2019.04.01.9
Files > Volume 4 > Vol 4 No 1 2019
INVESTIGATION / RESEARCH
Mycorrhizal symbiotic effectiveness as a tool for decision making in restauration of the tropical dry forest

Jorge A. Sierra-Escobar1, y John Alexander Ortíz-Correa2
Available from: http://dx.doi.org/10.21931/RB/2019.04.01.9
ABSTRACT
A greenhouse experiment was designed to determine the mycorrhizal symbiotic effectiveness in native mycorrhizal fungi population associated with different soil coverage in the Cesar department. The experimental design was completely randomized with nine treatments and six variations per treatment, 54 experimental units in all. Treatments consisted of combination of soils which contained a substrate from different mulches or soil coal mining (soil-coverage): natural forest (NF), transition soil (TS), a horizon (AH), mining waste (MW), palm (PM), pastures (PT), undisturbed soil (US), with its respective controls, positive Glomus mosseae (GM) and negative sterile substrate without inoculum (WI). The variables studied were foliar P content was monitored as a function of time; at harvest, shoot dry weight, shoot P content, and mycorrhizal colonization represented the time increments. The higher contents of P foliar obtained in the sampling period were for the positive control (GM) showing significant differences between soil-coverage, except for ST and US assessed on the sampling day 74. Shoot dry weight had a significant difference in GM, NF, TS, AH, PM and US treatments compared to the remaining three. Treatments with the most weight were US and GM (positive control). Mining waste (MW), PT and WI (negative control) had the lowest values in mass. As expected, shoot P content in the GM samples was higher and had significant differences compared to the other treatments. Soil-coverage closest to the positive control were NF, US, and TS. All assessed treatments showed mycorrhizal colonization except the negative control (WI). Three soil-coverages PM, PT, and US were similar to the positive control, with colonization percentages of 29, 24 and 48 respectively. In conclusion, this kind of research suggests that symbiotic effectiveness experiments are an excellent tool for the selection of native arbuscular mycorrhizal fungi. Besides, and as evidenced, soil-coverage NIT was statistically similar to the positive control (GM), which makes it a candidate for mass crude inoculum production for restoration purposes.
Keywords: arbuscular mycorrhiza, Glomus mosseae, mycorrhizal effectiveness.
RESUMEN
Se realizó un experimento para determinar la efectividad simbiótica micorrizal en diferentes suelos-cobertura del departamento del Cesar. Para el efecto se utilizó un diseño experimental completamente al azar con nueve tratamientos y seis repeticiones por tratamiento, para un total de 54 unidades experimentales. Los tratamientos consistieron en la combinación de muestras de suelos con un sustrato de crecimiento, procedentes de diferentes coberturas vegetales o suelos de minería de carbón (suelos-cobertura): bosque natural (NF), suelo de transición (TS), horizonte A (AH), residuos mineros (MW), palma (PM), pastos (PT), suelo no intervenido (US), con sus respectivos controles, positivo Glomus mosseae (GM) y negativo sustrato sin inocular (WI). Como variables respuesta se emplearon el contenido de P foliar, el P total, la masa seca aérea (MSA) y la colonización micorrizal. Los mayores contenidos de P foliar obtenidos en el periodo de muestreo fueron para el control positivo (GM) presentando diferencias significativas entre los suelos-cobertura, con excepción de TS y US evaluados en el día 74 del muestreo. En la masa seca aérea existieron diferencias significativas de los tratamientos GM, NF, TS, AH, PM y US comparados con los tres restantes. Los tratamientos con mayor masa fueron US y GM (control positivo). Por el contrario MW, PT y WI (control negativo) tuvieron los valores más bajos en cuanto a masa. Tal como se esperaba, en cuanto al P total absorbido, GM fue superior y tuvo diferencias significativas comparadas con los demás tratamientos, los suelo-cobertura más cercanos al control positivo fueron NF, US y TS. Todos los tratamientos evaluados exhibieron colonización micorrizal con excepción del control negativo (SI), se resaltan tres suelos cobertura PM, PT y US que se asemejan al control positivo, con porcentajes de colonización de 29, 24 y 48 respectivamente. En conclusión, esta clase de investigaciones sugieren que los experimentos de efectividad simbiótica son una excelente herramienta para la selección de inóculos de hongos micorricico arbusculares nativos. Además, y Tal como se evidencia en los presentes resultados, el suelo-cobertura US fue estadísticamente similar al control positivo (GM), lo que lo hace candidato para la producción en masa de inóculos crudos para fines de restauración.
Palabras clave: efectividad micorrizal, micorriza arbuscular, Glomus mosseae
INTRODUCTION
The tropical dry forest (td-F) is an intermediate form between the tropical savanna and the tropical rainforest1. These tropical ecosystems are the most biologically diverse in the world2 and belong to a vegetative formation between 0 and 1000 m.a.s.l, with temperatures above 24 ºC and with two or three times of drought per year3, 4, 5, 6, 7. Nearly 42% of the tropical and subtropical habitats belongs to td-F8. South America represents 22% of the total forest area7. Miles et al.9 estimated that more than half of the td-F remaining in the world (54,2%) is located in South America. In Colombia, the td-F is the second largest ecosystem in existence occupying 24.97 % of the country10. Over the last century, the biodiversity in these ecosystems has been deteriorating because of human activity, replacement of forest by agricultural fields, cattle, and mining. Such is the advance of urbanization and industrialization processes11,12.
According to Janzen3 the td-F is considered one of the most fragile ecosystems, due to the slow regeneration capacity and the persistent deforestation threats. These are both natural and anthropic issues. Moreover, the dry conditions which they are subjected, seedling recruitment and the growth rates are lower than the rainforest13,14. The td-F has become one of the most threatened ecosystems in the world3,11.
The td-F in the Cesar department is not unaffected by the changes caused by different anthropic activities to the soil. Soils in these areas have different uses with negative consequences. Everyday activities such as farming, raising cattle, growing oil palm crops, corn crops, as well as cotton production represents 44% of national productions in its best times. Cotton and rice production were essential for the Cesar department’s development in 1976, using 52% of the agricultural land15. As of late, coal open pit mining has been the primary source of income for the area.
Coal mining started in the 1980s and was already an underway in the departments of Cesar, La Guajira, and Córdoba. Mining presented a significant dynamic growth in the regions and economic sustainability. According to Sánchez et al16, between 1988-2003, Colombian coal production grew 39%, reaching 47 million tons in 2003. Production grew to 96%, achieving an extraction of 19 million tons in the same year. Therefore, the department of Cesar yielded 40% of the Colombian coal in 200315. Coal mining is one of the largest industries in the department of Cesar and is one of the most significant sources of environmental deterioration. The mining exploitation system involves sinkholes up to 400 m deep, making dramatic changes in vegetation and soil. Once the coal has been extracted, the soil layers are mixed and piled up in mounds up to 400 m high called dumps. The high demand of mineral resources generates disturbances in the ecosystems. It also changes the natural hydrology, reliefs, soil, biological communities, land uses which in turn change human population activities17. It also makes temperature increase and acidity, reduces the soil humidity, organic matter and nutrient content. All these factors make it difficult to recover the vegetal coverage18, 19.
Open pit mining has a wide range of environmental impacts that are sometimes irreversible in the environment, unlike other productive processes, this takes place in a finite period of time20. The mine operation and closure plans, which in Colombia are established in laws 685 of 2001, provide guidelines for the mining owner to establish rehabilitation activity and restore vegetal restoration of the affected areas by the mining extraction processes. Moreover, the law 99 of 1993 made a change in the execution of mining projects in Colombia, establishing mechanisms and technical instruments for any activity that may cause severe deterioration to renewable natural resources or the environment or introduces considerable or notorious modifications to the landscape21.
Vegetation is one of the areas most affected by mining. Evidence that plants show effects is in their growth due to the low availability of nutrients present in soils, especially degraded soils. Plant size becomes limited, or they mature in poor form. In order to reverse this effect, plants perform beneficial symbiosis with microorganisms found in the soil making mutualistic associations. This association is established between the plants and specific groups of soil fungi that are found mainly in the rhizosphere. One of the most common mutualistic relationships is mycorrhizal symbiosis. The word mycorrhiza has a Greek origin and means a symbiotic association between a fungus (mycos) and the root (rhizo) of plants. The existence of several types of mycorrhiza, the endomycorrhiza or arbuscular mycorrhizal fungi (AMF), is the largest group forming associations with the 73% of the plant species of the world, mainly tropical22. AMF allows plants to improve their nutritional development and overcome stress phenomena in the colonization of terrestrial ecosystems. This is due to the roots ability for symbiosis establishment23. Extraradical hyphae can explore larger volumes of soil and reach sites where the root is not able to penetrate24, 25, 26, increasing the nutrient uptake, mainly from P27, 28, 29, 30. This P uptake in the soil by the AMF and the subsequent translocation and transfer to the host plant allows it to obtain a level of equivalence or higher than that obtained by non-associated plants with AMF 31,32,33. Regardless of the nutritional role, the AMF colonization contributes significantly to improve soil structure, increasing the plant’s resistance to biotic and abiotic stress and favors the establishment of interactions with other beneficial microorganisms27, 34, 35, 36, 37, 38. Techniques have been developed to assess the AMF and learn the state of these fungi in the soil. One of these is the mycorrhizal symbiotic effectiveness technique that was initially developed by Habte and collaborators39 of the University of Hawaii. This is based on the theory which is defined as the ability of soil or an inoculum to successfully perform mycorrhizal symbiosis, which is reflected in the infectivity and effectiveness of the infective propagules of the sample. Subsequently, the technique was adjusted by Habte and Osorio40. It is clear that in this technique no defined range measures the effectiveness, it is measured indirectly with the statistical analysis that is presented in the response variables such as leaf P, air dry mass and percentage of mycorrhizal colonization. This technique requires reference controls (positive and negative) used in the experimental design. We expect to determine mycorrhizal symbiotic effectiveness as a tool for decision making in the restoration of the tropical dry forest. We think that with the knowledge of microorganism from the soil, especially AMF, we could start the restoration process adequately. Therefore, we should choose the appropriate native inoculum.
MATERIALS AND METHODS
Study area. The department of Cesar is located in the northwest area of Colombia, limited to the north by the Guajira, to the east by the northern of Santander and Venezuela, to the south by Santander and the west by Bolivar and Magdalena. It has an average temperature between 28 and 30 °C and an average annual rainfall of 1940 mm, a bimodal regime with two wet periods between April and June in which 31% of annual rainfall occurs and between August and November with 53% of the annual rainfall 41. It is located in a tropical dry forest life zone8. Here, we worked with several soils or materials, as described in Table 1, comparing areas of the department of Cesar, which were called soil-coverage to facilitate the understanding of the results. Samples were taken from soil-coverage areas of the Calenturita mine at different points, proportional to the production stage (sterile material, horizon A, not used). Also, samples of the most representative soils of the department were also used from: natural forest, stubble, palm, and grass.
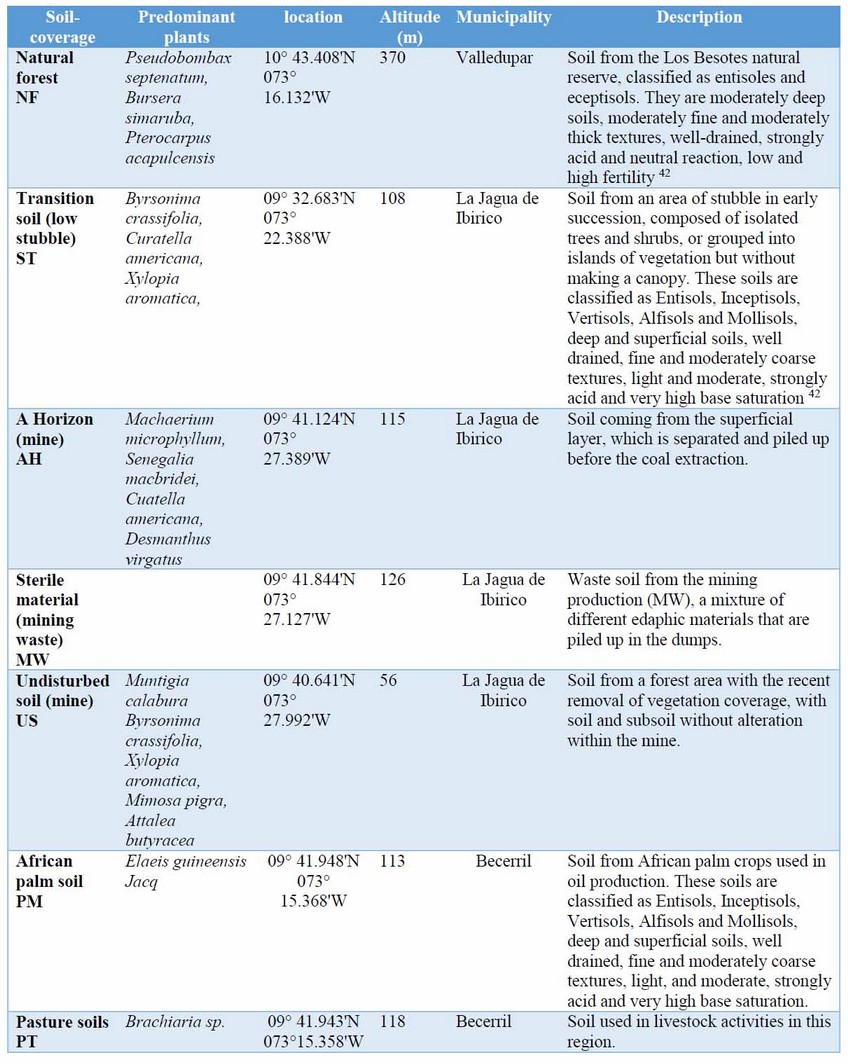
Table 1: coordinates of the different soils or materials (soil-coverage) from the department of Cesar.
Experiment area. The symbiotic effectiveness experiment was carried out in the greenhouse of the Las Mercedes farm, owned by the Universidad Católica de Oriente (6 ° 1'32.3 "N, 75 ° 10' 4.5" W, the altitude of 1116 m.a.s.l) Cocorna (Antioquia Colombia). It has an average temperature of 24 °C and an average annual rainfall of 4,200 mm; the site is located in a predominately humid forest life zone8.
Growth substrate. The growth substrate, was a mixture between soil and sand in a 6-4 proportion (v / v) respectively, as suggested by Habte and Osorio40. The soil sample corresponded to an A horizon of Andisol (supplied by a commercial company). Sand 68%, Loam 24%, Clay 8%, texture Frank-Sandy (Boyucos), pH 5.0, organic matter 3.4% (by ignition); Calcium, Magnesium and Potassium, 0.180, 0.06 and 0.05 cmolckg-1 (ammonium acetate1M, pH 7), Al 0.4 cmolckg-1 (1M KCl); Phosphorus 105 mg kg-1 (Bray ll), Sulfur 24 mg kg-1 (Calcium Phosphate 0.008M), Iron, Manganese, Copper and Zinc 210, 5, 8 and 6 mg kg-1 (modified Olsen); Boron 0.1 mg kg-1 (hot water); Nitrate 49.1 mg kg-1 (Aluminum Sulfate 0.025M); Cationic Exchange Capacity 17.07 cmolckg-1, electrical conductivity C.E 0.1 dS / m.cmolckg-1, electrical conductivity C.E 0.1 dS /m.
We adjusted the pH of the growth substrate to 6.0 with 2g of CaCO3 per kg of soil. We used dolomite lime (57% CaCO3 and 38% MgCO3) in order to improve the concentration of Mg in the substrate. For lime concentrations determination, we made an incubation curve of CaCO3 according to the Uchida & Hue43 methods. The growth substrate was disinfected by vaporization, and a week later it was sterilized in an autoclave at 120 °C and 0.1 MPa, for an hour. In order to establish the substrate solution phosphorus concentration of 0.02 mg / L, the optimal level for mycorrhizal activity44. An isotherm of P sorption (Figure 1), was based on the methods proposed by Fox & Kamprath45.
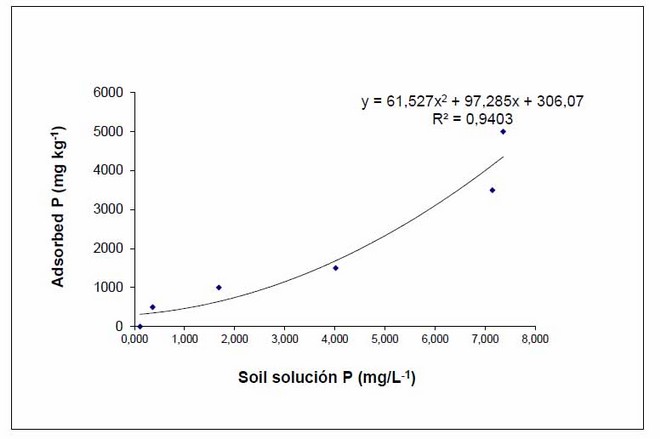
Figure 1: isotherm of P sorption of the growth substrate
Sources of inoculum (soil-coverage). The growth substrate was transferred to 54 pots (11.5x15 cm) with a mass of 800 g / pot. 350 g was directly added to each pot, and the remaining 450 g of the substrate was inoculated and mixed uniformly with 20 g of each of the sources of inoculum (soil-cover), previously mentioned. This with the purpose of facilitating contact of the root with each inoculum. Likewise, we used a crude inoculum of Glomus mosseae (GM) as a positive control.
This mycorrhizal inoculum was purchased from the International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM) and later multiplied in sorghum by a commercial company. It contained spores, fragments of infected roots and hyphae of the fungus suspended in a solid matrix composed of soil and sand. As a negative control, we used the same growth substrate, that is, without inoculation (WI), which received 20 g of sterile substrate. The negative control was the same growth substrate without inoculation (WI), which received 20 g of sterile substrate. For each of the soils or materials (soil-coverage), we performed soil analysis, in order to record its properties, and facilitate the final analysis (Table 2).
This mycorrhizal inoculum was purchased from the International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi (INVAM) and later multiplied in sorghum by a commercial company. It contained spores, fragments of infected roots and hyphae of the fungus suspended in a solid matrix composed of soil and sand. As a negative control, we used the same growth substrate, that is, without inoculation (WI), which received 20 g of sterile substrate. The negative control was the same growth substrate without inoculation (WI), which received 20 g of sterile substrate. For each of the soils or materials (soil-coverage), we performed soil analysis, in order to record its properties, and facilitate the final analysis (Table 2).
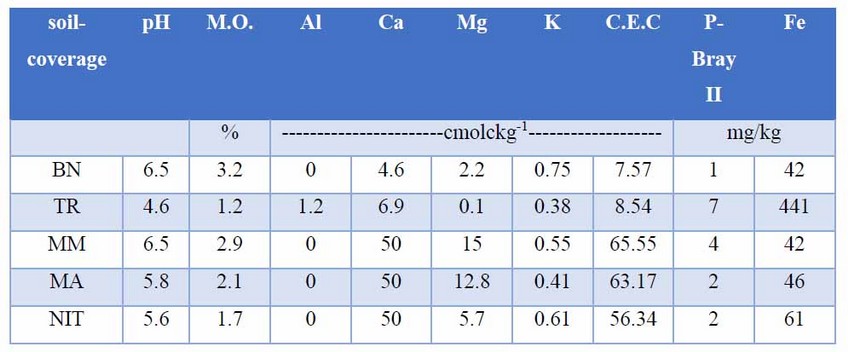
Table 2: Soil or material analysis (soils-coverage) of the department of Cesar
Indicator plant. Leucaena leucocephala var. K11 was used as a trap species or indicator plant because it is highly dependent on the mycorrhizal condition and has rapid growth40. Certified L. leucocephala seeds were introduced to decrease the experimental variability. Seeds were scarified with H2SO4 for 20 min, washed 6 times with deionized water44 and placed in a humid chamber with filter paper for germination. Once pre-germinated, one seed was placed per pot or experimental unit in the different treatments (soil-cover). The second week after planting, each experimental unit received 10 cm3 of the P-free Hogland nutrient solution in the following doses (mg L-1): N 50, K 132, Mg 106, S 204, Zn 10, Cu 5, B 0.8 and Mo 0.5. Plants were stored under natural light for growth and were periodically watered to maintain them between 50 and 60% of the maximum water retention capacity.
Experimental design. A completely randomized experimental design with nine treatments and six repetitions per treatment, for a total of 54 experimental units was analyzed.
Response variables
AMF spore extraction was used a sub-sample of each soil-coverage. Each subsample was transferred to a beaker with 300ml of running water and 0.15g of sodium pyrophosphate. It was stirred and left to rest for 5 minutes; the supernatant was passed through a battery of sieves (250, 106 and 53 μm), and centrifuged with a sugar solution at 70% for 5 min. Later the sedimented product of the centrifugation was collected on filter paper40. We are concluding with the spores count in the stereoscope. Leaf P content was measured through frequent monitoring (every 21 days) as a function of time in the youngest mature leaf following using the non-destructive sampling method Habte et al. 46. P determination was carried out using the molybdate blue method47, after reducing the leaf samples to ash in a muffle at 500 °C for 3 hours48. Shoot dry weight (SDW); plants were harvested at 95 days after the experiment planting. SDW determination was made after drying the plant material at 60 °C for 72 hours40. Mycorrhizal colonization, this was determined by the method of plates49 from the number of positive fields (presence of arbuscular, vesicles and hyphae) inside the root, for this, the finest roots were rinsed with KOH at 10%50 and then stained with a 0.15% acid fuchsin in lactic acid51. The total P content absorbed (TPC) was estimated through the concentration of P in the fourth pineal at the time of harvest46, as described above.
Analysis of results. We made and verified the statistical analyzes and the assumptions, to verify that the standardized residuals are normally distributed with zero mean and variance one, the standardized residuals were plotted with the independent variable. Data were subjected to Duncan's multiple range test and Fisher's LSD test, which a level of significance of P £ 0.05. We used the statistical packages R wizard and Statgraphics. Obtained data from the aerial dry mass, was necessary to perform a natural logarithmic transformation (Ln) to meet the conditions of normality and homogeneity of variance (ANOVA).
RESULTS
The soil-coverage with the most significant amount of spores were GM, TS, PM, and US with values of 11500, 3360, 2070, and 1060 respectively. The number of spores in the sample of soil from the palm crop (PM) is striking, which did not show up in the other results. As will be seen later (Table 3).
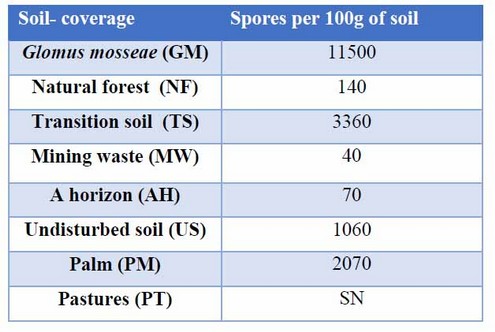
Table 3: AMF spore extraction in different soil-coverage. Without data (WD)
The positive control (GM) was statistically superior in leaf P content in time in comparison with the other treatments (soils-coverage), only on day 74 the TS and US treatments had no statistical differences with GM. Conversely, the negative control showed, in general, the lowest concentration of leaf P content, followed by PT and MW. It is noteworthy that the three soils with the least anthropic intervention (US, NF, and TS) showed a similar tendency in terms of leaf P content in the first two samples (days 31 and 54) with very similar values to the negative control (WI). Apparently, from day 60, all these treatments had significant increases of P until the end of the experiment, presenting significant differences compared to WI (Figure 2).
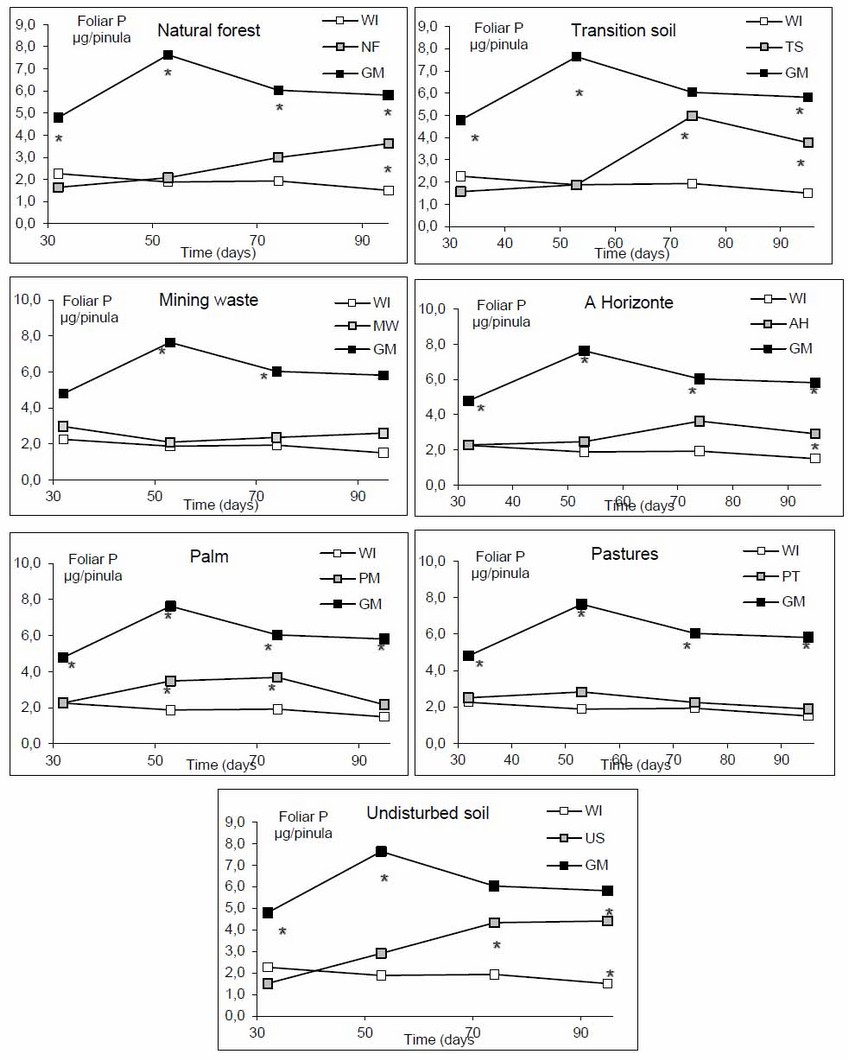
Figure 2: Leaf P content evaluated over time according to the inoculation with different sources of inoculum (soil-coverage). Asterisk indicates significant differences.
The total P content absorbed (TPC) at the end of the harvest, the GM positive control had significant differences compared to the other assessed soil-coverage treatments. In addition, we found that treatments or soil-coverage NF, TS and US had a similar behavior to the GM positive control in the contents of total P. As for the WI treatment (negative control), it obtained the lowest contents of total P content absorbed, followed by the soil-coverage MW, PM, and PT with similar results (Figure 3).
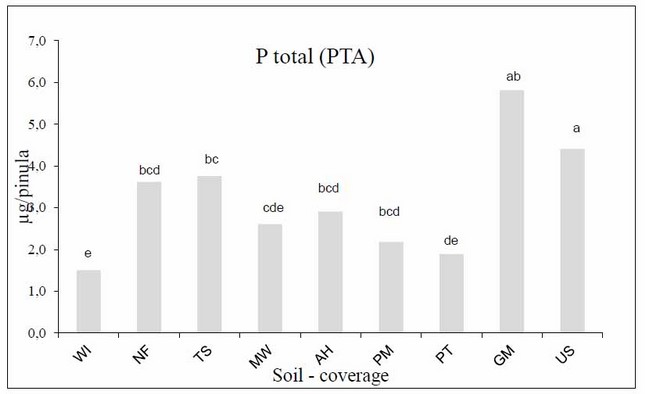
Figure 3: Total P (PTA) in the Leucaena pineal at the end of the harvest in the different sources of inoculating (soils-coverage). Columns with different lowercase letters indicate significant differences (Duncan, P £ 0.05).
The treatment with the shoot dry weight (SDW) was US, which even exceeded the positive control (GM), although not with significant differences. Overall, the remaining soil-coverage presented two tendencies, one similar to the positive control (GM), grouped in TS, NF, AH, and PM, where the soil-coverage is again found from soils not much intervened and another similar to the negative control (WI) next to the MW and PT treatments (Figure 4).
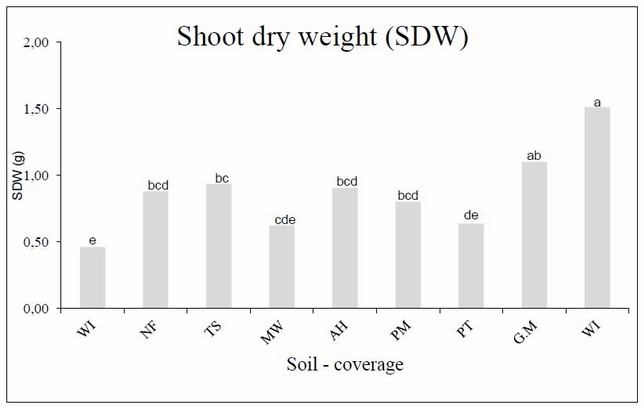
Figure 4: Shoot dry weight (SDW) of Leucaena at the end of the harvest in the different sources of inoculate (soils-coverage). Columns with different letters indicate significant differences (Duncan, P £ 0.05).
Mycorrhizal colonization was present in all the evaluated treatments, with the exception of the negative control WI, as expected. In addition, three soils-coverage PT, PM and WI showed similarity to the GM positive control; these coverage-soils obtained colonization percentages of 24, 29 and 48 respectively (Table 4).
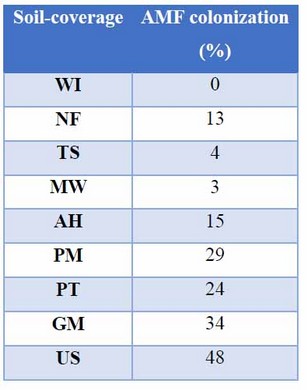
Table 4: Percentage of mycorrhizal colonization present in the Leucaena root in the different inoculum sources (soil-coverage).
Soil-coverage
DISCUSSION
Results indicate that the AMF of the different soil-coverage has very variable dynamics52 since in general two clearly defined trends were presented, two sets of data were grouped, the first (US, NF, and TS) associated to the positive control and the other remaining group associated with the negative control. These results are possibly due to the lack of infective mycorrhizal propagation absent in the ground coverage of the second group. This behavior has been reported by Osorio et al.53. Although with opposite tendencies, which found that forests, stubbles and forest plantations of high areas (low montane forests) had a similar behavior to the negative control. On the contrary crops and pastures evaluated by these researchers, had similar tendencies to the positive control (Glomus aggregatum).
From the ecological perspective Stürmer & Siqueira52 found that the conversion of primary forest to different land uses (secondary forest, pastures, agroforestry systems and crops) apparently does not reduce the diversity of AMF in the Brazilian Amazon, this could explain in part , because soil-coverage US, NF and TS, showed similarity with the positive control in terms of SDW and leaf P. On the other hand, Lovera & Cuenca54 found a contrary effect, when they compared a natural savanna versus a disturbed savanna in Venezuela, where a significant decrease in soil spore diversity was observed in the disturbed savanna.
Previously mentioned, it is noteworthy that the undisturbed soil-coverage (US) from the area of the Calenturita mine, presented values very similar to the positive control. This soil was not disturbed at the time of the samples. Therefore, it is assumed that the diversity of AMF and the number of spores in the sample were not affected.
Glomus mosseae inoculation increased significantly in the Leucaena plants, the leaf P contents, and the SDW plant growth, with respect to the plants that were inoculated with the soil-coverage. Similar behavior was showed by Jaramillo et al55. They used Glomus aggregatum as a positive control in the evaluation of several soils from oil palm, secondary forest, and soil degraded by alluvial mining. It is noteworthy that in the study by Jaramillo et al55, the soils from the palm and the alluvial mining showed low mycorrhizal symbiotic effectiveness, with values very similar to those presented here (Figure 3 and 4).
The low symbiotic effectiveness of the soil-coverage of African palm (PM) and pasture (PT) is possibly due to the management of these soils, which includes the use of pesticides and tillage. Both of which negatively affect the diversity of AMF and its number of effective propagules. There are several documents reported by literature confirming these effects54, 56, 57, 58. Likewise and as expected, the mixture of soils and materials from the coal mining operation negatively affected the soil-coverage sterile material (MW) and A horizon (AH) resulting in low symbiotic effectiveness. The negative consequences of mining on microorganisms have been well documented by different authors59. Finally, with the obtained results here, some soil coverage (US, NF, and TS) can be selected that have high symbiotic effectiveness, and that can be used (after multiplying the crude inoculum) in restoration and reforestation processes. Studies carried out by Souza et al60 suggest that the same degraded areas, such as those subjected to mining activities, may contain efficient AMF populations, contributing positively to rehabilitation.
CONCLUSIONS
Assessing treatments, we found that in general there are significant differences between two groups of data, first was evidenced that the soil-coverage US, NF, and TS had similar results to the positive control (GN), but MW, PM, PT and AH had behaviors similar to the negative control.
According to the data found, the soil-coverage with the best mycorrhizal symbiotic effectiveness were Undisturbed Soil, (US), Natural Forest (NF) and transition soil (TS). They have a high potential to be used in fundamental processes of ecological restoration.
ACKNOWLEDGMENTS
This work is part of a project funded by Colciencias entitled "Fitorregeneración de suelos disturbados por explotación minera " for this reason we thank both Colciencias and the Directorate of research and development of the Universidad Católica de Oriente for the support in the completion these researches. In addition, we appreciate the support of the Estudios Florísticos group, belonging to the Ingeniería Ambiental and the Facultad de Ingenierías from the Universidad Católica de Oriente
REFERENCES
1. Sánchez F., J. Alvarez, C. Ariza & A. Cadena. 2007a. Bat assemblage structure in two dry forests of Colombia: Composition, species richness, and relative abundance. Mammalian Biology. 2007a; 72 (2): 82–92
2. Calle, Z. Diversidad biológica y diálogo de saberes, memorias del curso de campo sobre biodiversidad y recursos genéticos indígenas y campesinos. 1 ª ed. Cali: Maestría en desarrollo sostenible de sistemas agrarios. 1994; 142
3. Janzen, D.H. Management of habitat fragments in a tropical dry forest: growth. Ann. Missouri Botanical Garden. 1988; 75: 105-116
4. Espinal, L. S. Geografía ecológica del departamento de Antioquia. Revista de la Facultad Nacional de Agronomía. 1985; 38 (1): 24-39
5. Instituto Alexander Von Humboldt (IAVH). Caracterización ecológica de cuatro remanentes de Bosque seco Tropical de la región Caribe colombiana. Grupo de Exploraciones Ecologicas Rapidas, IAVH, Villa de Leyva. 1997; pag. 76
6. Instituto Alexander Von Humboldt (IAVH). El Bosque Seco Tropical (bs-T) en Colombia. IAVH, Villa de Leyva, Colombia. 1998.
7. Murphy P.G. & A.E. Lugo. Ecology of tropical dry forest. Annual Review of Ecology and Systematics. 1986; 17: 67-88.
8. Holdridge L. Life Zone Ecology. Tropical Science Center, San José, Costa Rica. 1967.
9. Miles L., A.C. Newton, R.S. DeFries. A global overview of the conservation status of tropical dry forests. Journal of Biogeography. 2006; 33 (3): 491-505.
10. Gonzáles, S. M. & W. Devia. Caracterización fisionómica de la flora de un bosque seco secundario en el corregimiento de Mateguadua, Tuluá Valle. Cespedesia. 1994; 20(66): 35-65.
11. Maass J.M., H.D. Mooney, & E. Medina. Conversion of tropical dry forest to pasture and agriculture.in S. H. Bullock, and editors. Seasonally Dry Tropical Forests. Cambridge University Press, New York. 1995; Pages 399-422
12. Rudell, T. K., Bates, D. & Machinguiashi, R. A tropical forest transition agricultural change, out-migration, and secondary forest in the Ecuadorian Amazon. Annals of the Association of American Geographers. 2002; 92(1), 87-102
13. Gerhardt, K. Seedling development of four tree species in secondary tropical dry forest in Guanacaste, Costa Rica.Doctoral Dissertation. Uppsala University, Uppsala. 1994; 142
14. McLaren, K. P. & M. A. McDonald. The effects of moisture and shade on seed germination and seedling survival in a tropical dry forest in Jamaica. Forest Ecology and Management. 2003; 183: 61-75.
15. Bonet, J. Minería y desarrollo económico en el Cesar. Banco de la República. 2007; 1-31.
16. Sanchez, F., Mejía, C., & Herrera, F. "Impato de las regalías del carbón en los municipios del Cesar 1997-2003. Cuadernos PNUD, Investigaciones sobre desarrollo regional, Bogota. 2005.
17. Arias, M.A. & Barrera, J. Caracterización florística y estructural de la vegetación vascular en áreas con diferente condición de abandono en la cantera soratama, localidad de usaquén, Bogotá. Universitas Scientiarum. Edición especial II - Restauración Ecológica de Canteras. Dpto. de Biología. 2007; Vol 12 25-45
18. Jha, A.K. & Singh, S. Spoil characteristics and vegetation development of an age series of mine spoils in a dry tropical environment. Vegetatio. 1991; 97: 63-76.
19. BRADSHAW A. Restoration of mined lands-using natural processes. Ecological Engineering. 1997; 8: 255-269.
20. Arango Aramburo, M., & Olaya, Y. (2012). Problemática de los pasivos ambientales mineros en Colombia. Gestión y Ambiente. 2012; 15 (3), 125-133.
21. República de Colombia. Ley 99 de 1993, por medio del cual se expide el Código de Recursos Naturales. 1993
22. Brundrett Mark C. Micorrhizal associations and other means of nutrition of vascular plans: understanding the global diversity of host plants by resolving conflicting information and developing reliable means. Plan Soil. 2009; 320:37-77.
23. Allen, M. The ecology of arbuscular mycorrhizas: a look back into the 20 th century and a peek into the 21th. Mycological Research. 1996; 100 (7) pp. 769-782.
24. González, Ch. M.; Gutiérrez, C. M. y Wright, S. Hongos micorrízicos arbusculares en la agregación del suelo y su estabilidad. Terra Latinoamer. 2004; 22:507-514.
25. Aseri, G. K.; Jain, N.; Panwar, J.; Rao, A. and Meghwal, P. R. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of pomegranate (Punica granatum L.). Sci. Hort. 2008; 117:130-135.
26. Cornejo, P.; Rubio, R.; Castillo, C.; Azcón, R. and Borie, F. Mycorrhizal effectiveness on wheat nutrient acquisition in an acidic soil from southern Chile as affected by nitrogen sources. J. Plant Nutr. 2008; 31:1555-1569.
27. Sieverding, E. Vesicular-Arbuscular mycorrhiza management. Editorial GTZ, Eschbor. 1991; 57-72
28. Jakobsen, I.; L.K. Abbott, and A.D. Robson. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 2. Hyphal transport of 32P over defined distances. New Phytologist. 1992b; 120: 509-516.
29. Johansen, A.; I. Jakobsen, and E.S. Jensen. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 3. Hyphal transport of 32P and 15N. New Phytologist, 1993; 61-68.
30. Schachtman, D.; Reid, R. and Ayling, S. M. Phosphorus uptake by plants: from soil to cell. En: Plant Physiology. 1998. Vol. 116; p. 447-453.
31. Abbott, L. K. y A. D. Robson. The role of vesicular arbuscular mycorrhizal fungi in agriculture and the selectios of fungi for inoculation. Journal Agric. 1982; 33: 389 – 408.
32. Lopes, E.S; J.O. Siqueira, and L. Zambolim. Caracterizaçao das micorrhizas vesicular-arbuscular (MVA) e seus efeitos no crescimento das plantas. Revista Brasileira de Ciência do Solo. 1983; 7: 1-19.
33. Hooker, J.E., Jaizme-Vega, M., Atkinson, D. Biocontrol of plant pathogens using arbuscular mycorrhizal fungi. In: Gianinazzi, S., Schüepp, H. (Eds.), Impact of Arbuscular Mycorrhizas on Sustainable Agriculture and Natural Ecosystems. Birkhäuser Verlag, Basel, Switzerland. 1994; pp. 191–200.
34. Haselwandter, K. and G. D. Bowen. Mycorrhizal relations in trees for agroforestry and land rehabilitation. Forest Ecology and Management. 1996; 81: 1-17.
35. Barea, J.M; R. Azcón, and C. Azcón-Aguilar. Mycorrhizosphere interactions to improve plant fitness and soil quality. Antonie van Leewenhoek. 2002; 81: 343-351.
36. Mansfeld-Giese, K; J. Karsen, and L. Bødker. Bacterial populations associated with mycelium of the arbuscular mycorrhizal fungus Glomus intrarradices. FEMS Microbiology Ecology. 2002; 41: 133-140.
37. Elsen, A.; H. Baimey; R. Swennen, and D. De Waele. Relative mycorrhizal dependency and mycorrhiza-nematode interaction in banana cultivars (Musa spp.) differing in nematode susceptibility. Plant and Soil. 2003; 256: 303-313
38. Johansson, F., R. Leslie, and R. Finlay. Microbial interactions in the mycorrhizosphere and their signicance for sustainable agriculture. Plant and Soil. 2004; 0: 1–21
39. Habte, M. and A. Manjunath. Soil solution phosphorus status and mycorrhizal dependency in Leucaena leucocephala. Applied and Environmental Microbiology. 1987; 53: 797-801.
40. Habte, M. and N.W. Osorio. Arbuscular Mycorrhizas: Producing and applying Arbuscular Mycorrhizal Inoculum. University of Hawaii, Honolulu. 2001; 47 p.
41. CORPOCÉSAR Informe Anual de la Actividad Meteorológica de la Cuenca del Río César. Informe interno. 1997; pp. 87-103.
42. INSTITUTO GEOGRÁFICO AGUSTÍN CODAZZI (IGAC). Estudio general de suelos y zonificación de tierras. Departamento del César, Escala 1:100.000. Bogotá D.C. 1997.
43. Uchida, R. and N.V. Hue. Plant nutrient management in Hawaii´s soils, approaches for tropical and subtropical agriculture. pp. 101-111. In: Silva, J.A. and R. Uchida (eds.). College of Tropical Agriculture and Human Resources. University of Hawaii at Manoa. 2000; 158 p.
44. Habte, M. and A. Manjunath. Categories of vesicular-arbuscular mycorrhizal dependency of host species. Mycorrhiza. 1991; 1: 3-12
45. Fox, R. and E. Kamprath. Phosphate sorption isotherms for evaluating the phosphate requirements of soils. Soil Science Society of America Proceedings. 1970; 34: 902-907.
46. Habte, M.; R. Fox, and R. Huang. (1987). Determining vesicular-arbuscular micorrizal effectiveness by monitoring P status of subleaflets of indicator plants. Communications in Soil Science and Plant Analysis.1987; 18: 1403-1420.
47. Murphy, J. and J.P. Riley. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta. 1962; 27:31-35.
48. AZIZ, T. and M. HABTE. Determining vesicular-arbuscular micorrizal effectiveness by monitoring P status of leaf disk. Can. J. Microbiol. 1987; 33: 1097-1101.
49. Sanchez de P., M. Gomez, E; Muñoz, J.; Barrios, E.; Praguer, M.; Bravo, N.; El-Sharkawi, M.; Perez, J.; Asakawa, N; Marmolejo, F.; Cadavid, L.; Quintero, R.; Miranda, C.; Mier, C.; Reyes JT.; Torres, R.; Zapata, C.; Tofiño, R.; Benjumea, C.; Diaz, G.; Trujullo, L.; Bonilla, F.; Espinosa, J.; Rodriguez, H.; Garcia, H.; Triana, W.; Carlosama, C. y Vargas, N. Las endomicorrizas expresión bioedáfica de importancia en el trópico. Universidad Nacional de Colombia, Sede Palmira. 2007b; 351 p.
50. Phillips, J.M. and D.S. Hayman. improved procedures for clearing and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society. 1970; 55: 158-161.
51. Kormanik, P.P., A.C. McGraw, and R.C. Schultz. 1980. Procedure and equipment for staining a large number of plant samples for endomycorrhizal assay. Can. J. Microbiol. 1980; 26: 536-538.
52. Stürmer, S.L. & J.O. Siqueira. Species richness and spore abundance of arbuscular mycorrhizal fungi across distinct land uses in Western Brazilian Amazon. Mycorrhiza (2011). 2010; 21:255–267
53. Osorio N, M Díez, J. Sierra & L Paternina. Consideraciones ecológicas sobre la asociación micorrizal en suelos de la región alto andina. En: León JD, editor. Ecología de bosques andinos. Experiencias de investigación. Medellín (Colombia): La Carreta Editores. 2008; p. 181-200
54. Lovera, M y G. Cuenca. Diversidad de hongos micorrízico arbusculares (HMA) del suelo de una sábana natural y sabana perturbada de la Gran Sabana. Venezuela. Interciencia. 2007; Volumen 32 # 2.
55. Jaramillo Padilla, S., Silva Benjumea, J., & Osorio Vega, N. Potencial simbiotico y efectividad de hongos micorrizo arbusculares de tres suelos sometidos a diferentes usos. Revista Faculta Nacional de Agronomia, Medllin. 2004
56. Rilling, M. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecology Letters. 2004; 7: 740-754.
57. St-John, T. V. Soil disturbance and the mineral nutrition of native plans. 2nd Native Plant Revegetation Symposium. 1987; 34-39 p.
58. Munyanziza E.; H. K. Kehri y D.J. Bagyaraj. Agricultural intensification, soil biodiversity and agro-ecosystem function in the tropics: the role of mycorrhiza in crop and tree. Applied Soil Ecology. 1997; 6: 77-85.
59. Silvia DM, Fuhrmann JJ, Hartel PG, Zuberer YD. Principles and applications of soil microbiology 2nd Edition. Ed. Pearson Prentice Hall. New Jersey. United States of America. 2005
60. Souza Moreira, Fátima Maria de, Donizetti Santos, José Geraldo, Siqueira, José Osvaldo, Eficiência de Fungos Micorrízicos Arbusculares Isolados de Solos de Áreas de Mineração de Bauxita No Crescimento Inicial de Espécies Nativas. Revista Brasileira de Ciência do Solo [en linea] 2008, 32:[Fecha de consulta: 3 de agosto de 2016] Disponible en:<http://www.redalyc.org/articulo.oa?id=180214230014> ISSN 0100-0683
Received: 18 January 2019
Approved: 28 February 2019
Jorge A. Sierra-Escobar1, y John Alexander Ortíz-Correa2
1. Docente Asociado, grupo de estudios florísticos, Facultad de Ingenierías Universidad Católica de Oriente (UCO)
2. Estudiante de ingeniería Ambiental Universidad Católica de Oriente.