2023.08.01.53
Files > Volume 8 > Vol 8 No 1 2023
Evaluation of serum Interleukin 36 in Iraqi patients with Rheumatoid arthritis
1,2 Department of Biology, College of Science, Mustansiriyah University, Baghdad, Iraq
3 College of Medicine, University of Baghdad, Baghdad, Iraq
Corresponding author email : [email protected], [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.53
ABSTRACT
Rheumatoid arthritis is a worldwide inflammatory chronic autoimmune disease with varying severity. Due to no definitive cure for this disease, current therapies aim to decrease the pain and slow further damage. The interleukin (IL)‐36 cytokine was little known for its role in rheumatoid arthritis; this research aimed to evaluate the serum IL36 levels in RA patients compared to healthy controls. This study included 80 patients with rheumatoid arthritis registered at the Rheumatology Clinic in Baghdad teaching hospital. The patients were divided into three groups based on the treatments received. Group 1 included patients treated with biological therapy (etanercept, adalimumab), Group2 patients with non-biological treatment (methotrexate hydroxychloroquine and prednisone), Group3 patients without any treatment and compared with Group 4 healthy control group. Patients is all groups were assessed for their serum IL-36 concentration; the mean IL-36 serum level was significantly higher in three groups of RA patients which include the group of patients treated with biological therapy (Enbrel (etanercept) and Humira (adalimumab) means were (1132.41±475.2,), and group of non-biological therapy patients (Methotrexate hydroxychloroquine and prednisone) (G2) means was 553.95±307, than patients' group without any treatment (G3) means was 1044.01±575.3 compared to the control (341.38±113.1) p-value> 0.00001. The patient's age and BMI were not significantly different between three groups of patient Rheumatoid arthritis. Parameters for this disease also were tested which include RF, CRP, ESR, anti-CCP and disease activity score-28 (DAS 28), there were significant differences when compared with the control group. IL-36 serum level was significantly higher in three groups of rheumatoid arthritis than those in controls, and when compared between three patients groups there was less concentration in the non-biological therapy treatment group means was 553.95±307 than in the rest of the patient groups,biology tratment, without any treatment, means were (1132.41±475.2, 1044.01±575.3) respectively. This study found that Rheumatoid arthritis patients' serum IL36 levels increased, where a non-biologic therapies reduced this cytokine. IL-36's pathogenic involvement in Rheumatoid arthritis needs more study.
Keywords: Rheumatoid arthritis, IL-36, IL-1,C‐reactive protein, RF, ESR and anti-CCP.
INTRODUCTION
Rheumatoid arthritis (RA) is a worldwide inflammatory chronic autoimmune disease with varying severity, and due to no definitive cure for this disease, the goals of current therapies are to decrease the pain and slow further damage1. Rheumatoid arthritis affects small joints after that, larger joints, finally leading the tendons and ligaments weaken, and the cartilage and bone of joints are distortion and erosion which cause severe pain for a patient, and affects on heart, kidneys, lungs skin, and eyes2. Commonly autoimmune diseases are characterized by an excessive immune response and cause deterioration of specific or multiple tissues and organs 3 , and generally believed that cytokines implicated in each phase of the pathogenesis of RA, such as IL-18, IL-17, IL-16, IL-12, IL-10, IL-8, IL-7, IL-6, IL-1, IFN-gamma, etc4. Interleukin 36 is an inflammatory cytokine, a member of the IL1 family, composed of agonists IL36α, IL36β, IL36γ, antagonist IL-36 receptor IL36Ra and accessory protein (IL-1RAcP)5, chromosome 2 carries the genes of the IL-36 family6, expressed and act on the barrier sites of the body on a variety of cells including epithelial ( synoviocytes, keratinocytes, and skin, lung, and gut cells) and immune cells (T lymphocytes, antigen-presenting cells)7. IL-36 cytokines are regulated immune responses in a specific tissue; the upregulation of IL36 through CD80, CD86, MHCII of dendritic cells induces the production of several proinflammatory cytokines3 such asIL1β, IL6, IL8, IL17, and TNFα and IFNγ in the pathogenesis of inflammatory diseases, in lung tissue, joint synovium (arthritis), colonic mucosa tissues ( bowel diseases)and skin lesion 8and may have diagnostic and/or therapeutic relations with inflammatory diseases7. In RA patients, IL-36α, IL-36R, and IL-36Ra were detected in the synovial tissues and may be correlated with its pathogenesis8,6. In the synovium of RA patients, IL-36α, IL-36β, IL-36γ, and IL-36Ra are correlated and upregulated with the expression of IL-1β, Chemokine ligand 3, Chemokine ligands 4 and Macrophage colony-stimulating factor8, detected the IL-36 cytokines in the synovium of RA patients which induce production of proinflammatory mediators by synovial fibroblasts. Still, there were no effects when the blockade of IL-36 receptors in the arthritis of mouse models the production of proinflammatory mediators by synovial fibroblasts9. Found the circulating IL-36 levels were significantly higher among Juvenile Idiopathic Arthritis children10. The present study examined the serum concentration of IL-36 in rheumatoid arthritis patients.
MATERIALS AND METHODS
All serum samples of 80 rheumatoid arthritis patients (11 males and 69 females) who attended the Rheumatology Clinic in Baghdad teaching hospital and diagnosis was under the supervision of Dr. Muhammad Hadi Al-Assami, Consultant Rheumatologist, according to the revised American College of Rheumatology 2010 criteria, based on 4 factors which are the distribution of affected joints number tender joint and swollen, Serology test results rheumatoid factor (RF), anti-cyclic citrullinated protein antibody (ACCP), Acute-phase reactant test results( such as ESR, CRP etc.), the duration of symptoms, the score >6weak constitutes a classification of RA
Clinicians used criteria to diagnose rheumatoid arthritis. Presence of at least one clinical tumor in the participant), up to three points are assigned depending 11; it was done by the consultant medical staff at the clinic, in addition to using the kits for laboratory tests to confirm the presence of arthritis such as anti-cyclic citrullinated peptide antibodies (anti-CCP) kit (commercially available kits by indirect enzyme-linked immunosorbent assay MyBioSource, USA), rheumatoid factor (RF) and C-reactive protein (CRP) kits (latex slide agglutination tests by Agappe Diagnostics Switzerland GmbH for semi-quantitative detection). The serum concentrations of IL-36 were measured by using the Competitive ELISA kit (MyBioSource, USA, sensitivity = 1.0 ng/ml)) according to the manufacturer's instructions.
Disease Activity Score were measured based on the estimation of 28 of joints (DAS28) with the number of swollen joints and erythrocyte sedimentation rate (ESR)(by the Westergren method), and it was calculated using the following equation:
DAS 28 = 0.56 × √(tender 28 joint count) + 0.28 × √(swollen 28 joint counts) + 0.70 × ln(erythrocyte sedimentation rate (ESR), mm/hr) + 0.014 × general health12,13,14,
after obtaining all patients' data, patients were categorized into patients treated with biological treatment(Enbrel (etanercept) and Humira (adalimumab)G1(38), non-biological treatment (Methotrexate hydroxychloroquine and prednisone) G2 (26), and without treatment G3 (16), also patients identified 2 specific groups: first, patients with positive anti-CCP test and second, patients who had the negative anti-CCP test. While 36 healthy control group G4 individuals were selected according to strict conditions, they didn't suffer from any disease symptoms and were negative for CRP, RF, ESR and anti-CCP tests.
Patients and control individuals were obtained under the Ministry of Health and Mustansiriyah University approved protocols, informed consent was obtained from the ministry of health, and a written all participants acceptance.
Statistical analysis
The statistical analyses were performed using the SPSS 20 (2022))Statistical Package For the Social Sciences, also known as IBM SPSS Statistics) analysis of variance (ANOVA) test to compare various groups with each other for numerical variables, which were expressed as mean + standard error (SE), and the LSD test was used to calculate the significant differences between the tested mean, the letters (A, B, C, and D for column and a, b, c and d rows) represented the levels of significant, highly significant start from the letter (A or a) and decreasing with the last one. Similar letters mean no significant differences between the tested mean; all data showed normal distribution using the Shapiro-Wilk test. The categorical variables were expressed as a percentage and used chi-square tests of goodness of fit and independence.
RESULTS
The demographic and clinical features of the 80 patients with rheumatoid arthritis included in this study are displayed in Table 1 according to the above-mentioned division in the materials and methods. There were no significant differences observed in the age at sample collection between the patients with RA and the healthy control group (P > .05). The mean age of the three patient groups: treated with the biological treatment group was 43.8±2.3, the non-biological treatment group was 46.1±2.2, and the non-treatment group was 40.3±3.2 and36 healthy controls with a mean age of 46.7±2.9 years (Table 1).
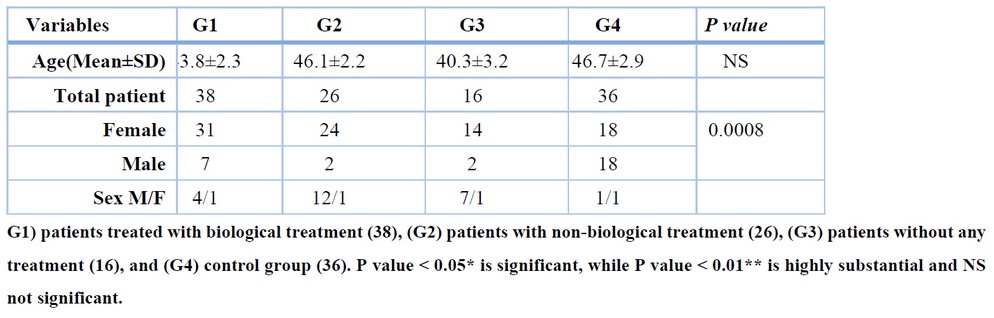
Table 1. Demographic characteristics of RA patients and healthy controls
The selection of patients, according to what was mentioned in the materials and methods, from patients who visit a specialized center for rheumatic diseases and who were diagnosed according to the revised American College of Rheumatology 2010 criteria by specialists and the general parameters for this disease also were tested which include RF, CRP, ESR, anti-CCP and disease activity score-28 (DAS 28), and there were significant differences when compared with the control group because the latter was chosen with great caution table 2. It excluded anyone who gave a positive result to any of those above to confirm that the control group members were free from RA table 2.
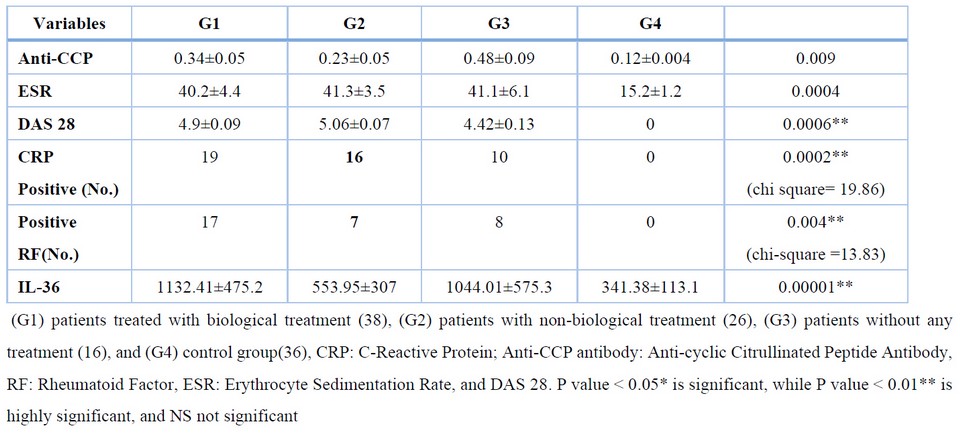
Table 2. Laboratory characteristics of the rheumatoid arthritis
The mean IL-36 serum level was significantly higher in three groups of RA patients which include the group of patients treated with biological therapy (Enbrel (etanercept) and Humira (adalimumab)) (G1), the group of non-biological therapy patients (Methotrexate hydroxychloroquine and prednisone) (G2), and the patients' group without any treatment (G3) (1132.41±475.2, 553.95±307, 1044.01±575.3) respectively, compared to the control (341.38±113.1), (Fig. 1).
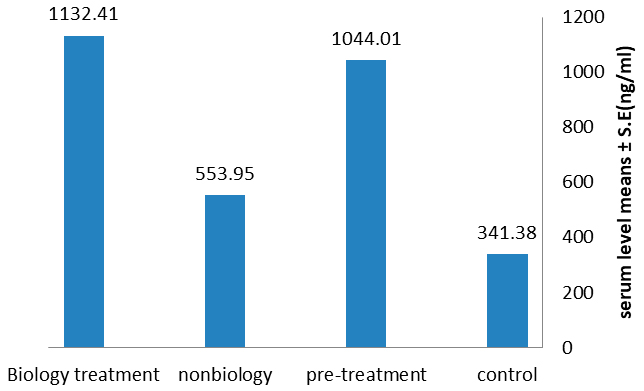
Figure 1 . Distribution of serum levels IL 36 in three patients versus controlIL-36 concentration in the studied groups; patients treated with biological treatment, non-biological treatment, pre-treatment, and control group.
DISCUSSION
There were significant differences between patients with rheumatoid arthritis and normal controls in terms of sex (Table I); the ratio of female to male was 6:1 in this study, and in general, according to previous studies, women are about three times more likely to suffer from this disease, and the effect of the disease is also different between the two sexes, and this may be due to physiological differences between the sexes, such as the difference in hormonal content, the difference in behavior, and the role of genes and heredity 15,16. RA can be triggered by the production of autoantibodies (anti-citrullinated protein antibodies ACPA) against citrullinated peptides which are distributed throughout the whole body that activate MHC class II-dependent T cells that induce B cells to produce more ACPA 17. The mean IL-36 serum level was significantly higher in three groups of RA patients, these results are in line with a previous study, soon published, where it was found that interleukin-36 increases in patients with Juvenile Idiopathic Arthritis10, High levels of interleukin36 were observed in patients and mouse models with osteoarthritis through joint destruction and found that transforming growth factor-beta (TGF-β) receptor type 2 signaling dampened IL-36 signaling in healthy joints18, found that IL-36 upregulated in the synovial tissue of patients with RA and they added that particularly linked to the inflammatory processes in the synovial tissue through promotes the expression of proinflammatory cytokines19, this confirmed by found that the serum IL-36 higher in SLE patients with arthritis than in those without arthritis20, IL-36 acts as a proinflammatory by magnifying inflammatory responses and triggering further inflammatory mediators and causing excessive immune infiltration and tissue damage 21,22 Also noted in table 1 interleukin-36 was less concentrated in the non-biological therapy (hydroxychloroquine and prednisone) treatment group (553.95±307) than the rest of the patient groups (1132.41±475.2, 1044.01±575.3) figure 1, (methotraxate ,prednisone) decreases inflammation and suppresses the immune system through binding with specific nuclear receptors and cause altered gene expression and inhibition of proinflammatory cytokine production23 while hydroxychloroquine treatment prevents trained immunity Many causes depend on the severity of the disease, the stage of the disease , the effect of the treatment and the dose, the physiological factors, the age 24 and this confirmed recently by Devarajan and Vaseghi in their study that hydroxychloroquine impair host immunity in response to SARS-CoV-2 25 and that demonstrates this results.
According to previous articles, the uses of these two treatments lead to a decrease in the production of inflammatory cytokines; prednisone has a significant effect on inhibiting the immune response through its effect on immune cells and also can reduce the concentration of inflammatory cytokines 26and hydroxychloroquine is an antimalarial drug and, is now used as an immunomodulator agent for rheumatic autoimmune disorders, such as primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis through the inhibition of antigen presentation, B- and T-cell activation, NOX signaling and rebalances Treg/Th17 cell ratio and these effects on different immune cells cause decreased in production and release of proinflammatory cytokines27,28.
CONCLUSIONS
This study demonstrated that RA patients displayed increasing in serum IL‐36 and it was also found that non-biological treatments have a role in reducing this cytokine. Further studies are needed to explain a more detailed IL-36 pathogenic role in RA.
Abbreviations: Rheumatoid arthritis (RA), Anti-Cyclic Citrullinated Peptide antibodies (Anti-CCP), anti-citrullinated protein antibodies (ACPA), Disease active score (DAS 28), Cluster of Differentiation (CD), C-Reactive Protein (CRP), Interleukin (IL), Major Histocompatibility Complex (MHC), Rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), Systemic Lupus Erythematosus (SLE), Intreleukin-1 receptor accessory protein (IL-1RAcP)
Funding
No fund received: for this article.
Informed consent to publish
Not applicable.
Ethical statements for human/animal experiments
The study was approved by institutional ethics committee "University of Mustansiriyah" and informed consent was obtained in written by each individual participants. Each participant was known about the study follow up before enrolling for the study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments: We thank Mustansiriyah University in Baghdad /Iraq) for its support to achievement this work.
REFERENCES
1. Bullock J, Rizvi SA, Saleh AM, Ahmed SS, Do DP, Ansari RA, Ahmed J. Rheumatoid arthritis: a brief overview of the treatment. Medical Principles and Practice. 2018;27(6):501-507. https://doi.org/10.1159/000493390
2. Lee JE, Kim IJ, Cho MS, Lee J. A case of rheumatoid vasculitis involving hepatic artery in early rheumatoid arthritis. Journal of Korean medical science. 2017 Jul 1;32(7):1207-10.
3. Chen WJ, Yu X, Yuan XR, Chen BJ, Cai N, Zeng S, Sun YS, Li HW. The Role of IL-36 in the Pathophysiological Processes of Autoimmune Diseases. Frontiers in Pharmacology. 2021:2643..https://doi.org/10.3389/fphar.2021.727956
4. Burska A, Boissinot M, Ponchel F. Cytokines as biomarkers in rheumatoid arthritis. Mediators of inflammation. 2014 Mar 9;2014, 545493. https://doi.org/10.1155/2014/545493.
5. Zhou L, Todorovic V. Interleukin-36: structure, signaling and function. Protein Reviews. 2020:191-210.
6. Yuan ZC, Xu WD, Liu XY, Liu XY, Huang AF, Su LC. Biology of IL-36 signaling and its role in systemic inflammatory diseases. Frontiers in immunology. 2019:2532.
7. Buhl AL, Wenzel J. Interleukin-36 in infectious and inflammatory skin diseases. Frontiers in Immunology. 2019:1162.
8. Ding L, Wang X, Hong X, Lu L, Liu D. IL-36 cytokines in autoimmunity and inflammatory disease. Oncotarget. 2018 Jan 5;9(2):2895.
9. Schmitt V, Hahn M, Kästele V, Wagner O, Wiendl M, Derer A, Taddeo A, Hahne S, Radbruch A, Jäck HM, Schuh W. Interleukin‐36 receptor mediates the crosstalk between plasma cells and synovial fibroblasts. European journal of immunology. 2017 Dec;47(12):2101-12.
10. Jamal QW, Alubaidi G, Humadi Y. Level of Interleukin-35, Interleukin-36, and the Interleukin-35/Interleukin-36 Ratio in Juvenile Idiopathic Arthritis. Open Access Macedonian Journal of Medical Sciences. 2021 Sep 2;9(A):741-7.
11. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham III CO, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & rheumatism. 2010 Sep;62(9):2569-81.
12. Prevoo ML, Van'T Hof M, Kuper HH, Van Leeuwen MA, Van De Putte LB, Van Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 1995 Jan;38(1):44-8.
13. Balsa A, Carmona L, González-Alvaro I, Belmonte MA, Tena X, Sanmartí R, EMECAR Study Group. Value of Disease Activity Score 28 (DAS28) and DAS28-3 compared to American College of Rheumatology-defined remission in rheumatoid arthritis. The Journal of rheumatology. 2004 Jan 1;31(1):40-6.
14. Schmitt V, Hahn M, Kästele V, Wagner O, Wiendl M, Derer A, Taddeo A, Hahne S, Radbruch A, Jäck HM, Schuh W. Interleukin‐36 receptor mediates the crosstalk between plasma cells and synovial fibroblasts. European journal of immunology. 2017 Dec;47(12):2101-12.
15. Nourisson C, Soubrier M, Mulliez A, Baillet A, Bardin T, Cantagrel A, Combe B, Dougados M, Flipo RM, Schaeverbeke T, Sibilia J. Impact of gender on the response and tolerance to abatacept in patients with rheumatoid arthritis: results from the 'ORA'registry. RMD open. 2017 Nov 1;3(2):e000515.
16. Intriago M, Maldonado G, Cárdenas J, Ríos C. Clinical characteristics in patients with rheumatoid arthritis: differences between genders. The Scientific World Journal. 2019 Jul 3;2019.
17. Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone research. 2018 Apr 27;6(1):1-4.
18. Li T, Chubinskaya S, Esposito A, Jin X, Tagliafierro L, Loeser R, Hakimiyan AA, Longobardi L, Ozkan H, Spagnoli A. TGF-β type 2 receptor–mediated modulation of the IL-36 family can be therapeutically targeted in osteoarthritis. Science translational medicine. 2019 May 8;11(491):eaan2585.
19. Frey S, Derer A, Messbacher ME, Baeten DL, Bugatti S, Montecucco C, Schett G, Hueber AJ. The novel cytokine interleukin-36α is expressed in psoriatic and rheumatoid arthritis synovium. Annals of the rheumatic diseases. 2013 Sep 1;72(9):1569-74.
20. Mai SZ, Li CJ, Xie XY, Xiong H, Xu M, Zeng FQ, Guo Q, Han YF. Increased serum IL-36α and IL-36γ levels in patients with systemic lupus erythematosus: association with disease activity and arthritis. International immunopharmacology. 2018 May 1;58:103-8.
21. Aoyagi T, Newstead MW, Zeng X, Nanjo Y, Peters-Golden M, Kaku M, Standiford TJ. Interleukin-36γ and IL-36 receptor signaling mediate impaired host immunity and lung injury in cytotoxic Pseudomonas aeruginosa pulmonary infection: Role of prostaglandin E2. PLoS pathogens. 2017 Nov 22;13(11):e1006737.
22. Hahn M, Frey S, Hueber AJ. The novel interleukin-1 cytokine family members in inflammatory diseases. Current opinion in rheumatology. 2017 Mar 1;29(2):208-13.
23. Heming N, Sivanandamoorthy S, Meng P, Bounab R, Annane D. Immune effects of corticosteroids in sepsis. Frontiers in Immunology. 2018:1736.
24. Devarajan A, Vaseghi M. Hydroxychloroquine can potentially interfere with immune function in COVID-19 patients: Mechanisms and insights. Redox Biology. 2021 Jan 1;38:101810.
25. Rother N, Yanginlar C, Lindeboom RG, Bekkering S, van Leent MM, Buijsers B, Jonkman I, de Graaf M, Baltissen M, Lamers LA, Riksen NP. Hydroxychloroquine Inhibits the trained innate immune response to interferons. Cell Reports Medicine. 2020 Dec 22;1(9):100146.
26. Shams S, Martinez JM, Dawson JR, Flores J, Gabriel M, Garcia G, Guevara A, Murray K, Pacifici N, Vargas MV, Voelker T. The therapeutic landscape of rheumatoid arthritis: current state and future directions. Frontiers in Pharmacology. 2021 May 28;12:1233.
27. Mohammadpour F, Kargar M, Hadjibabaie M. The role of hydroxychloroquine as a steroid-sparing agent in the treatment of immune thrombocytopenia: a review of the literature. Journal of research in pharmacy practice. 2018 Jan;7(1):4.
28. Nirk EL, Reggiori F, Mauthe M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO molecular medicine. 2020 Aug 7;12(8):e12476.
Received: December 30, 2022 / Accepted: February 20, 2023 / Published:15 February 2023
Citation: Mohammed W T, Mahmood Alubadi A E, Munshed Alosami M H. Evaluation of serum Interleukin 36 in Iraqi patients with Rheumatoid arthritis. Revis Bionatura 2023;8 (1) 53. http://dx.doi.org/10.21931/RB/2023.08.01.53
