospora sp. and Entrophospora sp.) was taken. The product was sieved to 1mm to remove large particles. Between 7-8 g of this product was added to each well and mixed with the substrate.
Trichoderma asperellum (Ta) inoculum.
The fungus was grown in PDA medium (potato dextrose agar) for five days. Then a spore solution was made in sterile distilled water and inoculated into a bag with rice as substrate. It was incubated at in room temperature with photoperiod 12:12 for 14 days. After this, sterile distilled water was added, it was mixed very well with the rice to obtain the spore suspension, and the solution was filtered in a clean beaker passing through gauze. Then serial dilutions were made, and a spore count was performed in a Neubauer chamber to find the concentration. With this data, the fungus solution to be used was prepared at a concentration of 1x108 spores/mL. 5 mL of the spore suspension of the fungus T. asperellum was inoculated per well.
The Agricultural and Environmental Bacteriology Research Group of the Universidad de Antioquia donated all the microorganisms used.
Beneficial microorganisms' experiments.
Effects of PAACd and AMF.
The genotype used in this experiment was CCN51 for two months. The effect of PAACd, AMF, and the mixture of both microorganisms and control were evaluated on survival percentage. The experiment was laid out in CRD, the factor type of substrate, with four treatments. Each treatment had 10 experimental units for a total of 40 plantlets.
Effects of PAACd and Ta.
The genotypes used in the experiment were CCN51, CNCh12, and CNCh13. The effect of PAACd and Ta, the mixture of microorganisms and control, was evaluated on survival percentage and leaf area (cm2). The experiment was laid out in a CRD with the factor type of substrate with four treatments. Each treatment had 10 experimental units for a total of 40 plantlets.
Effects of AMF and phosphor (P) concentrations
Since a negative effect on mycorrhizal fungi colonization has been described when phosphorus concentrations are greater than 0.02 mg/L., the amount of phosphorus present in BS was quantified. For this, the methodology described by Osorio (2017) was followed, which consists of taking 3 g of an essential dry substrate and transferring them to centrifuge tubes, subsequently adding 30 mL of CaCl2 0.01 M and two drops of toluene for inhibition of microbial activity. The tubes were shaken horizontally for 1 hour, centrifuged at 5000 rpm for 15 minutes, and the supernatant passed through Whatman No. 1 filter paper. The concentration of P was determined by the molybdate blue method by making measurements in a spectrophotometer at a wavelength of 890 nm.
Different concentrations of P in the irrigation solution and AMF quantity (g) were evaluated. In the fertilization process, the Hoagland solution was prepared at 1:10 by modifying the amount of phosphorus as K2HPO4 salt at 0%, 50% and 100%. The fertilization was carried out biweekly, adding 5mL per plant for up to 60 days. The plantlets received irrigation with water as needed. The AMF inoculation consisted of applying the commercial formula in different quantities of 0g, 2g, and 3g, which were sieved and mixed with the BS. The effect of AMF quantity (0g, 2g, 3g) and phosphor concentrations (P) (0%, 50% and 100%) were evaluated on the percentage of mycorrhizal infection and survival percentage. The experiment was laid out in a CRD with nine treatments. Each treatment had 10 experimental units for a total of 90 plantlets.
Statistical analysis
Results were subjected to an analysis of variance (ANOVA), and a comparison test was performed based on residuals to test normality (Shapiro-Wilk test). Additionally, the variance homogeneity test (Levene's test) was performed. Likewise, the comparison of means was carried out through Tukey or Dunnet's test. If they were not normal, the Kruskal-Wallis and Mann-Whitney tests were used. On the other hand, for comparisons between 2 treatments, the t-test was used for parametric data and the Wilcoxon test for non-parametric data. For unequal numbers, an analysis of variance across the generalized linear model (GLM) was performed for some stages with a Poisson variable response distribution. The Pearson Chi-square (χ2) test was carried out regarding the qualitative data. Dispersion, box, and whisker diagrams were used. A significance of 95% was determined for all comparisons using the R project v3.6.1 software.
RESULTS
In vitro growth
Effects of culture media on embryo maturation.
In this experiment, there was a higher average in the number of true leaves per plantlet in the EM2-MF transition. An average of 3.12, 3.12, and 4.10 was obtained at 30, 60, and 90 days, respectively, with significant differences concerning EM2-MM6 (Figure 1 A). For the stem height in the EM2-MF medium, an average of 3.64 cm was obtained at 60 days of cultivation with differences with respect to 2.91 with EM2-MM6 (Figure 1 B). For root length, 4.07 cm was obtained for EM2-MF and 4.4 cm for EM2-MM6, without a difference (Figure 1 C). Consequently, the appropriate medium for somatic embryo maturation was the transition between EM2 and MF medium for the CCN51 genotype with sufficient fibrous and tap root development (Figure 1 S1).
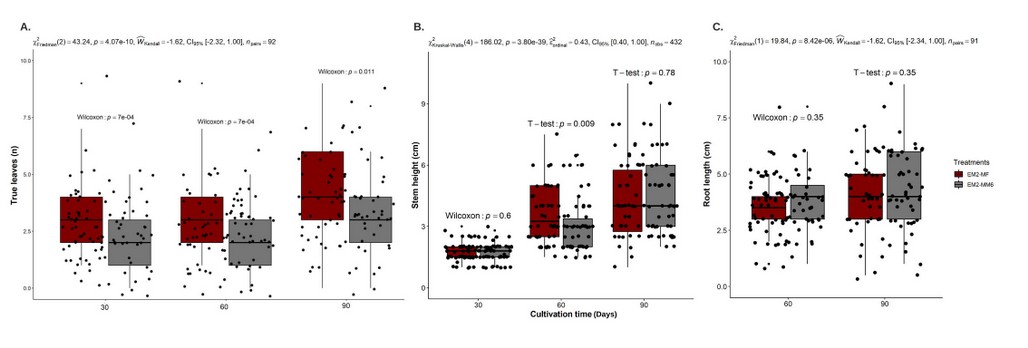
Figure 1. In the maturation stage of somatic embryogenesis development for the CCN51 genotype. Effects of culture media transition EM2-AMF and EM2-MM6 on (A) number of leaves (n) per plantlet at 30, 60, and 90 days of time cultivation, (B) height of stem (cm) at 30, 60 and 90 days of time cultivation and (C) root length (cm) at 60, and 90 days of time cultivation.
Effects of culture media on the development of plantlet.
At this stage, the average number of leaves per plantlet was 4.77 obtained in the ENR8 medium, 4.55 in the MF medium, and 4,26 in the ENR6 medium without a difference (Figure 2 A). Likewise, the highest values of height stem were 4.42 cm in the MF medium and 4.03 cm in the ENR8 medium (Figure 2 B), and finally, the most excellent root length of 5.67 cm was obtained in the MF medium, followed by 4.31 cm in ENR8 (Figure 2 C). Therefore, the adequate development of the plantlets was achieved in general in the MF medium, with results consistent with the experiment detailed in the maturation assay.
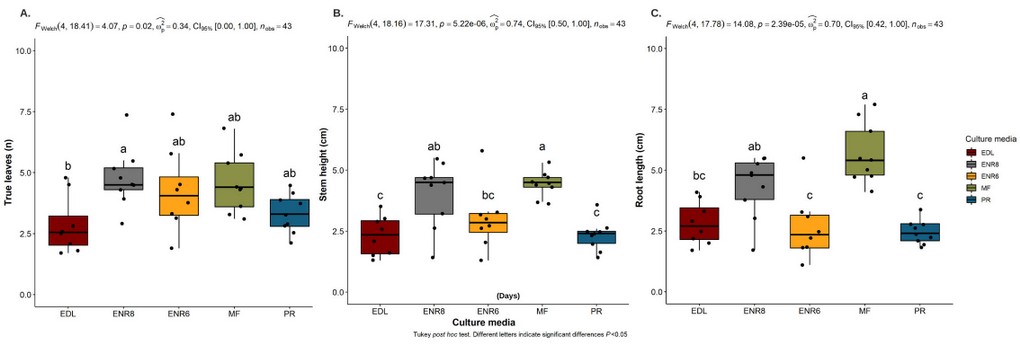
Figure 2. In the germination stage of somatic embryogenesis development for CCN51 genotype. Effects of culture media (ENR6, AMF, ENR8, EDL, PR) on (A) number of true leaves (n), (B) height stem (cm), (C) root length (cm).
Ex vitro adaptation
BS on survival plantlets
During the evaluation of the BS substrate's effect on the ex vitro adaptation process, it was possible to verify that the conductivity of the substrate is critical in the process since only the addition of organic matter (MO) can significantly alter this parameter. In previous experiments, using irrigation solutions such as full and half concentration MS and DKW salts or including MO in the essential substrate, it was found that the electrical conductivity increased more than twice the ideal for cocoa (Table 3). Due to this condition, the plants stopped their growth and development; on the contrary, the mortality was 100%. Likewise, in previous trials, it was found that the corrections in the conductivity of the BS substrate supplemented with different plant growth-promoting components like AMF and OM had a positive effect on survival. In addition, the formation of the white root is observed, like a characteristic of healthy roots for cacao (Table 1 S1, Figure 2 S1).
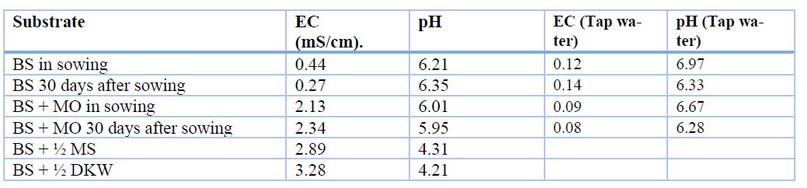
Table 3. Conductivity and pH results in basal substrate supplemented with organic matter and irrigation with ½ de MS and DKW macronutrients and micronutrients.
Minimum morphological quality characteristics
Identifying the plantlet's minimum morphological quality characteristics for survival during the adaptation process was possible. CCN51 plantlets must have at least: 3.98 cm of height stem, five true leaves with 3.58 cm of length and 1.76 of width, and prominent radical development of 6.71 cm root length with both primary pivotal roots and secondary roots (Table 4).
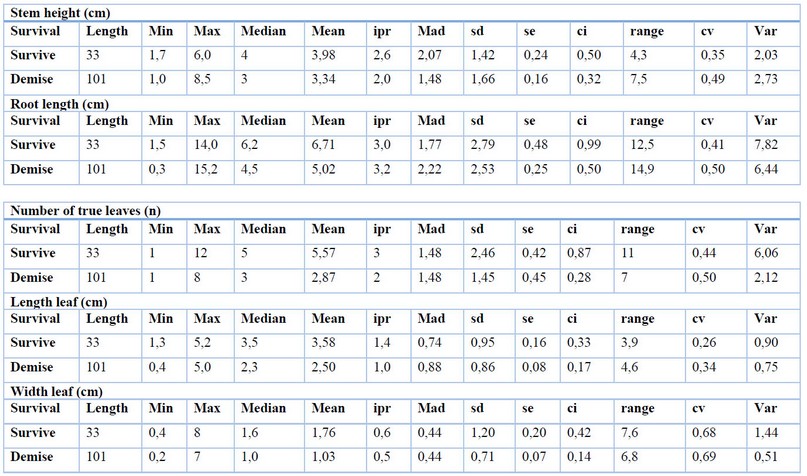
Table 4. Central tendency measures for minimum morphological quality characteristics of CCN51 in vitro-plantlets to continue ex vitro adaptation.
Effect of PAACd and AMF mix on plantlet's survival.
In the effects of PAACd and AMF on the survival of plantlets, no association was found between the different treatments evaluated and the survival. The highest survival percentage of 62.5% was obtained with the substrate supplemented with PAACd + AMF, the same result was obtained with only PAACd (Figure 3B).
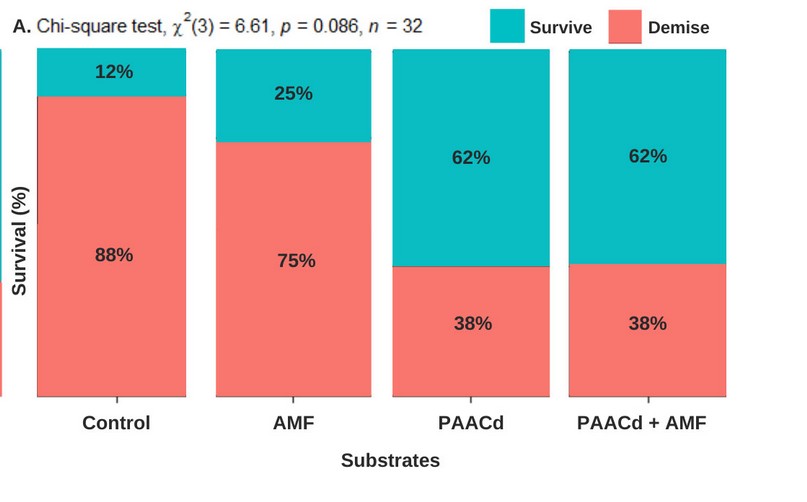
Figure 3. Effects of Pseudomonas ACC desaminase (PAACd), mycorrhizal fungi (AMF), and mix of Pseudomonas ACC desaminase (PAACd), mycorrhizal fungi (AMF) and control.
Effects of PAACd and Ta on plant survival and leaf area
For the leaf area, an average of 20.41 cm2 was obtained in the substrate supplement with PAACd + Ta for the CNCh12 genotype, with a significative difference from the control. The same result was for CCN51, with an average of 37.65 with PAACd + Ta but without significant differences between PAACd and control. For the CNCh13 genotype, an average of 14.64 was obtained with PAACd, with a significative difference from the control (Table 5). In the survival percentage, 83% was obtained with PAACd + Ta for CNCh12, 67% was obtained with PAACd for CNCh13, and 67% was obtained with PAACd and Ta for CCN51 but without significative differences (Figure 3 S1).
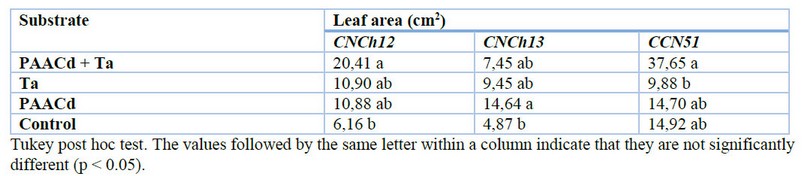
Table 5. Effects of Pseudomonas ACC deaminase (PAACd) and Trichoderma asperellum (Ta) on leaf area of regenerated plantlets for CNCh12, CNCh13, and CCN51 genotype.

Figure 4. In vitro plantlets of CCN51 in (A) EM2-AMF transition media during maturation stage; (B) Conversion to plantlet in MF culture media; (C) Transparent dome used for the first day of ex vitro adaptation process and holes for 15 days; (D) Experiment of beneficial microorganism Pseudomonas ACC desaminase (PAACd), mycorrhizal fungi (AMF) and Trichoderma asperellum (Ta) on survival and leaf area; Plantlets with pivotal and fibrous roots of (E) CNCh12 and (F) CNCh13; Cocoa seedling with adequate state of development and vigor with at least one month of ex vitro adaptation process ready to be transplanted from the bag (40cm h x 15 cm d).
Effects of AMF and P concentrations on survival and mycorrhizal infection
The phosphorus absorbance measurements recorded from known concentrations allowed the drawing of a standard calibration curve (Figure 4 S1), on which the phosphorus concentration in the substrate was determined. For the BS sample, an absorbance of 0.1139 was obtained, which corresponds to a concentration of 0.2651 mg/L of P.
The percentage of mycorrhizal infection in the treatments with 2g and 3g was significantly different from to control, with an average of 74% of condition for three phosphor concentrations (Figure 5). Survival of 100% of the plantlets was observed in the treatments of 2g AMF + 50% P and 3g AMF + 50% P, results that reveal the importance of phosphorus for the process of adaptation of cocoa plantlets to the CCN51 genotype (Figure 5 S1).
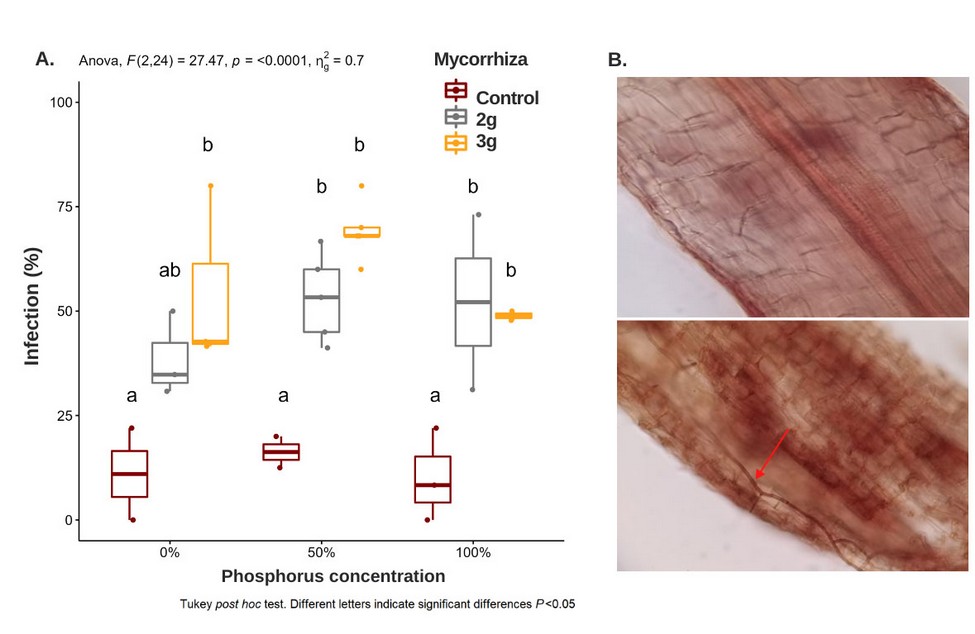
Figure 5. Effects of AMF (0g, 2g y 3g) and different concentrations of phosphorus (0%, 50% and 100%) in the Hoagland nutrient solution (1:10) on the percentage of infection. B. Above: the root of control plant without structures of mycorrhizal fungi. Under: plant root treated with 2g of AMF and Hoagland's solution with 50% P with hyphae traversing the root.
DISCUSSION
Cacao somatic embryogenesis process is divided into six well-defined steps induction, expression, multiplication, maturation, germination, and plant conversion 44 . All these steps are mediated by a complex regulatory network where genetic and epigenetic mechanisms regulate different genes at the transcriptional level 45 . In this work, the acclimation process is addressed from the stage of embryo maturation. Conceptually, the developmental stages of the somatic and zygotic embryo are divided into two main metabolic stages; the first is a morphogenetic stage that is characterized by cell division and the onset of cell differentiation; the second is a metabolic stage or maturation phase that is characterized by biochemical activities, which involves the accumulation of significant storage products and the preparation for desiccation, dormancy, and germination/conversion 46,47 . In this last phase, somatic embryos achieve both morphological and physiological maturity, guaranteeing satisfactory post-embryonic performance. Therefore, the conversion potential is programmed during embryo maturation 48 .
Embryo maturation
Better characteristics were obtained in the plants from embryo maturation in the transition between EM2-MF medium. The EM2 medium first reported by Fontanel et al. (2002) 36 has been effective for the maturation different researchers have often modified it according to the genotype of interest. On the other hand, the MF medium is a modification of the MM6 medium that was first reported by Fontanel et al. (2002) 36 and later modified by Henao et al. (2018) 35 . However, the MF medium has not been as widely used as the ED medium initially reported by 37 , a medium without growth regulators for the maturation process. Both MF and MM6 medium have NAA and GA3, a combination of growth regulators suitable for different cocoa genotypes, such as CCN51.
Plantlet development
In the maturation of the embryo and the conversion to plantlet, the MF medium had the best results in the number of leaves and length of stem and root. The results obtained with the MF medium were like the results with the ENR8 medium. The ENR8 medium does not have the growth regulators NAA-GA3; instead amino acids. The continuation of embryo development and conversion in the MF medium result from the effect of the NAA and GA3, activated carbon and other culture medium components, such as high concentrations of macroelements, especially nitrogen as NH4NO3 and high carbon concentrations like glucose. NAA and GA3 have also been used by Iracheta et al. (2019) 50 to facilitate cotyledonary embryo development plus 3.7 µM of abscisic acid. Various authors have developed somatic embryos in the culture medium with MS salts supplemented with NAA and GA3 51 . In addition, GA3 has also been used to elongate and produce embryos and plantlets in other species 52 . Furthermore, activated carbon can significantly affect embryo development because it can absorb substances, such as toxic metabolites and phenolic exudates, which inhibit the process, preventing correct maturation 53 .
The biggest bottleneck in the embryogenic process is presented in the maturation and germination stage. Genotype plays a dominant role in somatic embryogenesis response and in the conversion rate of embryos 54 . The embryo-to-plant conversion rate reached 20 to 40% with a very strong genotype dependency and high batch-to-batch variability 10,44,55 .
Beneficial microorganisms
The inoculation with beneficial microorganisms improves the characteristics of the SE cocoa plants in the evaluated genotypes. With mycorrhizal fungi, better physiological characteristics were observed in the roots of the plantlets of the CCN51, CNCh12 and CNCh13 genotypes. Respect mycorrhizal fungi; they are known as a biostimulant and are anchored on their potential to increase plant nutrient uptake, improve plant resilience to drought, and reduce pesticides and inorganic fertilizers 33 . For abiotic stresses, the mechanisms of adaptation of mycorrhizal fungi to these stresses are generally linked to increased hydromineral nutrition, ion selectivity, gene regulation, production of osmolytes, an extension of the root absorbing area, and the synthesis of phytohormones and antioxidants. These benefits are influenced by its ability to colonize its host plant, a phenomenon that depends on the fungal genotype, the soil characteristics and the plant genotype 30 .
For the CCN51 genotype, using mycorrhizal fungi allows a better characteristic in root development was observed. This has been evidenced in other plants but directly in seeds with four selected microorganisms (Pseudomonas chlororaphis MA342, P. fluorescens CHA0, Clonostachys pityriasis IK726d11 and Trichoderma harzianum T22), generating a decrease in seedling mortality and stimulation of establishment 56 . Directly in cocoa plants at the greenhouse level, it was shown that inoculation with a mixture of microorganisms such as Trichoderma sp., Candida utilis and Pseudomonas putida had better behavior in these plants compared to the control 57 . Also, in cocoa with Trichoderma spp. was possible to obtain a beneficial association, and the association could be a first step in developing of biocontrol strategies against diseases 58 . In another study, colonization of cocoa seedlings by the Trichoderma hamatum isolate DIS 219b was shown to enhance seedling growth, alter gene expression, and delay the onset of cocoa drought response in leaves at the molecular physiological and phenotypic level 58 . With the Pseudomonas ACC deaminase promoting plant growth from seed has been observed, specifically in the production of tomato roots 59 . Likewise, in cocoa, Pseudomonas ACC deaminase significantly affected on plant height, number of leaves, stem diameter, wet weight and dry weight of roots, number of roots, and root volume 60 . In the present study, different responses with each genotype were obtained; however, an improvement in survival was observed in the acclimation process for CNCh12 y CNCh13, as well as in the CCN51 genotype.
Furthermore, an improvement in plant height, number of leaves, and root length were observed. The results obtained for CCN51 agree with other reports 61–63 . Chavez-Jalk et al. (2022) 62 used T. harzianum with a 100% colonization of the root hairs and trichomes on stems, and 65 used strains of Trichoderma and arbuscular mycorrhizal fungi obtaining a higher yield of cocoa beans. This could be because Trichoderma has different auxin production mechanisms that, when entering into symbiosis with the root, improve the agronomic characteristics of the cocoa plant in such a way that when developing a greater amount of root, this has greater ease of absorption of the nutrients available in the soil; in addition, Trichoderma together with the microorganisms creates associations that help increase the rhizosphere of the soil, degrading the organic matter in less time and allowing the plants to extract the nutrients with a greater degree of assimilation 66,67 . On the other hand, the positive effects on plantlet development occur through the reduction of ethylene, a hormone that negatively affects root growth under biotic and abiotic stress 68,69 .
Phosphate is the second essential nutrient required by plants, and its bioavailability is associated with increased plant growth 70 . Only a low percentage of this amount of P (15–30%) can be taken by plants, while the remaining part is converted into insoluble complexes 71 . Therefore, increasing P-use efficiency is a major challenge in intensive agricultural production systems. In this regard, using rock phosphate as substrates in P solubilization by microorganisms is a promising strategy 72 . This strategy aims to reduce P adsorption and precipitation by promoting P sources with a low solubility instead of soluble P sources 73 . The result showed that the survival of plantlets was more significant in the treatments with 2g and 3g of mycorrhiza and 50% phosphorus. Therefore, for the CCN51 genotype, AMF can make an alternative to reduce phosphorus loss by improving P-use efficiency, plant health, and growth 74 .
The concentration of P in the basal substrate was 0.2651 mg/L, and the concentration of P in the Hoagland solution negatively affected the plantlets' survival (Data not shown). However, when the concentration of P in the Hoagland solution was modified to half of KH2PO4 and the substrate was supplemented with mycorrhizae, the effect on plant growth and survival was positive. As described by Osorio (2012) 75, for correct AMF colonization in plant roots, the substrate must have a phosphorus (P) concentration of 0.02 mg/L, and low concentrations of 0.001-0.005 mg/L do not allow a response to the inoculation of AMF, while very high concentrations above 0.2 mg/L inhibit the effectiveness of the fungus. According to Quiñones et al. (2012) 76 , the mechanism of the dose-effect on mycorrhizae is not precise. Still, it seems to depend on the interaction of the fungus and the plant species since different species of mycorrhizae are tolerant to high concentrations. The results of this study show that cocoa seedlings obtained by somatic embryogenesis inoculated with mycorrhizae were favored in terms of survival by phosphorus concentrations higher than 0.02 mg/L in the substrate, even requiring an additional supply of phosphorus in irrigation.
Based on the results obtained, it is proposed one possibility for the future is to carry out tests where plant defenses are reinforced by inoculating competent plant growth-promoting microorganisms from the in vitro phase to the ex vitro phase through a process called digitization 77 . These could act as biostimulants or biocontrol agents and help deal with biotic and abiotic stressors 78–80 . Finally, work on rhizosphere bacteria and fungi has already shown potential in managing various agricultural problems. Their use in the form of biofertilizers and biopesticides has resulted in a lesser reliance on synthetic agrochemicals 81 .
CONCLUSION
In ex vitro adaptation processes of cocoa plantlets obtained by SE, the adequate physiological development of plantlets is essential in embryo maturation and conversion to plantlet. In the present work, it was possible to address critical factors affecting the growth of cocoa plantlets from ES in vitro to ex vitro transition. It was possible to establish efficient culture media for embryo maturation, plantlet conversion and development, as well as some parameters that affect development in ex vitro conditions. The culture of the embryos in the cotyledonary state in the EM2 and MF media, and with subsequent subculture in the MF medium, allows plants with prominent root development, stem elongation and leaf formation to continue to the greenhouse adaptation phase. The use of mycorrhizae in the ex vitro adaptation stage significantly improved the survival percentages, thus advancing the production process. It was found that, although the concentrations in the substrate were greater than 0.2 mg/L, a concentration reported as high and that can affect mycorrhizal infection, in the case of cocoa plantlets and the mycorrhizae used, there was no affectation in the infection for the mycorrhizae.
For this reason, it is recommended that for each genotype, it should be established how much phosphorus in the substrate and the irrigation solution affect mycorrhizal infection and plant growth. These results will help optimize beneficial microorganisms' use in SE plantlets production and the subsequent physiological performance of plants under nursery and open field conditions. Also, it is a significant starting point to carry out in other cacao genotypes, achieving increasing survival and contributing to the establishment of complete production protocols via ES.
Supplementary Materials: The following are available online at www.revistabionatura.com/xxx/s1, Figure 1 S1. Association of culture media transition EM2-AMF and EM2-MM6 (Secondary embryogenesis-maturation) and the fibrous root and tap root percentage in CCN51 genotype plantlets, Table 1 S1 Effect of BS (Basal substrate) supplemented with MZ and OM on plant survival of CCN51 genotype, Figure 2 S1. Effects of mycorrhizae (MZ) and organic matter (MO) increased in the percentage of BS substrate on white roots in CCN51 genotype. Green: Survival, Pink: Demise. Figure 3 S1. Effects of Pseudomonas ACC deaminase (PAACd) and Trichoderma asperellum (Ta) supplemented in the substrate on survival (%). A. Effects on CNCh12 genotype. B. Effects CNCh13 genotype. C. Effects CCN51 genotype. Figure 4 S1. Figure 4. Standard calibration curve of phosphorus (P) measurement in the basal substrate (BS). Figure 5 S1. Figure 5. Effects of AMF (0g, 2g y 3g) and different concentrations of phosphorus (0%, 50% and 100%) in the Hoagland nutrient solution (1:10) on the percentage of survival.
Author Contributions: Conceptualization, Ana María Henao Ramírez and Aura Inés Urrea Trujillo; methodology and software, Julian David Morales Muñoz, Ruth Tatiana Hernández Hernández; validation and formal analysis, Juliam David Morales Muñoz, Ana María Henao Ramírez; investigation, resources, data curation, writing—original draft preparation, Ana María Henao Ramírez; writing—review and editing and supervision, Aura Inés Urrea Trujillo. All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by the General Royalties System - Science, Technology, and Innovation Fund with the Center of Agrobiotechnological Development and Innovation– CEDAIT- BPIN 2016000100060, National Planning Department, Office of the Governor of Antioquia, Universidad de Antioquia, Universidad Católica de Oriente, and Compañía Nacional de Chocolates.
Acknowledgments: We would like to thank the Laboratory of Plant Physiology and Plant Tissue Culture of the Universidad de Antioquia. A special acknowledgment to Universidad de Antioquia and Granja Yariguíes – Compañia Nacional de Chocolates.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Patalinghug M. Status of Cacao (Theobroma cacao L.) production on its challenges and prospect in Zamboanga del Norte Province in the Philippines. International Journal of Agricultural Technology. 2022;18(3).
2. Nair K. Cocoa (Theobroma cacao L.). In: Nair KP, ed. Tree Crops . Springer International Publishing; 2021:153-213. doi:10.1007/978-3-030-62140-7_5
3. Sayago-Ayerdi S, García-Martínez D, Ramírez-Castillo A, Ramírez-Concepción, H. Viuda-Martos M. Tropical Fruits and Their Co-Products as Bioactive Compounds and Their Health Effects: A Review. Foods. 2021;10(8). doi:10.3390/foods10081952
4. Cádiz-Gurrea M, Fernández-Ochoa A, Leyva-Jiménez F, et al. LC-MS and Spectrophotometric Approaches for Evaluation of Bioactive Compounds from Peru Cocoa By-Products for Commercial Applications. Molecules. 2020;25(14). doi:10.3390/molecules25143177
5. Ebuehi OAT, Anams C, Gbenle OD, Ajagun-Ogunleye MO. Hydro-ethanol seed extract of Theobroma cacao exhibits antioxidant activities and potential anticancer property. J Food Biochem. 2019;43(4):e12767. doi:https://doi.org/10.1111/jfbc.12767
6. Singh M, Agarwal S, Agarwal M, Rachana. Benefits of Theobroma cacao and Its Phytocompounds as Cosmeceuticals. In: Swamy MK, ed. Plant-Derived Bioactives. Springer Singapore; 2020:509-521. doi:10.1007/978-981-15-1761-7_21
7. Liaqat H, Parveen A, Kim SY. Neuroprotective Natural Products' Regulatory Effects on Depression via Gut–Brain Axis Targeting Tryptophan. Nutrients. 2022;14(16). doi:10.3390/nu14163270
8. Boriollo MFG, Alves VE, Silva TA, et al. Decrease of the DXR-induced genotoxicity and nongenotoxic effects of Theobroma cacao revealed by micronucleus assay. Brazilian Journal of Biology. 2021;81(2):268-277. doi:10.1590/1519-6984.223687
9. Méndez-Hernández HA, Ledezma-Rodríguez M, Avilez-Montalvo RN, et al. Signaling Overview of Plant Somatic Embryogenesis. Front Plant Sci. 2019;10. doi:10.3389/fpls.2019.00077
10. Guillou C. Theobroma cacao: Somatic Embryogenesis. In: Guillou C, Verdier D, eds. Somatic Embryogenesis. Vol 2527. ; 2022:69-81.
11. Garcia C, Marelli J, Motamayor J, Villela C. Somatic Embryogenesis in Theobroma cacao L. In: Loyola-Vargas V, Ochoa-Alejo N, eds. Plant Cell Culture Protocols, Methods in Molecular Biology. Vol 1815. Springer Science; 2018:227-245. doi:https://doi.org/10.1007/978-1-4939-8594-4_15
12. Henao-Ramírez A, Urrea-Trujillo A. Somatic Embryogenesis for Clonal Propagation and Associated Molecular Studies in Cacao (Theobroma cacao L.). In: Chong P, Newman D, eds. Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery. Springer, Cham; 2020:63-102. doi:https://doi.org/10.1007/978-3-030-51358-0_5
13. Garcia C, Corrêa F, Seth F, et al. Optimization of somatic embryogenesis procedure for commercial clones of Theobroma cacao L . Afr J Biotechnol. 2016;15(36):1936-1951. doi:10.5897/AJB2016.15513
14. Traore A. Somatic Embryogenesis, Embryo Conversion, Micropropagation and Factors Affecting Genetic Transformation.Pdf. Pennsylvania State University; 2000.
15. Manlé TE, Kouassi KM, Soumahoro BA, Koné T, Koffi KE, Koné M. Effect of water stress induced by polyethylene glycol 6000 on the somatic embryos conversion into whole plantlets in cocoa (Theobroma cacao L.). Int J Biol Chem Sci. 2021;15(1):12-25. doi:10.4314/ijbcs.v15i1.2
16. Goenaga R, Guiltinan M, Maximova S, Seguine E, Irizarry H. Yield performance and bean quality traits of cacao propagated by grafting and somatic embryo-derived cuttings. HortScience. 2015;50(3):358-362. doi:10.21273/hortsci.50.3.358
17. Pospisilova J, Synkova H, Haisel D, Semoradova S. Acclimation of plantlets to ex vitro conditions: effects of air humidity, irradiance, co2 concentration and abscisic acid. In: Acta Horticulturae. International Society for Horticultural Science (ISHS), Leuven, Belgium; 2007:29-38. doi:10.17660/ActaHortic.2007.748.2
18. Figueira A, Janick J. Somatic embryogenesis in cacao (Theobroma cacao L.). 1995;2:291-310.
19. Aguilar ME, Villalobos VM, Vasquez N. Production of cocoa plants (Theobroma cacao L.) via micrografting of somatic embryos. In Vitro Cellular & Developmental Biology - Plant. 1992;28(1):15-19. doi:10.1007/BF02632186
20. Jones J, Zhang E, Tucker D, et al. Screening of cultivars for tissue culture response and establishment of genetic transformation in a high-yielding and disease-resistant cultivar of Theobroma cacao. In Vitro Cellular and Developmental Biology - Plant. 2022;58(1):133-145. doi:10.1007/s11627-021-10205-0
21. Hazarika BN. Acclimatization of tissue-cultured plants. Curr Sci. Published online 2003:1704-1712.
22. Tarigan D, Siregar H, Utami S, Basyuni M, Novita A. Seedling Growth in Response to Cocoa (Theobroma cacao L.) for The Provision of Guano Fertilizer and Mycorrhizal Organic Fertilizer in the Nursery. Proceedings of the International Conference on Sustainable Agriculture and Natural Resources Management. 2018;(January):47-50.
23. Aggangan NS, Cortes AD, Reaño CE. Growth response of cacao (Theobroma cacao L.) plant as affected by bamboo biochar and arbuscular mycorrhizal fungi in sterilized and unsterilized soil. Biocatal Agric Biotechnol. 2019;22:101347. doi:https://doi.org/10.1016/j.bcab.2019.101347
24. Groppa MD, Benavides MP, Zawoznik MS. Root hydraulic conductance, aquaporins and plant growth promoting microorganisms: A revision. Applied Soil Ecology. 2012;61:247-254. doi:https://doi.org/10.1016/j.apsoil.2011.11.013
25. Balkrishna A, Sharma IP, Arya V, Sharma AK. Biologicals and their plant stress tolerance ability. Symbiosis. 2022;86(3):243-259. doi:10.1007/s13199-022-00842-3
26. Schmidt JE, DuVal A, Isaac ME, Hohmann P. At the roots of chocolate: understanding and optimizing the cacao root-associated microbiome for ecosystem services. A review. Agron Sustain Dev. 2022;42(2):14. doi:10.1007/s13593-021-00748-2
27. Mahendra R, Chauhan N, Sharma JB, Rana K, Bakshi M. Ex-vitro Establishment of Tissue Cultured plants in Fruit Crops-A Review. Int J Curr Microbiol Appl Sci. 2020;9(11):3321-3329. doi:10.20546/ijcmas.2020.911.397
28. Bailey BA, Strem MD, Wood D. Trichoderma species form endophytic associations within Theobroma cacao trichomes. Mycol Res. 2009;113(12):1365-1376. doi:https://doi.org/10.1016/j.mycres.2009.09.004
29. Song Z, Bi Y, Zhang J, Gong Y, Yang H. Arbuscular mycorrhizal fungi promote the growth of plants in the mining associated clay. Sci Rep. 2020;10(1):2663. doi:10.1038/s41598-020-59447-9
30. Diagne N, Ngom M, Djighaly PI, Fall D, Hocher V, Svistoonoff S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity (Basel). 2020;12(10). doi:10.3390/d12100370
31. Boubekri K, Soumare A, Mardad I, et al. The Screening of Potassium- and Phosphate-Solubilizing Actinobacteria and the Assessment of Their Ability to Promote Wheat Growth Parameters. Microorganisms. 2021;9(3). doi:10.3390/microorganisms9030470
32. Borden KA, Thomas SC, Isaac ME. Variation in fine root traits reveals nutrient-specific acquisition strategies in agroforestry systems. Plant Soil. 2020;453(1):139-151. doi:10.1007/s11104-019-04003-2
33. Snoeck D, Koko L, Joffre J, Bastide P, Jagoret P. Cacao Nutrition and Fertilization BT. In: Lichtfouse E, ed. Sustainable Agriculture Reviews. Springer International Publishing; 2016:155-202. doi:10.1007/978-3-319-26777-7_4
34. Jaimez RE, Barragan L, Fernández-Niño M, et al. Theobroma cacao L. cultivar CCN 51: a comprehensive review on origin, genetics, sensory properties, production dynamics, and physiological aspects. PeerJ. 2022;9:1-23. doi:10.7717/peerj.12676
35. Henao A, De-La-Hoz T, Ospina T, Atehortúa L, Urrea A. Evaluation of the potential of regeneration of different Colombian and commercial genotypes of cocoa ( Theobroma cacao L .) via somatic embryogenesis. Sci Hortic. 2018;229:148-156. doi:10.1016/j.scienta.2017.10.040
36. Fontanel A, Gire S, Labbe G, et al. In vitro multiplication and plant regeneration of Theobroma cacao L. via stable embryogenic calli. IAPTC Congress, Plant Biotechnology 2002 and beyond. Published online 2002:23-28.
37. Li Z, Traore A, Maximova S, Guiltinan M. Somatic embryogenesis and plant regeneration from floral explants of cacao (Theobroma cacao L.) using thidiazuron. In vitro Cell Dev Biol. 1998;34:293-299. doi:https://doi.org/10.1007/BF02822737
38. McGranahan GH, Driver JA, Tulecke W. Tissue Culture of Juglans. In: J.M B, D.J. D, eds. Cell and Tissue Culture in Forestry. Springer, Dordrecht; 1987:232-248. doi:10.1007/978-94-017-0992-7
39. Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473-497.
40. Lloyd G, McCown B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. 1980;30:421-427.
41. Guillou C, Fillodeau A, Brulard E, et al. Indirect somatic embryogenesis of Theobroma cacao L. in liquid medium and improvement of embryo-to-plantlet conversion rate. In Vitro Cellular and Developmental Biology - Plant. 2018;54(4):377-391. doi:10.1007/s11627-018-9909-y
42. Vargas JRN, Solis EC, Salgado MDO. Diagnóstico de la calidad del suelo y el agua en una parcela de cacao en Huehuetan, Chiapas, Mexico. In: Quinto Congreso Nacional de Riego y Drenaje. COMEII; 2019:1-12.
43. Barrezueta S. Construcción de Indicadores Agrarios Para Medir La Sostenibilidad de La Producción de Cacao En El Oro, Ecuador. Universidade Da Coruña; 2018.
44. Henao A, Palacio D, Urrea A. Cost Analysis of Cacao (Theobroma cacao L.) Plant Propagation through the Somatic Embryogenesis Method. Bionatura. 2022;7(2):1-13. doi:10.21931/rb/2022.07.02.2
45. Garcia C, Furtado de Almeida AA, Costa M, et al. Single-base resolution methylomes of somatic embryogenesis in Theobroma cacao L. reveal epigenome modifications associated with somatic embryo abnormalities. Sci Rep. 2022;12(1):15097. doi:10.1038/s41598-022-18035-9
46. Noah AM, Niemenak N, Sunderhaus S, et al. Comparative proteomic analysis of early somatic and zygotic embryogenesis in Theobroma cacao L. J Proteomics. 2013;78:123-133. doi:https://doi.org/10.1016/j.jprot.2012.11.007
47. Winkelmann T. Somatic Versus Zygotic Embryogenesis: Learning from Seeds BT - In Vitro Embryogenesis in Higher Plants. In: Germana MA, Lambardi M, eds. Springer New York; 2016:25-46. doi:10.1007/978-1-4939-3061-6_2
48. Juarez-Escobar J, Bojórquez-Velázquez E, Elizalde-Contreras, JM. Guerrero-Analco JA, Loyola-Vargas VM, Mata-Rosas M, Ruiz-May E. Current Proteomic and Metabolomic Knowledge of Zygotic and Somatic Embryogenesis in Plants. Int J Mol Sci. 2021;22(21). doi:10.3390/ijms222111807
49. Henao A, De-La-Hoz T, Ospina T, Garcés L, Urrea A. Evaluation of the potential of regeneration of different Colombian and commercial genotypes of cocoa (Theobroma cacao L.) via somatic embryogenesis. Sci Hortic. 2018;229:148–156. doi:10.1016/j.scienta.2017.10.040
50. Iracheta L, Cruz L, Lopez P, Avendaño C, Ortiz S. 2iP and brasinosteroids promote somatic embryogenesis induction in Theobroma cacao L. Agroproductividad. 2019;12(1):65-70. doi:https://doi.org/1010.32854/agrop.v0i0.1340
51. Urbanek A, Zechmann B, Müller M. Plant regeneration via somatic embryogenesis in Styrian pumpkin: Cytological and biochemical investigations. Plant Cell Tissue Organ Cult. 2004;79(3):329-340. doi:10.1007/s11240-004-5177-0
52. Kordestani G, Karami O. Picloram-induced somatic embryogenesis in leaves of strawberry (fragaria ananassa l.). Acta Biol Crac Ser Bot. 2008;50(1):69-72.
53. López A, Carreño J, Martínez A, Dabauza M. High embryogenic ability and plant regeneration of table grapevine cultivars (Vitis vinifera L.) induced by activated charcoal. Vitis - Journal of Grapevine Research. 2005;44(2):79-85.
54. Guillou C, Fillodeau A, Brulard E, et al. Nestlé Cocoa plan: Cocoa propagation by somatic embryogenesis. In: Park YS, Bonga JM, eds. Proceedings of the Third International Conference of the IUFRO on "Woody Plant Production Integrating Genetic and Vegetative Propagation Technologies ". Vol 1. IUFRO; 2015:75-80.
55. Osorio T, Henao A, de la Hoz T, Urrea A. Propagation of IMC67 Plants, Universal Cacao (Theobroma cacao L.) Rootstock via Somatic Embryogenesis. International Journal of Fruit Science. 2022;22(1):78-94. doi:10.1080/15538362.2021.2023067
56. Bennett AJ, Whipps JM. Beneficial microorganism survival on seed, roots and in rhizosphere soil following application to seed during drum priming. Biological control. 2008;44(3):349-361.
57. Cortes-Patiño S, Vesga-Ayala N, Sigarroa-Rieche A, Moreno-Rozo L, Cardenas-Caro D. Substrates inoculated with microorganisms for cocoa plants development (Theobroma cacao L.) during nursery stage. Bioagro. 2015;27:151-158.
58. Cortes-Patiño S, Vesga-Ayala N, Sigarroa-Rieche A, Moreno-Rozo L, Cardenas-Caro D. Substrates inoculated with microorganisms for cocoa plants development (Theobroma cacao L.) during nursery stage. Bioagro. 2015;27:151-158.
59. Orozco-Mosqueda Ma del C, Duan J, DiBernardo M, et al. The Production of ACC Deaminase and Trehalose by the Plant Growth Promoting Bacterium Pseudomonas sp. UW4 Synergistically Protect Tomato Plants Against Salt Stress. Front Microbiol. 2019;10. doi:10.3389/fmicb.2019.01392
60. Irawan T, Soelaksini L, Nuraisyah A. The growth response of cocoa (Theobroma cacao L.) growth with various concentrations of PGPR (Plant Growth Promoting Rhizobacteria) cocoa roots. Jurnal Ilmiah Hijau Cendekia. 2022;7(1):7-17. doi:10.32503/hijau.v7i1.2205
61. Cargua J, Echeverria C, Cedeño G. Effectiveness of biochar and biofertilizers in the growth and quality of cocoa seedlings. Revista ESPAMCIENCIA. 2020;11:95-100.
62. Chavez-Jalk A, Leiva S, Bobadilla LG, Vigo CN, Arce M, Oliva-Cruz M. Effect of Endophytic Trichoderma sp. Strains on the Agronomic Characteristics of Ecotypes of Theobroma cacao L. under Nursery Conditions in Peru. Gahlaut V, ed. International Journal of Agronomy. 2022;2022:5297706. doi:10.1155/2022/5297706
63. Irawan T, Soelaksini L, Nuraisyah A. The growth response of cocoa (Theobroma cacao L.) growth with various concentrations of PGPR (Plant Growth Promoting Rhizobacteria) cocoa roots. Jurnal Ilmiah Hijau Cendekia. 2022;7(1):7-17. doi:10.32503/hijau.v7i1.2205
64. Chavez-Jalk A, Leiva S, Bobadilla LG, Vigo CN, Arce M, Oliva-Cruz M. Effect of Endophytic Trichoderma sp. Strains on the Agronomic Characteristics of Ecotypes of Theobroma cacao L. under Nursery Conditions in Peru. Gahlaut V, ed. International Journal of Agronomy. 2022;2022:5297706. doi:10.1155/2022/5297706
65. Tuesta-Pinedo A, Trigozo-Bartra E, Cayotopa-Torres J, et al. Optimization of organic and inorganic fertilization cocoa (Theobroma cacao L.) with the inclusion of Trichoderma endophyte and arbuscular mycorrhizae. Revista Tecnologia en Marcha. 2017;30:67-78.
66. Naik K, Mishra S, Srichandan H, Singh PK, Sarangi PK. Plant growth promoting microbes: Potential link to sustainable agriculture and environment. Biocatal Agric Biotechnol. 2019;21:101326. doi:https://doi.org/10.1016/j.bcab.2019.101326
67. Hipólito-Romero E, Carcaño-Montiel MG, Ramos-Prado JM, Vázquez-Cabañas EA, López-Reyes L, Ricaño-Rodríguez J. [Effect of mixed edaphic bacterial inoculants in the early development of improved cocoa cultivars (Theobroma cacao L.) in a traditional agroforestry system of Oaxaca, Mexico]. Rev Argent Microbiol. 2017;49(4):356-365. doi:10.1016/j.ram.2017.04.003
68. Soumare A, Diédhiou AG, Arora NK, et al. Potential Role and Utilization of Plant Growth Promoting Microbes in Plant Tissue Culture. Front Microbiol. 2021;12. doi:10.3389/fmicb.2021.649878
69. Cortes AD, Opulencia RB, Aggangan NS. Characterization of plant growth promoting diazotrophic bacteria isolated from cacao (Theobroma cacao l.) rhizosphere treated with bamboo biochar and arbuscular mycorrhizal fungi. Philipp J Sci. 2020;149(4):1063-1070.
70. Fonseca AA, Santos DA, Passos RR, Andrade FV, Rangel OJP. Phosphorus availability and grass growth in biochar-modified acid soil: A study excluding the effects of soil pH. Soil Use Manag. 2020;36(4):714-725. doi:https://doi.org/10.1111/sum.12609
71. Singh H, Reddy MS. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur J Soil Biol. 2011;47(1):30-34. doi:https://doi.org/10.1016/j.ejsobi.2010.10.005
72. Soumare A, Boubekri K, Lyamlouli K, Hafidi M, Ouhdouch Y, Kouisni L. From Isolation of Phosphate Solubilizing Microbes to Their Formulation and Use as Biofertilizers: Status and Needs. Front Bioeng Biotechnol. 2020;7. doi:10.3389/fbioe.2019.00425
73. Soltangheisi A, Santos VR dos, Franco HCJ, et al. Phosphate Sources and Filter Cake Amendment Affecting Sugarcane Yield and Soil Phosphorus Fractions. Rev Bras Cienc Solo. 2019;43. doi:10.1590/18069657rbcs20180227
74. Soumare A, Boubekri K, Lyamlouli K, Hafidi M, Ouhdouch Y, Kouisni L. Efficacy of phosphate solubilizing Actinobacteria to improve rock phosphate agronomic effectiveness and plant growth promotion. Rhizosphere. 2021;17:100284. doi:https://doi.org/10.1016/j.rhisph.2020.100284
75. Osorio W. Use of mycorrhiza-forming fungi as a biotechnological alternative to promote seedling nutrition and growth. Manejo Integral Suelo Nutr Veg. 2012;1(2):1-4.
76. Quiñones E, Hernández E, Rincon G, Ferrera R. Interaction of arbuscular mycorrhizal fungi and phosphate fertilization in papaya. Terra Latinoam . 2012;30(2).
77. Kanani P, Modi A, Kumar A. Biotization of endophytes in micropropagation: A helpful enemy. In: Kumar A, Singh VKBTME, eds. Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead Publishing; 2020:357-379. doi:https://doi.org/10.1016/B978-0-12-818734-0.00015-2
78. Hamid B, Zaman M, Farooq S, et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability. 2021;13(5). doi:10.3390/su13052856
79. Cataldo E, Fucile M, Mattii GB. Biostimulants in Viticulture: A Sustainable Approach against Biotic and Abiotic Stresses. Plants. 2022;11(2). doi:10.3390/plants11020162
80. Dubey A, Kumar A, Khan ML. Role of Biostimulants for Enhancing Abiotic Stress Tolerance in Fabaceae Plants BT - The Plant Family Fabaceae: Biology and Physiological Responses to Environmental Stresses. In: Hasanuzzaman M, Araújo S, Gill SS, eds. Springer Singapore; 2020:223-236. doi:10.1007/978-981-15-4752-2_8
81. Mishra J, Singh R, Arora NK. Plant Growth-Promoting Microbes: Diverse Roles in Agriculture and Environmental Sustainability BT - Probiotics and Plant Health. In: Kumar V, Kumar M, Sharma S, Prasad R, eds. Springer Singapore; 2017:71-111. doi:10.1007/978-981-10-3473-2_4
Received: 26 September 2022 / Accepted: 15 October 2022 / Published:15 February 2023
Citation: Henao Ramírez A M, Morales Muñoz J D, Vanegas Villa D M, Hernández Hernández R T, Urrea-Trujillo A I. Regeneration of cocoa (Theobroma cacao L.) via somatic embryogenesis: Key aspects in the in vitro conversion stage and in the ex vitro adaptation of plantlets.Revis Bionatura 2023;8 (1) 10. http://dx.doi.org/10.21931/RB/2023.08.01.10
