2022.07.04.68
Files > Volume 7 > Vol 7 No 4 2022
Study on the anti-microbial effect of Sinigrin against some pathogenic bacterial species
1. Department of Biology, College of Science, University of Baghdad, Baghdad, Iraq,
* Correspondence: [email protected]; Tel.: +9647708820309; Iraq; 10061
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.68
ABSTRACT
The increasing anti-bacterial drug resistance is one of the biggest challenges facing doctors around the globe, so finding alternative treatments is one of the ideal options to overcome this problem. The cruciferous family is one of the wealthiest plants worldwide because it contains the most important secondary metabolites, glucosinolates, known for their anti-microbial properties. The present study aimed to evaluate the anti-bacterial effect of glucosinolates (Sinigrin) against eight bacterial isolates (Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Actinomyces, Proteus mirabilis and Streptococcus pneumoniae). The current study investigated six concentrations of pure Sinigrin (100, 300, 500, 700, 900, and 1100 µg/ml). The sensitivity of bacterial isolates to various antibiotics was tested by VITIK 2DensiCheck equipment. The anti-bacterial activity of Sinigrin was assessed using the agar diffusion method, and the microtiter plate method measured the minimal inhibitory concentration (MIC). The highest anti-bacterial effect of Sinigrin was observed against S. aureus, E. coli, and E. faecalis. The anti-bacterial activity started as lower as 100 µg/ml, while a moderate effect was seen against P. aeruginosa and K. pneumoniae at a concentration lower than 700 µg/ml. On the other hand, Sinigrin was not effective against Actinomyces, P. mirabilis, and S. pneumoniae. It can be concluded from the present study that Sinigrin has an anti-bacterial effect on some isolates of bacteria which suggests the possibility of using Sinigrin as alternative medicine in the future.
Keywords: Anti-bacterial activity, Agar well diffusion, Glucosinolates, Minimum inhibition concentration and antibiotic susceptibility, Sinigrin.
INTRODUCTION
The appearance of antibiotic-resistant microbes to multiple anti-microbial agents has become a life-threatening issue to public health. Therefore, several actions must be taken to reduce this problem, for example, by controlling antibiotic usage, developing research for enhancing the genetic mechanisms of resistance, and developing new synthetic natural anti-bacterial drugs since the ultimate goal is to offer appropriate and efficient anti-microbial drugs to thepatients1. Their botanical derivatives are safer in comparison with high efficacy2.
Antibiotics and other anti-microbial drugs have been used extensively in treating infectious disorders for many years to combat bacterial and fungal infections. Infectious organisms have been exposed to these substances on a large scale due to the widespread use of antibiotics, their repeat prescription in healthcare facilities, and an overuse of antibiotics in animal-rearing practices in agriculture. Because of this, some organisms have developed a resistance to them, and certain bacterial strains now significantly threaten overall health. Myrosinase-activated glucosinolates produce thiocyanates, isothiocyanates, and nitrile compounds. In vitro studies show that these chemicals are ideal for anti-bacterial testing in treating such infections3,4.
There are many diverse sources of natural anti-microbials, including plants, animals, bacteria, algae, and fungi. Among them, the advantages of glucosinolates and the products derived from them for human nutrition, plant defense, and as powerful anti-bacterial agents have gained recognition. Plants produce thousands of phytochemicals, many of which have nutritive value in both medicinal and health-promoting properties5. Sinigrin(allyl-glucosinolate or 2-propenyl-glucosinolate) is natural aliphatic glucosinolate present in cruciferous or Brassicaceae plants such as broccoli, broccoli sprouts, cabbage, mustered, Brussel sprouts, cauliflower and many others. These plants have essential components of a healthy diet, and their pic flavor profile with several physiological processes6. To activate, the glucosinolates molecules require conversion by the myrosinase enzyme to bioactive thiocyanate, isothiocyanate, and nitrile derivatives; therefore, glucoraphanin and Sinigrin are converted into bioactive sulphoraphanin (SFN) and allyl isothiocyanate (AITCs) with fungicidal and bactericidal properties.6 Melrose in 2019 illustrated sinigrin uses and benefits in biomedicine.4 Previous studies showed Sinigrin's role in modulating the immune system.7 Therefore, the present study aims to evaluate the potential anti-microbial activity of sinigrin secondary metabolite against different bacterial isolates, which may suggest the possibility of bringing it as an alternative treatment in the future.
MATERIALS AND METHODS
Preparation of glucosinolates (sinigrin) solutions
Pure Sinigrin (extracted from the mustard plant/ Brassicaceae family) was purchased from Sigma-Aldrich. The stock solution of 3 mg/ ml of Dimethyl sulfoxide (DMSO) (99 %, v/v) (Fluka) was prepared. Different concentrations of Sinigrin were prepared from stock solution (100, 300, 500, 700, 900, and 1100 μg/mL). The dilutions were prepared by using (DMSO) (99 %, v/v) (Fluka) aseptically. The Sinigrin was dissolved in a sterile container and shaken gently at room temperature.8
Bacterial strains
Eight isolates of pathogenic bacterial species, including Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Actinomyces, Proteus mirabilis, Streptococcus pneumoniae (blood agar was used in growing the fastidious isolated bacteria) were isolated and identified in the Microbiology Laboratory. Department of Biology, College of Science, University of Baghdad, Baghdad, Iraq, in the level 2 biosafety lab. Basic biochemical tests and microscopic examination were used to identify the bacterial isolates. VITEK 2 fluorescence system (ID-GNB card) was used to confirm the identification of bacterial isolates.9
Preparation of bacterial suspension and growth medium
All bacterial isolates were stored for a long time at −80 °C in a sterile cryovial containing 30 % glycerol (v/v, glycerol/overnight bacterial growth). Before starting the experiments, a loop full of bacterial isolates was inoculated onto Mueller-Hinton Agar (MHA) and incubated at 37 oC overnight. The suspension of bacterial growth was prepared by mixing the loop full of the isolated colony with sterile normal saline. The normal saline was used to adjust the number of bacteria to be 1.5 x 108 c.f.u/ml by using a 0.5 McFarland tube.10
Antibiotic susceptibility
VITEK2DensiCheck (bioMe'rieux) technology was employed to test the susceptibility of isolated bacteria to various antibiotics, Cefazolin, Ceftazidime, Ceftriaxone, Cefepime, Imipenem, Gentamicin, Ampicillin, Amoxicillin/Clavulanic Acid, Ampicillin/Sulbactam, Piperacillin/Tazobactam. The CLSI 2021 manual was followed for the selected drugs.11
The anti-bacterial effect of Sinigrin against bacterial isolates
Diffusion method
The anti-microbial effect of different concentrations of Sinigrin was evaluated by measuring their clear inhibition zone against eight bacterial isolates cultured onto Mueller-Hinton Agar plates. A hundred microliter of standard bacterial inoculum 1.5 x 108 of each bacterial isolate was separated onto Mueller-Hinton Agar plates. A sterile crock-poorer made several 0.45 mm diameter wells on Mueller-Hinton Agar plates. Fifty microliters of the serial concentrations (100, 300, 500, 700, 900, and 1100 μg/mL) of Sinigrin were put into the six wells, and 50 µl of DMSO was placed in the seventh well (as control). The plates were incubated overnight at 37 C. Scales were used to measure the clear inhibition zones.
Minimum inhibition concentration (MIC) of Sinigrin
The minimum inhibition concentration (MIC) of pure Sinigrin was determined using a microtiter plate. A hundred microliters of each concentration of Sinigrin (100, 300, 500, 700, 900, and 1100 μg/mL) was added into the wells of microtiter plates, 100 µl of sterile tryptic soya broth (double concentration of TSB) and 10 µl of overnight growth of bacterial isolates (1.5 x 108 c.f.u/ml). In control wells, 100 µl of DMSO was used instead of the sinigrin solution. Three duplicates of each trial were made. The microtiter plates were shaken gently and incubated overnight at 37 C.10
Statistical analysis
Statistical analysis was done by using Origin 8 software. The data were expressed as means ± SE. The chi-square was used to evaluate the difference between the diameters of the test (different concentrations of Sinigrin) and the diameters of wells that were filled with DMSO (control negative). A value of P<0.05 was considered to be statistically significant.
RESULTS
The anti-microbial effect of Sinigrin
The anti-bacterial effect of Sinigrin was estimated in this study against eight bacterial isolates that were isolated and diagnosed in the microbiology laboratory at the Department of Biology, College of Science, University of Baghdad. Table 1 shows that the different concentrations of Sinigrin have affected the growth of five bacterial isolates (S. aureus, E. coli, P. aeruginosa, K. pneumoniae, and E. faecalis) but not against three isolates (Actinomyces, S. pneumoniae, and P. mirabilis). It was shown that the highest anti-bacterial effect of Sinigrin was seen in the case of S. aureus starting from the lowest concentration of 100 µg/ml though the effect was not in a concentration-dependent manner. Similarly, in the case of E. coli but the highest concentrations of Sinigrin (900 µg/ml and 1100 µg/ml) were non-significant. In the cases of P. aeruginosa and K. pneumoniae, the anti-bacterial effect of Sinigrin starts at 700 µg/ml concentration upwards, and the effect was in a concentration-dependent manner. In the case of E. faecalis, the effect of Sinigrin appeared at low concentrations, i.e., at 100 µg/ml, 300 µg/ml, and 500 µg/ml. No anti-bacterial effect of Sinigrin was observed at high concentrations, i.e., at 700 µg/ml, 900 µg/ml, and 1100 µg/ml. Finally, the growth of Actinomyces, S. pneumoniae, and P. mirabilis isolates was not affected (Figure 1).
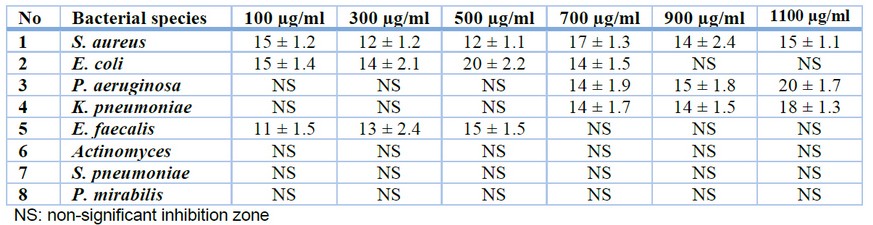
Table 1. Anti-microbial activity of different concentrations of Sinigrin expressed as the diameter of inhibition zones in millimeters (mm) against 8 bacterial species. Each treatment was performed in triplicate.

Figure 1. The well-diffusion method was used to measure the anti-bacterial effect of different concentrations of Sinigrin (well 1, 100 μg/ml; well 2, 300 μg/ml; well 3, 500μg/ml; well4, 700 μg/ml; well 5, 900 μg/ml; well 6, 1100 μg/ml) in Mueller-Hinton Agar (MHA) that was inoculated (spared) previously inoculated with a and b (S. aureus); c and d (E. coli); e (K. pneumoniae); f and g (E. faecalis); h (P. mirabilis).
MICs of Sinigrin against the bacterial species
The MIC method was used to evaluate the anti-microbial effect of pure sinigrin standard solution against different bacterial isolates of S. aureus, E. faecalis, E. coli, K. pneumoniae, P. aeruginosa, Actinomyces, P. mirabilis, and S. pneumoniae. The current study showed that the MICs of Sinigrin against S. aureus, E. coli, and E. faecalis were 300µg/ml, while the MICs of Sinigrin against K. pneumoniae and P. aeruginosa were 300 and 700 µg/ml respectively. The MIC method showed no anti-bacterial effect of Sinigrin against Actinomyces, P. mirabilis, and S. pneumoniae.

Table 2. Minimum inhibition concentrations (MICs) of Sinigrin against 8 bacterial species. Each treatment was performed in triplicate.
DISCUSSION
Previous studies were focused on the treatment application of Sinigrin because of the safe and non-toxic effect of this substance as it is extracted from plant 12. Several studies highlighted the therapeutic impact of Sinigrin; Tanaka et al. (1992) established the anticancer effect of Sinigrin,13 while the anti-inflammation effect was attributed by Lee and Lee (2015).14 As for the anti-oxidant activity, it was reported by Ippoushi et al. (2010). The main effect of this herbal extract was the anti-microbial effect.15 Several studies reported the anti-bacterial effect of Sinigrin. Other investigators highlighted the anti-bacterial impact of Sinigrin against E. coli,16 Bacillus subtilis, and Listeria monocytogenes,17 hence the development of resistance to the conventional antibiotic by pathogenic bacteria makes it necessary to find alternative anti-microbials to eradicate them.18
The use of different serial concentrations was done according to the manufacturer company of sinigrin phytochemicals are routinely classified as anti-microbials based on susceptibility tests that produce inhibitory concentrations in the range of 100 to 1,000 μg/mL, which is why this range was used in the present study.19,20 In the current study, eight bacterial isolates were isolated and identified using biochemical tests and VITEK 2 DensiCheck (bioMe'rieux) technology. The diffusion method was used to check the anti-bacterial effect of different concentrations of Sinigrin. As illustrated above, the results showed that Sinigrin has an anti-bacterial effect on five isolates. Sinigrin is not usually an anti-microbial substance; when it is enzymatically hydrolyzed from allyl isothiocyanate, it exhibits potent anti-microbial activity against food spoilage and pathogenic organisms.21 Allyl isothiocyanate molecules showed anti-microbial effects against E. coli O157:H7 at low pH. Based on the results that proteins and sulphydryl compounds could suppress various isothiocyanates, it was hypothesized that the mechanism of isothiocyanates' anti-microbial activity was related to the intracellular inactivation of sulphydryl-enzymes 9, 22. It is known that the thioredoxin system has an essential role in DNA formation, which suggests that the anti-bacterial activity of allyl isothiocyanate could be related to the inhibition of DNA formation. Allyl isothiocyanate inhibits the catalysis of thioredoxin reductase and acetate kinase, which are responsible for essential metabolic reactions in bacteria. Thus, it can be proposed that allyl isothiocyanate has many targeted anti-microbial activities, as they can cause enzymatic inhibition and membrane damage.16,17 A study by Herzallah and Holley (2015) evaluated the use of carboxymethyl cellulose (CMC) nanoparticulate on the anti-microbial activity of CMC films containing Sinigrin against E. coli O157:H7 on fresh beef.23 An investigation by Lara-Lledo and their group found that pure Sinigrin after hydrolysis can interfere with the metabolism of bacteria and inhibit the monocytogenes growth.14,18
In the novelty of the current study, different concentrations of pure Sinigrin were used to check the anti-bacterial effect of Sinigrin against other bacterial isolates, and two methods to approve the anti-bacterial effect of Sinigrin in vitro were used as well.13
CONCLUSIONS
The current study has shed light on one of the most important natural compounds, glucosinolates. Glucosinolates (from the Brassicaceae family) and their hydrolysis products (such as Sinigrin) have decisive bioactive benefits, one of which is their anti-microbial properties. We have discussed Sinigrin's anti-bacterial effect (at low concentrations) against different bacterial isolates such as S. aureus, E. coli, and E. faecalis. In contrast, moderate concentrations were effective against P. aeruginosa and K. pneumoniae. Further studies on glucosinolates and their hydrolysis products are recommended to investigate their anti-microbial effect, which can be a valuable approach to enhance the therapeutic activities of such critical secondary metabolites.
Author Contributions: Alaa M. Hasan conducted the research experiments and wrote the manuscript, and Jenan A. Ghafil, conducted the research experiments, data interpretation, statistical analysis, and writing the manuscript.
Funding: Self-funding.
Institutional Review Board Statement: This work was approved by the Institutional Review Board Statement of the Department of Biology, College of Science, University of Baghdad (No. 1045).
Acknowledgments: We sincerely thank the management of the Department of Life Sciences for their support in the laboratory experiments carried out in the department's laboratories.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Tiwaria P, Tushar K, Varsha S, Hanhong B, Vinay K. Plant synthetic biology for producing potent phyto-antimicrobials to combat anti-microbial resistance. Biotechnol Adv. 2021; 48:107729.
2. Shin J, Vasantha-Srinivasan P, Kwang-sun K. The multi-faceted potential of plant-derived metabolites as anti-microbial agents against multidrug-resistant pathogens. Microbial Pathogenesis. 2018; 116:209-214.
3. Dias C, Aires A, Bennett RN, Rosa EA, Saavedra MJ. First study on antimicriobial activity and synergy between isothiocyanates and antibiotics against selected Gram-negative and Gram-positive pathogenic bacteria from clinical and animal source. Med Chem. 2012; 8:474–480.
4. Melrose J. The Glucosinolates: A Sulphur Glucoside Family of Mustard Anti-Tumour and Antimicrobial Phytochemicals of Potential Therapeutic Application. Biomedicine. 2012; 7: 62.
5. Briones-Herrera A, Eugenio-Perez D, Reyes-Ocampo JG, Rivera-Mancia S, Pedraza-Chaverri J. New highlights on the health-improving effects of sulforaphane. Food Funct. 2018; 9:2589–2606.
6. Mazumder A, Dwivedi A, du Plessis J. Sinigrin and Its Therapeutic Benefits. Molecules. 2016; 21:416.
7. Walter NS, Gorki V, Chauhan M, Dhingra N, Kaur S. Sinigrin in combination with artesunate provides protection against lethal murine malaria via falcipain-3 inhibition and immune modulation. Int Immunopharmacol. 2021;101:108320. doi: 10.1016/j.intimp.2021.108320. Epub 2021 November 3. PMID: 34741871.
8. Jen JF, Lin TH, Huang JW, Chung WC. Direct determination of Sinigrin in mustard seed without desulfatation by reversed-phase ion-pair liquid chromatography. J Chromatography A. 2002;912:363–368.
9. Gatsing D, Tchakoute V, Ngamga D, Kuiate JR, Tamokou JD, Nji- Nkah BF. In vitro anti-bacterial activity of Crinum Purpurascens Herb. leaf extract against the Salmonella species causing typhoid fever and its toxicological evaluation. Iran J Med Sci. 2009; 34:126–36.
10. Zgair AK, Ghafil JA, Radif HM, Radhi SN, Hafiz MH, Albaayit SFA. Moxifloxacin reduces Stenotrophomonas maltophilia adhesion to mouse intestinal tract in vitro. Pak J Pharm Sci. 2017; 30:1753-1757. PMID: 29084698.
11. Khan A, Pettaway C, Dien Bard J, Arias CA, Bhatti MM, Humphries RM. Evaluation of the Performance of Manual Antimicrobial Susceptibility Testing Methods and Disk Breakpoints for Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2021;65(5):e02631-20. doi: 10.1128/AAC.02631-20. Epub ahead of print. Erratum in: Antimicrob Agents Chemother. 2021 May 18;65(6): PMID: 33558287; PMCID: PMC8092892.
12. Dufour V, Stahl M, Baysse C. The anti-bacterial properties of isothiocyanates. Microbiol. 2015; 61:229–243.
13. Tanaka T, Kojima T, Morishta Y, Moori H. Inhibitory effects of the natural products indole-3-carbinol and Sinigrin during initiation and promotion phases of 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. J Cancer Res. 1992;83:835–842.
14. Lee HW, Lee KR. Effect of Sinigrin on vascular cell adhesion molecule-1 expression in TNF-α-stimulated mouse vascular smooth muscle cells via downregulation of NF-κB signalling pathways. FASEB J. 2015; 29:593.15.
15. Maria M, Leoni O, Lori R, Cembali T. Antifungal vapour–phase activity of allyl–isothiocyanate against Penicillium expansum on pears. Plant Pathol. 2002;51:231–236.
16. Cordeiro RP, Wu C, Holley RA. Contribution of endogenous plant myrosinase to the anti-microbial activity of deodorized mustard against Escherichia coli O157: H7 in fermented dry sausage. Int J Food Microbiol. 2014; 189:132–138.
17. Mazumder A, Dwivedi A, du Plessis J. Sinigrin and Its Therapeutic Benefits. Molecules. 2016; 21:416. doi: 10.3390/molecules21040416. PMID: 27043505; PMCID: PMC6273501.
18. Ippoushi K, Takeuchi A, Azuma K. Sinigrin suppresses nitric oxide production in rats administered intraperitoneally with lipopolysaccharide. Food Chem. 2010;120:1119–1121.
19. Simões M, Bennett RN, Rosa EA. Understanding anti-microbial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep. 2009;26:746–757.
20. Borges A, Ana CA, Carla F, Maria JS, Lúcia CS, Manuel S. Antibacterial activity and mode of action of selected glucosinolate hydrolysis products against bacterial pathogens. J Food Sci Technol. 2015;52:4737- 4748.
21. Luciano FB, Holley RA. Enzymatic inhibition by allyl isothiocyanate and factors affecting its anti-microbial action against Escherichia coli O157:H7. Int J Food Microbiol. 2009;131:240–245.
22. Zgair AK, Chhibber S. Stenotrophomonas maltophilia flagellin is involved in bacterial adhesion and biofilm formation. Microbiol. 2013;82:647–651. https://doi.org/10.1134/S0026261713050172.
23. Herzallah S, Holley R. Use of a nanoparticulate carboxymethyl cellulose film containing Sinigrin as an anti-microbial precursor to kill Escherichia coli O157:H7 on fresh beef. Lett Appl Microbiol. 2015;61:139–145.
Received: September 22, 2022 / Accepted: October 18, 2022 / Published:15 November 2022
Citation: Hasan, A.M.; Ghafil, J.A. Study the anti-microbial effect of Sinigrin against some pathogenic bacterial species.Revis Bionatura 2022;7(4) 68. http://dx.doi.org/10.21931/RB/2022.07.04.68
