2022.07.04.25
Files > Volume 7 > Vol 7 No 4 2022
Impact of varying amounts of Moringa oleifera seed powder in the diet on a few aspects of common carp growth L. Cyprinus carpio
Jaber Bander Zghair Al-Rawashi1, Ali Hussain Salman1, Jassim Kassim Al-Gharawi1
1Animal Production Department, Agriculture College, Al-Muthanna University, Iraq.
Corresponding author: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.25
ABSTRACT
The current study was conducted in experimental cages in a mud pond, First Agricultural Research and Experiment Station, Agriculture College, Al-Muthanna University, to determine the effect of different levels of Moringa oleifera seed powder on the diets of common carp. A total of 75 common carp fish with an average weight of 65.08±0.42 g were used; it was randomly distributed to 5 treatments with three replicates (5 fish for each replicate). The fish that were fed on experimental diets was divided into five equal therapies in terms of protein percentages, different in the proportions of adding Moringa seed powder; the rate of seeds added to the treatments was 0, 0.5, 1, 1.5 and 2%, respectively, the fish were fed on the experimental diets at 5% of the live weight, divided into four meals a day. The results showed a significant superiority of T2 and T3 treatments compared with other therapies on growth parameters (final weight, weight gain, daily growth rate, specific and relative), and give the best feed conversion ratio, the highest food conversion and protein efficiency ratio. Indicates that adding Moringa seed powder to diets at rates of 0.5 and 1% led to fish growth promotion and increased utilization of feed intake.
Keywords: Moringa oleifera, growth parameters, common carp Cyprinus carpio L.
INTRODUCTION
The world faces significant challenges in increasing food production, eradicating famine, and achieving food security in developing countries and the poorest in the world, coinciding with the increasing population growth1. The world population is expected to reach 9.8 billion in 20502. Aquaculture, one of the fastest growing and developing sectors of animal food production, plays an essential role in providing a cheap and continuous source of animal protein to meet human nutritional needs worldwide3,4,5.
Fish farming is one of the developing food industries; despite the progress made in recent years, it suffers from many problems and obstacles that limit production and profits. It causes substantial economic losses, such as the manufacture of fish feed and its high cost, because feeding fish represents 60% of the fish production cost of fish farms6. Putting specialists in this field before a significant challenge in selecting feed ingredients should be suitable, available, naturally available, environmentally friendly and low cost7. Most traditional feed sources, whether plant or animal, such as fishmeal, soybeans, peanuts, etc., face competition in other uses such as direct human consumption or as feed for other animal species8,9.
These problems and obstacles affecting the development and sustainability of fish farming have opened new perspectives for researchers to test and use other medicinal plants as less competitive alternatives to traditional and low-priced feed ingredients. It promotes growth and increases production. One of the most famous plants that have been used is Moringa oleifera; it is called the tree of life or the miracle tree because of its importance and versatility10. Foidl et al.11 indicated that its original habitat is in the Himalayan regions of northwestern India; it grows in many tropical and subtropical countries. Their rapid growth and adaptability characterize Africa, Arabia and Southeast Asia to drought. Known to be of high nutritional value, every part of it can be used in different medical and industrial applications, such as the pharmaceutical industry or as food for humans and animals12.
Moringa seeds are characterized by high nutritional value because of their high protein content, characterized by their range of important essential amino acids. They are also a good source of fats, such as unsaturated fatty acids. In addition to carbohydrates, crude fiber and essential minerals contain a mixture of critical active compounds, such as phenols, flavonoids, tannins and saponins13. Moringa seeds enter the diets of many animals, especially fish, as a functional protein and nutritional supplement or as substitutes for fishmeal or soybeans; it promotes growth and improves digestibility14,15.
This study was conducted due to the scarcity of other studies related to Moringa seeds on its use in the diets of common carp Cyprinus Carpio L., one of the most important, commercialized and consumed fish species in the world16. Hence the importance of shedding light on the knowledge of the effect of this plant on fish diets. The present study aims to show the impact of Moringa seed powder in the diets of common carp fish on some parameters of their growth.
MATERIALS AND METHODS
The experiment was conducted at the first agricultural research and experiment station, Umm Al-Akf area, Al-Muthanna Governorate, from 1/10/2120 to 20/12/2021, in dugout ponds, 45 m long, 35 m wide and 1.5 m deep, it was about 570 m away from the Euphrates River, Atshan river. The experiment used fish farming cages consisting of two rectangular pieces of wood, 244 cm long and 122 cm wide. Eight circles were drilled in each piece with a diameter of 45 cm in two parallel rows, and the circular holes in the wooden cages were filled by installing 15 clip-on plastic cylindrical troughs with a diameter of 45 cm and a depth of 65 cm.
A total of Seventy-five Common carp Cyprinus carpio L., with an average weight of 65.08±0.42 g, were used, distributed randomly and evenly to the experimental cages ( 5 fish in each tank).
Moringa seeds were bought from local markets; after drying well by the sun, it was ground using a home grinding machine, then a sample was drawn from them to know their chemical composition (Table 1). Then it was added to other experimental diet components and distributed to treatments at rates 0, 0.5, 1, 1.5 and 2%, respectively (Table 2).
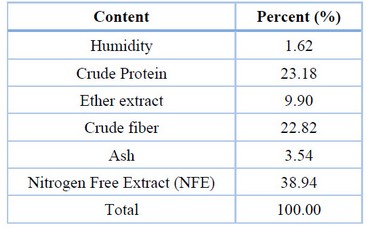
Table 1.The chemical composition of Moringa seeds.
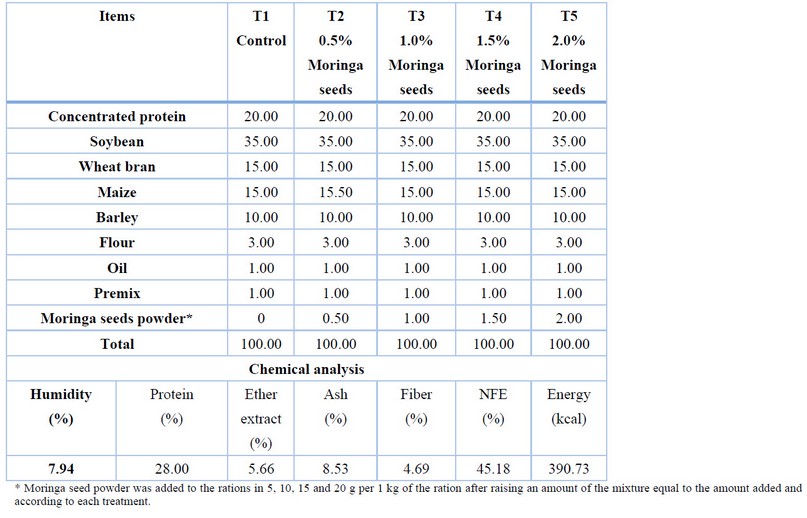
Table 2. The composition of the diets in the experiment.
Field experience
The experiment lasted for 82 days with localization; five different experimental formulas were used for the proportions of adding Moringa seed powder. The five experimental diets were T1: as the control diet, T2: 0.5%, T3: 1%, T4: 1.5% and T5: 2% Moringa seed powder, with a crude protein percentage of 28% in all diets, the experimental fish were fed 5% of their live weight, served four meals a day, the amount of feed was adjusted according to the periodic weight of the fish every ten days, weighing was done for the experimental fish, using a sensitive scale 500 g type DIGITAL SCALE, after drying it with a cotton cloth.
Growth parameters
Total weight gain (WG): WG = F.W-I.W (FW: Final weight, IW: Initial weight)
Daily Growth Rate (DGR): = (W2 – W1)/(T2 – T1) D.G.R
(W1: Initial weight, W2: final w eight (second), T2–T1: The duration of the trial or between the two weights).
Relative Growth Rate (RGR): RGR=(W2-W1)/W1×100
Specific Growth Rate (SGR): SGR=(Ln W2-LnW1)/(T2-T1)×100
Feed Conversion Ratio (FCR): FCR=(R(gm))/(G (gm))
(R: weight of food intake (gm), G: weight gain of fish (gm)).
Feed conversion efficiency (FCE): FCE=G/R×100
Protein Efficiency Ratio (PER): PER=(T.W.G)/(P.I)
(TWG: total weight gain (kg), PI: protein intake (kg)).
Statistical analysis
Randomized Complete Design (CRD) was used to study the effect of treatments on the studied traits; significant differences between means were tested using Duncan's17 multiple range test at a significance level of 0.05. The ready-made statistical program SPSS18 was used to analyze the data.
RESULTS
Table (3) shows that there were significant differences (P≤0.05) between the experimental treatments in the studied growth criteria, the fish of T3 significantly outperformed compare the other experimental treatments in the final weight, the highest rate was recorded and reached 186.80 g, the highest rate of weight gain was 121.78 g, it also recorded the highest daily growth rate of 1.74 g/day. T2 and T3 recorded the highest relative growth rate of 187.29 and 180.86%, respectively, and they recorded the highest specific growth rate of 1.47 and 1.50% / day for T2 and T3, respectively. The fish of T2 and T3 outperformed compare with the other experimental treatments on the feed conversion ratio, which amounted to 2.39 and 2.42 gm of feed/gm of weight gain, respectively, it also outperformed the feed conversion efficiency, which amounted to 41.38and 41.91% for T2 and T3, respectively, T2 and T3 were superiority on the percentage of protein efficiency, with values of 1.49 and 1.47 gm of weight gain/gm of protein intake, respectively.
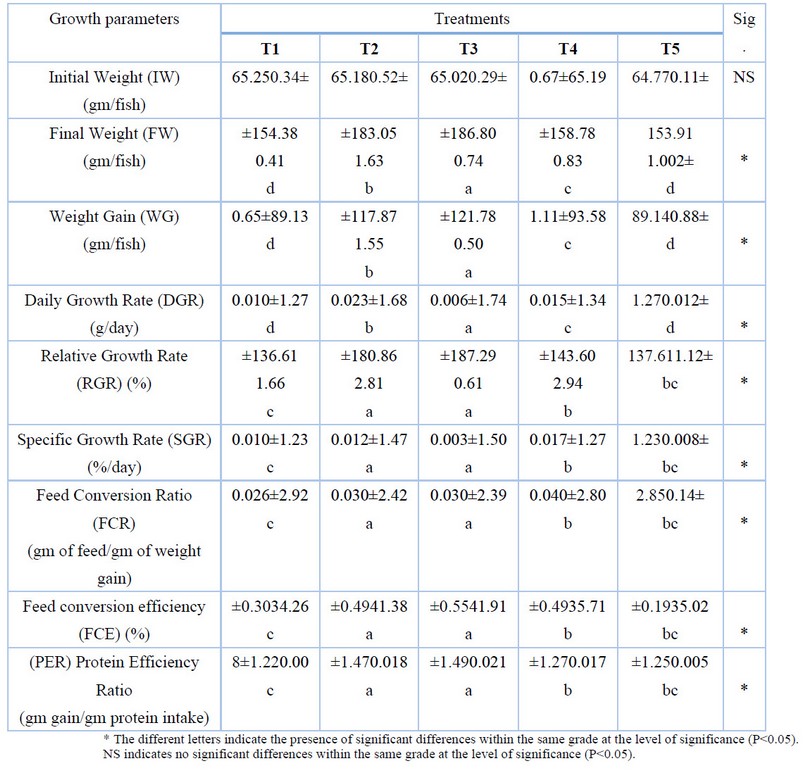
Table 3. Some growth parameters (mean ± standard error) of fish for experimental treatments.
DISCUSSION
The results in Table (3) show that the fishes of T2 and T3 treatments were superior in growth parameters (final weight, total weight gain, daily, relative and qualitative growth rates) compared to the other experimental treatments. The reason for this superiority and the positive results obtained may be due to the addition of Moringa at low and safe rates, have a clear impact on the growth parameters mentioned above, the chemical composition of Moringa seeds contains a good mix of essential amino acids important in the growth of the body, as well as being a good source of carbohydrates and raw fibers, it has a rich blend of vitamins such as vitamin C and E, and essential minerals such as calcium, phosphorous, magnesium and iron13,19. The high percentage of fat in it is one of the body's most important sources of energy, mainly unsaturated fatty acids, topped by oleic acid20. The results of the present study were close to the results of the study by Ayoola et al.21. These results were also in agreement with the findings of Yuangsoi and Masumoto22 in their study of common carp, which showed that the addition of Moringa leaves at 2%, or 20 g/kg, to the diets did not positively or negatively affect growth and digestion parameters.
Fish in treatments T2 and T3 were superior by recording the highest significant difference (P<0.05) in feed conversion ratio, feed conversion efficiency and protein efficiency ratio compared to other experimental treatments. We believe that the inclusion of moringa seed powder in the experimental diets at rates of 0. 5, and 1%, was the best in terms of its content of adequate, balanced and varied concentrations of nutrients, such as amino acids and active sub-stances essential for fish growth and health. It also enhanced the work and activity of the fish digestive system, improving feed digestion by stimulating the action of protein and fat-digesting enzymes, such as proteases, pepsin and lipases. It increased the ability to absorb and assimilate nutrients by increasing the length of the villi inside the intestine23. Thus, this leads to an improvement in the efficiency of feed consumption and conversion by increasing body weight and growth, as well as raising the digestibility and efficiency of the protein24, while the fifth treatment fish fed on a ration containing Moringa increased by 2%, the lowest values were obtained in the feed conversion ratio, feed conversion efficiency and protein efficiency ratio compared to the rest of the addition treatments, no significant differences were recorded between it and the control treatment, this may be due to the high levels of growth-inhibiting substances and anti-nutrients, with higher percentages of addition, as the presence of phytic acid in relatively high proportions, it has a direct effect on reducing the efficiency of the fish gut in splitting and digesting protein, thus, reducing the use of protein intake, during the formation of complexes with the protein as a result of its association with the enzyme trypsin, as well as the negative effect of phytic on reducing the availability and absorption of some nutrients necessary for growth25,26. We also believe that the reason is due to the lack of palatability of the feed due to the high concentration of saponins and alkaloids in diets containing relatively high percentages of Moringa seeds27,28. This irritates the lining of the mouth, leading to reduced appetite. In addition to the high fiber content in Moringa seeds, its presence at high rates hinders food consumption and digestion efficiency, creating a high bulk density within the intestines, and leading to reduced appetite29. The results of our current study were close to the findings of Akin-Obasola and Ajewole30; who showed that the addition of Moringa seed powder to African catfish rations in amounts of 6.25 and 9.37 g/kg of diet gave superiority in protein efficiency ratio and feed conversion ratio over the rest of the experimental treatments.
CONCLUSION
This study showed that Moringa seeds added to the diets of common carp fish at low rates, not exceeding 1.5%, had a positive and significant effect on all growth parameters studied in the experiment.
REFERENCES
1. FAO. The State of World Fisheries and Aquaculture: Contributing to Food Security and Nutrition For All, FAO Rome Italy 24, 2016. 200 pp.
2. UN. United Nations, Department of Economic and Social Affairs, Population Division. 2017. World Population Prospects. The 2017 Revision, Key Findings and Advance Tables. Working Paper No. ESA/P/WP/248. 2017.
3. Pradeepkiran, J.A. Aquaculture role in global food security with nutritional value: a review. Translational Animal Science, 2019. 3(2), 903-910.
4. Al Salman, N.T.Sh. and J.K.M. Al-Gharawi. Effect of Eucalyptus leaves water extract on some productive traits of broilers. Plant Archives Vol. 19, Supplement 1, 2019 pp. 920-923.
5. Gobi, N., Ramya, C., Vaseeharan, B., Malaikozhundan, B., Vijayakumar, S., Murugan, K., & Benelli, G. Oreochromis mossambicus diet supplementation with Psidium guajava leaf extracts enhance growth, immune, antioxidant response and resistance to Aeromonas hydrophila. Fish & shellfish immunology, 2016. 58, 572-583.
6. Gabriel, U. U., Akinrotimi, O. A., Bekibele, D. O., Onunkwo, D. N., & Anyanwu, P. E. Locally produced fish feed: potentials for aquaculture development in subsaharan Africa. African Journal of Agricultural Research, 2007. 2(7), 287-295.
7. Shahzad, M. M., Hussain, S. M., Akram, A. M., Javid, A., Hussain, M., Shah, S. Z. H., & Chaudhary, A. Improvement in nutrient digestibility and growth performance of Catla catla fingerlings using phytase in Moringa oleifera leaf meal based diet. Pak. J. Zool, 2020. 52(1):112-117.
8. Egwui, P. C., Mgbenka, B. O., & Ezeonyejiaku, C. D. Moringa plant and it use as feed in aquaculture development: a review. Animal Research International, 2013. 10(1), 1673-1680.
9. Adeshina, I., Sani, R. A., Adewale, Y. A., Tiamiyu, L. O., & Umma, S. B. Effects of dietary Moringa oleifera leaf meal as a replacement for soybean meal on growth, body composition and health status in Cyprinus carpio juveniles. Croatian Journal of Fisheries: Ribarstvo, 2018. 76(4), 174-182.
10. Quattrocchi, U. CRC World Dictionary of Plants Names Common Names, Scientific Names, Eponyms, Synonyms and Etymology., 3.CRC Press. 2000. P. 1731
11. Foidl, N., Makkar, H. P. S., & Becker, K. The potential of Moringa oleifera for agricultural and industrial uses. What development potential for Moringa products, 20. 2001.
12. Falowo, A. B., Mukumbo, F. E., Idamokoro, E. M., Lorenzo, J. M., Afolayan, A. J., & Muchenje, V. Multi-functional application of Moringa oleifera Lam. in nutrition and animal food products: A review. Food research international, 2018.106, 317-334.
13. Leon-Lopez, L., Escobar-Zuniga, Y., Milan-Carrillo, J., Dominguez-Arispuro, D. M., Gutierrez-Dorado, R., & Cuevas-Rodriguez, E. O. Chemical proximate composition, antinutritional factors content, and antioxidant capacity of anatomical seed fractions of Moringa oleifera. Acta universitaria, 30. 2020.
14. Yuangsoi, B., Klahan, R., & Charoenwattanasak, S. Partial replacement of protein in soybean meal by Moringa seed cake Moringa oleifera in bocourti's catfish Pangasius bocourti. Songklanakarin J. Sci. Technol, 2014. 36(2), 125-135.
15. Akangbe, E.E. Utllisation of Moringa oleifera lam. Seed as Protein source in the diets of Broiler Chickens (Doctoral dissertation). 2017.
16. FAO How to feed the world in 2050. Proceedings of the Expert Meeting on How to Feed the World in 2050. FAO Headquarters, Rome. 2009.
17. Duncan٫ D.B. Multiple rang and multiple F test. Biometrics٫ 1955. 11-19.
18. SPSS. SPSS users guide. Statistics version 20. Statistical Package Solution Service. 2012.
19. Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J., & Bertoli, S. Moringa oleifera seeds and oil: Characteristics and uses for human health. International Journal of Molecular Sciences, 2016. 17(12), 2141.
20. Barakat, H., & Ghazal, G. A. Physicochemical properties of Moringa oleifera seeds and their edible oil cultivated at different regions in Egypt. Food and Nutrition Sciences, 2016. 7(06), 472.
21. Ayoola, S. O., Kuton, M. P., & Shokefun, O. O. Evaluation of Nutritional Quality and Haematological Parameters of Moringa (Moringa oleifera) Lam Leaves in the Diet of African Catfish (Clarias gariepinus). Agrosearch, 2013. 13(1), 1-16.
22. Yuangsoi, B., & Masumoto, T. Replacing moringa leaf (Moringa oleifera) partially by protein replacement in soybean meal of fancy carp (Cyprinus carpio). Songklanakar in Journal of Science & Technology, 2012. 34(5:220-226.
23. Heidarieh, M., Mirvaghefi, A. R., Sepahi, A., Sheikhzadeh, N., Alishahbazfar, A., & Akbari, M. Effects of dietary Aloe vera on growth performance, skin and gastrointestine morphology in rainbow trout (Oncorhynchus mykiss). Turkish Journal of Fisheries and Aquatic Sciences, 2013. 13(2): 115-122.
24. Karthivashan, G., Arulselvan, P., Alimon, A., Safinar Ismail, I., & Fakurazi, S. Competing role of bioactive constituents in Moringa oleifera extract and conventional nutrition feed on the performance of Cobb 500 broilers. BioMed research international, 2015.
25. Hussain, S. M., Shahzad, M. M., Afzal, M., Javid, A., Mubarik, M. S., Shah, S. Z. H., ... & Riaz, D. Efficacy of phytase enzyme for increasing mineral digestibility of Cirrhinus mrigala fingerlings fed on soybean meal-based diet. Pakistan J. Zool, 2015. 47(6), 1807-1816.
26. Lei, X. G., Weaver, J. D., Mullaney, E., Ullah, A. H., & Azain, M. J. Phytase, a new life for an "old" enzyme. Annu. Rev. Anim. Biosci., 2013. 1(1), 283-309.
27. Al-Bayar, M. A., Abdulateef, S. M., Farhan, S. M., Shawkat, S. S. & Mohammed, Th. T. Role of Nitroglycerine injection in Japanese Quail (Coturnix japonica) testes tissues parameters. Indian Journal of Ecology. 2020, 47 (10): 251-255.
28. Anton, R., & Bernard, M. 2003. Plantes thérapeutiques: tradition, pratique officinale, science et thérapeutique. Édition Lavoisir, Paris. pp. 38–41.
29. Adewumi, A. A. Moringao leifera (Lam) as a Protein Supplement in Clariasgariepinus Diet. Advances in Research, 2014. 2(11), 580-589.
30. Akin-Obasola, B. J., & Ajewole, S. Nutrient Composition, Haematology and Growth Performance of Clarias gariepinus Post Juvenile fed Moringa oleifera Seed Meal. Journal of Children in Science and Technology (Jocist), 2018. 11(1), 19-24.
Received: July 20, 2022 / Accepted: October 15, 2022 / Published:15 November 2022
Citation: Al-Rawashi, J.B.Z.; Salman, A.H.; Al-Gharawi, J.K. Effect of different levels of Moringa oleifera seed powder to the diet on some growth parameters of common carp Cyprinus carpio L. Revis Bionatura 2022;7(4) 25. http://dx.doi.org/10.21931/RB/2022.07.04.25
