2022.07.02.39
Files > Volume 7 > Vol 7 No 2 2022
Middle Technical University, Medical Technical Institute –Baghdad.
*Correspondence author: Abeer J. Hassan [email protected],
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.39
ABSTRACT
The current work was done to determine the correlation between vitamin D and cystatin C as a predictor of kidney disease in males with diabetes mellitus. A total of 60 males were taken from October to December 2018. They were divided into 30 patients with type 2 diabetes and 30 healthy volunteers' as a control group. Glycated hemoglobin HbA1c, plasma glucose level, creatinine and urea were measured for all subjects. Also, levels of 25(OH) D and cystatin-c were determined using the enzyme-linked immune sorbent assay method. In this current study, as expected, patients with diabetes mellitus had significantly higher (p< 0.001) fasting blood glucose (FBG), body mass index (BMI), and glycated hemoglobin (HbA1c) levels as compared to the age-matched controls group. In addition, a significantly higher increase in the average level of urea, creatinine, and cystatin-c while significant higher decreases in vitamin D concentration compared to the patients group with the control group were found. A significant negative correlation was found between 25(OH) D and urea levels.
On the other hand, a significant positive correlation was observed between the 25(OH) D level and HbA1c, FBG, cystatin-c and creatinine. From the results of this work, vitamin D could be a valuable predictor of nephropathy in males with diabetes mellitus. Likewise, further work is required to suggest that vitamin D may be prone to nephropathy in all patients with diabetes mellitus by estimating cystatin C as a clinical risk for kidney function.
Keywords: Nephropathy, cystatin C, vitamin D.
INTRODUCTION
Nephropathy is a crucial chronic complication of type 2 and type 1 diabetes mellitus and is considered the first cause of chronic kidney disease worldwide. A third of people with diabetes will develop nephropathy after fifteen years of diagnosis1. Many causes such as genetic variability, diet and lifestyle have a role in developing nephropathy. Diabetes stimulates various biochemical, metabolic, and changes in the hemodynamics of kidneys. Major pathways to nephropathy include activation of polyol pathway, glomerular hyperfiltration, protein kinase C, oxidative stress by reactive oxygen species and advanced glycation end products2. Several markers can be an early predictor of renal damage, such as cystatin-c, nephrin, β-microglobulin and α-microglobulin in diabetes mellitus, both type 2 and type 13. Vitamin D belongs to fat-soluble steroid hormones, has a protective function against diabetes and cardiovascular disease, and regulates renal function, mineral metabolism, and immunity. Calcitriol can be hydroxylated in the liver to main form 25(OH) D then converted to 1, 25(OH) 2 D active form of VD in proximal renal tubules4. 25(OH) D is considered a precursor of the vitamin D active form, but when there is a very high vitamin D concentration, it will bind to the vitamin D receptor (VDR). The vitamin D receptor (VDR) is a nuclear receptor for 1-α, 25-dihydroxy vitamin D, an active metabolite of vitamin D, and its receptor contributes to inflammatory pathways. VDR was founded in the glomerular parietal epithelium, proximal tubular epithelium and podocytes in kidneys5. They decrease the vitamin D levels associated with insulin resistance and diabetes mellitus.
Therefore glucose concentration, renal function and obesity are risk factors for deficiency of vitamin D. Oxidative stress and inflammation are related to diabetic nephropathy pathogenesis and regulated by VDR6. Plasma 25 (OH) D concentrations are used to evaluate VD status. The reason is that 25(OH) D, which has a long half-life (about two weeks) and 25-hydroxylation, has not been under efficient metabolic control2. Cystatin C is a protein that is liberated from nucleated cells at a constant rate and is not affected by age, muscle mass, or gender and is excreted exclusively by glomerular filtration. Cystatin C is a more accurate marker to detect mild levels of kidney dysfunction and glomerular filtration rate (GFR) than creatinine7. Some studies have shown that cystatin C levels are stronger predictors of acute renal injury; increased cystatin C concentrations indicate impairment in GFR, renal dysfunction, and pathophysiological processes8, 9. The current study aimed to determine the correlation between vitamin D and cystatin C as predictors of kidney disease in males with diabetes mellitus.
MATERIALS AND METHODS
Sixty males were enrolled in "The National Diabetic Center (NDC) of Al-Mustansiriya University" from October to December 2018. They were divided into two groups 30 patients with type 2 diabetes with a mean age (of 47 ± 0.31) years and 30 healthy subjects as a control group with mean age (46± 2.99) years. Excluding patients with infection in the urinary tract, kidney failure, stage of heart failure, thyroid disease, and uncontrolled hypertension was done. A blood sample from all subjects was drawn to measure glycated hemoglobin HbA1c10, plasma glucose level11, creatinine12 and urea13. Levels of 25(OH) D and cystatin-c were also determined by ELISA technique14. And body mass index BMI was calculated for each male in the patient and control group.
Statistical test
All study data were submitted as (mean ± SD). Student's T-test was applied to compare patient groups with the healthy group; also, Pearson's coefficient was used for correlation analysis.
RESULTS
Results in table 1 summarized the anthropometric and metabolic characteristics of all participants. The average age of the volunteer group (46±2.99) was similar to the average age of the patient group (47±0.31); therefore, no significant difference was noticed when compared studied groups. As expected, patients with diabetes mellitus had significantly higher (p< 0.001) BMI, fasting blood glucose (FBG), and glycated hemoglobin (HbA1c) levels as compared to the controls group were found. Table 2 showed a highly significant increase in the average level of urea, creatinine, and cystatin-c and a highly significant decrease in vitamin D concentration compared to the patients group with the control group.
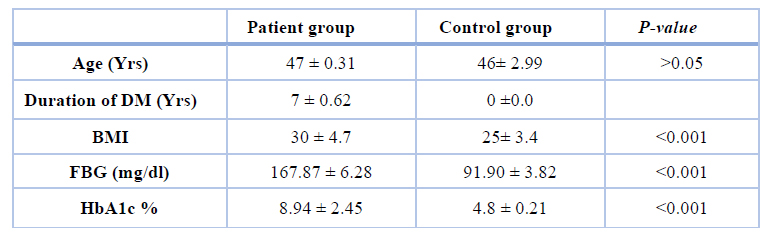
Table 1. Clinical characteristics of the patients and control groups.
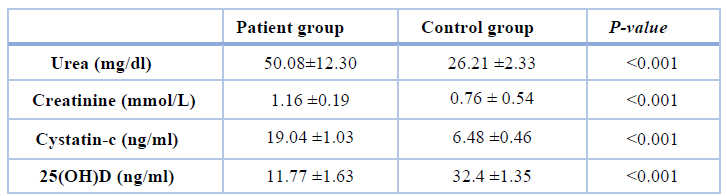
Table 2. Kidney biomarkers levels and VD concentration in all studied groups
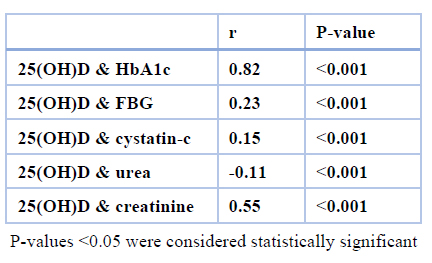
Table 3. Correlate vitamin D with some variables.
DISCUSSION
Research on vitamin D's effect on decreasing the complication of diabetes mellitus on kidney function is limited. So the objective of the present work was to illustrate the vitamin D relationships with some parameters such as cystatin C, urea and creatinine that measured renal function efficiency in diabetic Mellitus patients to avoid their development of nephropathy diabetes. In this study, the duration of diabetes mellitus in the patient's group was (7 ± 0.62) years, so these patients were at risk of nephropathy diabetes a few years later. As expected, the patient's group showed a highly significant increase in urea, creatinine and cystatin C concentration when compared with the control group. Our results are in agreement with other studies15-17. Creatinine is the blood depends on creatinine production, tubular handling, and kidney elimination. Some tubular proteins and enzymes are detectable in rising serum creatinine even before microalbuminuria, so tubular involvement may outstrip glomerular involvement.
On the other hand, cystatin C is a biomarker for determining kidney functions. It could be another tubular factor that clarifies the renal state in patients with diabetes and is considered a tool for the early detection of diabetic nephropathy18. As for the vitamin D concentration in our study's diabetes mellitus male group, the results indicated a highly significant decrease in 25(OH) D levels compared to the healthy male group. Also, a recent study has proposed a positive correlation between VD and cystatin C, urea, and creatinine. There is no available data in previous studies to find a relationship between vitamin D and cystatin C to predict the status of renal functions in diabetes mellitus male penitents.
The mechanisms of relation vitamin D deficiency with diabetes mellitus remain incompletely understood; maybe high glucose levels increase the synthesis of the angiotensin II hormone, which affects the contractile arteriole of the glomerulus. So, hemodynamic changes distinct by high filtration, high pressure and hypertransfusion in the early stage of diabetes mellitus will induce diabetes nephropathy development4. Also, 1, 25(OH) D increases the transcription of insulin receptor genes and suppresses the renin gene, reducing hyperglycemic induced increases in renin levels in pancreatic β cells9. Vitamin D in the blood binds to serum proteins either to D-binding protein or albumin to carry out its function. In diabetes mellitus patients, when microalbumin is lost with urine, that will decrease the concentration of serum albumin and explain decreasing in protein synthesis besides the significant loss in urinary albumin, which affects serum 25 (OH) D concentrations19.
CONCLUSION
Depending on the results of this work, vitamin D could be a valuable predictor of nephropathy in males with diabetes mellitus. Likewise, further work is required to suggest that vitamin D may be prone to nephropathy in all patients with diabetes by estimating cystatin C levels as a clinical risk for kidney function.
Author Contributions: collecting samples; Dunya NA Ahmed, investigation; Sarmad A Hazzaa; review and editing; Abeer J Hassan.
Funding: This research received no external funding.
Institutional Review Board Statement: The study was approved by the ethics committee of the Iraqi minister of health, and the study followed the principles stated in the Declaration of Helsinki.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Acknowledgments: to all subject those participants in this study.
Conflicts of Interest: The authors declare no conflict of interest
REFERENCE
1. Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018. Am J Kidney Dis. 2018; 71(6):884-895.
2. Mrunalini KC, Mahmoud IM, Tahir H, Mohammed J, Aadil SI. Vitamin D and its analogs in type 2 diabetic nephropathy: a systematic review. Journal of Diabetes & Metabolic Disorders 2015; 14:58: 2-10.
3. Zhang J, Liu J, Qin X. Advances in early biomarkers of diabetic nephropathy. Rev Assoc Med Bras. 2018; 64(1):85-92.
4. Min Zhang, Tiejun Liu, Wenge Li, Weijun G., Xusheng Y., Jianing Xi. Efficacy of vitamin D3 in patients with diabetic nephropathy: an updated meta-analysis. Iran Red Crescent Med J. 2017; 19(12):e64275.
5. Guan X, Yang H, Zhang W, Wang H, Liao L. Vitamin D receptor and its protective role in diabetic nephropathy. Chin Med J (Engl). 2014; 127(2):365.
6. Xiaoyan Xiao,Yajuan Wang,Yanlian Hou, Feng Han. Vitamin D deficiency and related risk factors in patients with diabetic nephropathy. Journal of International Medical Research 2016; 44(3): 673–684.
7. Yannick Stephan, Angelina R. Sutin, and Antonio Terracciano. Subjective age and cystatin c among older adults. Journals of gerontology: psychological sciences. 2019; 74(3):55-66.
8. Liu, C. K., Lyass, A., Massaro, J. M., D'Agostino, R. B. Sr, Fox, C. S., & Murabito, J. M.. Chronic kidney disease defined by cystatin C predicts mobility disability and changes in gait speed: The Framingham Offspring Study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences. 2014; 69: 301–307.
9. Yun KJ, Mi RK, Jung EH, Ji YM, and et. al. Cystatin c as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci. 2011; 26: 258-263.
10. Jeppson J.O., Kobold U., Barr J., Finke A., Weykamp C. Approved IFCC reference method for the measurement of HbA1c in human blood, Clin Chem Lab Med.2002; 40(1): 78- 89.
11. Bartham D., Trrinder P. An improved color reagent from the determination of blood glucose by the oxidation system, Analyst. 1972; 97: 142-145.
12. Bartels H, Bohmer M, Heierli C. Serum creatinine determination without protein precipitation, Clin Chim Acta. 1972; 73:193-7.
13. Fawcell JK and Scott JE. A rapid and precise method for the determination of urea, J Clin Pathol. 1960; 13(2): 156-159.
14. Votila M, Rouslahti E, Engvall E. Two site sandwich enzyme immunoassay with monoclonal antibodies to human. Alghafetoprotein J Immunl Methods. 1981; 42(1): 11-21.
15. Thethi TK, Bajwa MA, Ghanim H, Jo C, Weir M, Goldfine AB, et al. effect of paricalcitol on endothelial function and inflammation in type 2 diabetes and chronic kidney disease. J Diabetes Complications. 2015; 29(3):433–7.
16. Tiryaki O, Usalan C, Sayiner ZA. Vitamin D receptor activation with calcitriol for reducing urinary angiotensinogen in patients with type 2 diabetic chronic kidney disease. Ren Fail. 2016; 38(2):222–7.
17. Momeni A, Mirhosseini M, Kabiri M, Kheiri S. Effect of vitamin D on
proteinuria in type 2 diabetic patients. J Nephropathol. 2017; 6(1):10–4.
18. Topaloglu O, Evren B, Yologlu S. The Frequency of Vitamin D Deficiency in Obese Patients on Bariatric Surgery. Turk J Endocrinol Metab. 2019; 23:229-239.
19. Ock SY, Ha KH, Kim BK, Kim HC, Shim JS, Lee MH, Yoon YM, Kim DJ. Serum 25-hydroxyvitamin D concentration is independently inversely associated with insulin resistance in the healthy, non-obese Korean population. Diabetes Metab J. 2016; 40: 367-375.
Received: 1 December 2021 / Accepted: 9 March 2022 / Published:15 May 2022
Citation: Abeer J. Hassan , Sarmad Ajeel Hazzaa and Dunya Najim Alden Ahmed . Vitamin D a predictor Marker to kidney disease in male with type 2 diabetes mellitus . Revis Bionatura 2022;7(2) 39. http://dx.doi.org/10.21931/RB/2022.07.02.39
