2022.07.01.9
Files > Volume 7 > Vol 7 No 1 2022
Optimization of cephalosporin C acylase immobilization using crosslinked enzyme aggregates technique
Julkipli Julkipli1, Khaswar Syamsu1 and Ahmad Wibisana2,3,*
1 Biotechnology Study Program, Graduate School, IPB University, IPB Dramaga Campus, Bogor, Indonesia, 16680; [email protected]; [email protected]
2 The National Research and Innovation Agency (BRIN), Tangerang Selatan, Banten, Indonesia, 15314
3 Chemical Engineering Study Program, Pamulang University, Witana Campus, Tangerang Selatan, Banten, Indonesia, 15415
* Correspondence: [email protected]; Tel.: +6221-7560208
Available from: http://dx.doi.org/10.21931/RB/2022.07.01.9
ABSTRACT
Cephalosporin C acylase (CCA) is an essential enzyme for the one-step conversion of cephalosporin C into 7-aminocephalosporanic acid (7-ACA), an intermediate compound used to synthesize various semi-synthetic cephalosporin antibiotics. The industrial process prefers to use enzymes in immobilized form rather than soluble. A crosslinked enzyme aggregate (CLEAs) is a potential matrix-less enzyme immobilization technique to produce stable immobilized enzymes with high activity and low production costs. This study aimed to optimize the CCA immobilization using the CLEAs technique with Chitosan as a co-aggregate. The CCA lysate was obtained from harvesting CCA fermentation broth using a mutant strain of Escherichia coli through cell separation and lysis steps. Partially purified CCA by ammonium sulfate addition was conducted to obtain an active fraction of 20-60% saturation, followed by co-aggregation with Chitosan to form physical CCA aggregates. The aggregates were then immobilized by a crosslinking technique using glutaraldehyde to form CLEAs-CCA. Optimization of the immobilization process was carried out by Response Surface Methodology in three steps, (i) screening of the influencing factors, (ii) determining the level of the significant factors, and (iii) optimizing the immobilization condition. The CLEAs-CCA activity was used as a response parameter. Under optimum conditions, CLEAs-CCA activity obtained was 85.91 Ug-1.
Keywords. 7-aminocephalosporanic acid; cephalosporin C acylase; Chitosan; crosslinked enzyme aggregates; response surface methodology
INTRODUCTION
Cephalosporins are antibiotics with a broad antibacterial spectrum and almost cover 50% of β-lactam antibiotics humans use.1,2 Most of them are derived from 7-aminocephalosporanic acid (7-ACA), a precursor compound generated from hydrolysis of cephalosporin C (CPC), a natural antibacterial obtained from Acremonium chrysogenum fermentation.3 Therefore, it is essential to produce 7-ACA efficiently.
The industrial demands for efficient and environmentally friendly 7-ACA production have shifted from chemical to enzymatic processes. Initially, a two-step bioconversion using D-amino acid oxidase and glutaryl-7-aminocephalosporanic acid acylase was developed.4 Recently, one-step bioconversion using cephalosporin C acylase (CCA, EC 3.5.1.11) has paid the attention of researchers because it is more efficient.5
The industrial application of enzymes is preferred in the immobilized rather than soluble form due to the more efficient bioconversion process.6 Immobilization of enzymes on a matrix is a popular method. However, a dilution of enzyme up to 99% occurred, resulting in decreased activity.7 In addition, the matrix used causes an increase in the enzyme cost and generates a waste matrix generated from the inactive biocatalyst.8 To overcome these problems, enzyme immobilization by crosslinked enzyme aggregates (CLEAs) technique, a matrix-less immobilization approach, is a potential method to be applied.7 It showed a simple, fast, and efficient method to immobilize various enzymes.8-10 To the best of our knowledge, there is no publication on the application of CLEA for CCA immobilization.
The CLEAs formation involves two critical steps, i.e., enzyme precipitation to form aggregates and crosslinking of the aggregates.7 Ammonium sulfate and glutaraldehyde are often used as precipitation and crosslinker agents, respectively.9,11,12 However, crosslinking the enzyme with low superficial lysine residues with glutaraldehyde may cause weak enzyme binding, making it quickly released from its CLEAs.13 The addition of co-aggregates, such as bovine serum albumin, poly-ethyleneimine, and poly-lysine, could form larger aggregates size and higher stability.9,12,13 Chitosan is a relatively inexpensive biopolymer (polyaminosaccharides, copolymers of N-acetyl-D-glucosamine and N-amino-D-glucosamine) with high availability rich in reactive amino groups, potentials to mediate the formation crosslinks between the aggregates.14,15
In this research, optimization of the CCA immobilization by CLEAs technique using Chitosan as a co-aggregate was studied. Optimization was conducted with Response Surface Methodology (RSM), as it is an effective and widely used method for optimization in bioprocesses, including enzyme immobilization.16-18 CLEAs-CCA (Ug-1) activity was used as a response. The experiments were carried out in three steps, namely, (i) screening of the influencing factors using 2n-2 fractional factorial design (FFD), (ii) determining the level of significant factors using the steepest ascent method, and (iii) optimization of immobilization condition using RSM with Central Composite Design (CCD). CLEAs-CCA obtained were also evaluated for pH and temperature stability.
MATERIALS AND METHODS
Escherichia coli mutant as the CCA producer was provided by The National Research and Innovation Agency (BRIN), previously named was Biotech Center, Agency for the Assessment and Application of Technology - Indonesia. The culture was maintained in Miller Luria Bertani (LB) agar medium (Himedia, Mumbai, India) pH 7.0, containing 100 µgmL-1 ampicillin. The chemicals used were ammonium sulfate p.a (Merck, Germany), chitosan powder with a degree of deacetylation (DD) > 75% obtained from the local market, CPC-Na (Biorbyt, Netherlands), and 7-ACA (Tokyo Chemical Industry, Japan). Other chemicals such as 50% glutaraldehyde, absolute methanol, p-dimethylaminobenzaldehyde (p-DAB), NaOH granules, and acetic acid glacial were obtained from Sigma-Aldrich (Singapore).
CCA production
The CCA production method was adopted from Martius et al.19 A single colony of E. coli was selected and inoculated into 2 mL of fresh LB medium pH 7.0 containing ampicillin 100 µgmL-1 and cultivated at 37°C overnight with shaking at 170 rpm. The seed culture was then prepared by suspending 0.5 ml of the overnight culture to 2 ml fresh LB medium pH 7.0 containing ampicillin of 100 µgmL-1 and incubated at 37°C with shaking at 170 rpm in water bath. After two hours incubation (OD600 ± 0.6), 0.5 ml of the seed culture was inoculated into a fresh 50 ml LB pH 7.0 containing ampicillin of 100 µgmL-1 in a 250 ml Erlenmeyer flask and incubated at 37°C, shaken at 200 rpm until an OD600 of 0.6-0.8 was reached. The culture was then induced with lactose with a final concentration of 0.2% (w/v), and then cultivation was continued at 25oC with shaking at 200 rpm for 12 hours for CCA expression. The cells were harvested by centrifugation at 6,000 g, 4oC, for 10 minutes when the fermentation was finished. The pellets were then washed twice with a working buffer (0.1 M phosphate buffer pH 8.0) and then suspended in the same buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF). PMSF was used to inactivate the protease in cells. Cells were lysed by sonicating at 20 kHz, 25% amplitude, with a mode of 5 seconds “on” and 20 seconds “off” for 12 cycles using Misonix Sonicator XL2020 (Misonix, United States) in an ice bath. Cell debris was separated by centrifugation at 12,000 g, 4oC, for 5 minutes. CCA lysate was then stored at -20oC for further experiment.
Chitosan and glutaraldehyde solution preparation
The chitosan solution was prepared by Arsenault et al. method.17 Chitosan powder was dissolved in 0.1 M HCl to obtain a specific concentration, stirring for 30 minutes, and then sonicated for one hour to dissolve Chitosan completely. The pH of the solution was adjusted to 5.5 by adding 1 N NaOH. Glutaraldehyde solution with a particular concentration (%, w/v) was prepared by weighing 50% glutaraldehyde solution and diluted with a working buffer.
CCA partial purification
Simple partial purification of CCA was carried out by precipitation technique using ammonium sulphate (AS).20 AS powder was added slowly to 10 mL CCA lysate until it reached 20% saturation. The mixture was stirred continuously at a low speed at 4oC for 3 hours. The precipitate was separated by centrifugation at 10,000 g at 4oC for 15 minutes, and the supernatant was used for further fractionation. Precipitation was continued by adding AS to 60% saturation. The CCA aggregates of 20-60% saturation were separated by centrifugation and then resuspended in 40 mL of a working buffer. The suspension was used for CLEAs formation.
CLEAs-CCA formation method
The CLEAs formation method was adopted from Mageed et al.14 with slight modifications. A 0.5 mL chitosan solution was added to 1 mL of CCA aggregates suspension to obtain a final chitosan concentration in the range 1-5 mgmL-1 (x1) according to the FFD factor level (Table 1). Chitosan is insoluble at pH above 6.5 and will aggregate with CCA aggregates. The aggregation was conducted by shaking at 150 rpm for 30-90 minutes (x2). The co-aggregation allows CCA aggregates and Chitosan to interact electrostatically or hydrogen bonding. Furthermore, 0.5 mL of glutaraldehyde solution was added to obtain a final concentration in the range of 1-5 %, v/v (x3) and then shaking at 200 rpm. Variation in suspension pH (x4), crosslinking time (x5), and crosslinking temperature (x6) were carried out according to the range of each factor in the FFD (Table 1). In such a way, glutaraldehyde crosslinks of primary amine groups of enzyme and Chitosan. After the reaction was completed, the CLEAs-CCA was filtered using filter paper and washed with working buffer three times. The CLEAs-CCA was stored at -20oC for further analysis.
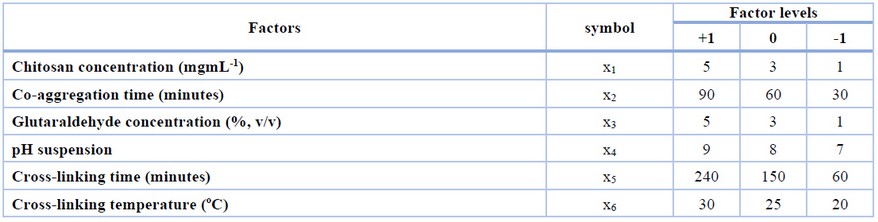
Table 1. Independent factors and their levels were used in the FFD experiment.
CLEAs-CCA activity determination
The CLEAs-CCA activity was determined using a micro-scale procedure according to Fernandez-Arrojo et al.21 with minor modifications. Twenty milligrams of CLEAs-CCA were added to a microtube containing 60 µL of CPC solution with a concentration of 20 µgmL-1 in a working buffer (0.1 M phosphate buffer pH 8.5). The mixture was incubated in an incubator shaker at 37oC for 5 minutes. After the reaction was completed, 20 μL of the reactant was taken and mixed with 280 µL of stop solution (the solution consists of 0.5% p-DAB in absolute methanol: 0.05 M NaOH: 20% acetate acid glacial with ratio 1:2:4). The solution was left for 10 minutes at room temperature to develop color, and then the absorbance was measured at 415 nm.
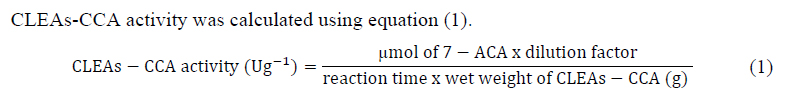
Screening of factors
In the first step, factors affecting CLEAs-CCA immobilization were screened using the FFD experimental design. The factor screening plays an important role when many potential factors influence the response while the resource availability is limited.22 FFD screened out the influencing factors based on their main effects and excluded their interaction.23 By preliminary literature review, six independent factors and their levels were selected, and the screening experiment design is presented in Table 1. The FFD experiments consist of 22 runs (16 runs of two-level fractional factorial (2n-2) and 6 center point runs). The experimental design and the results are shown in Table 2.
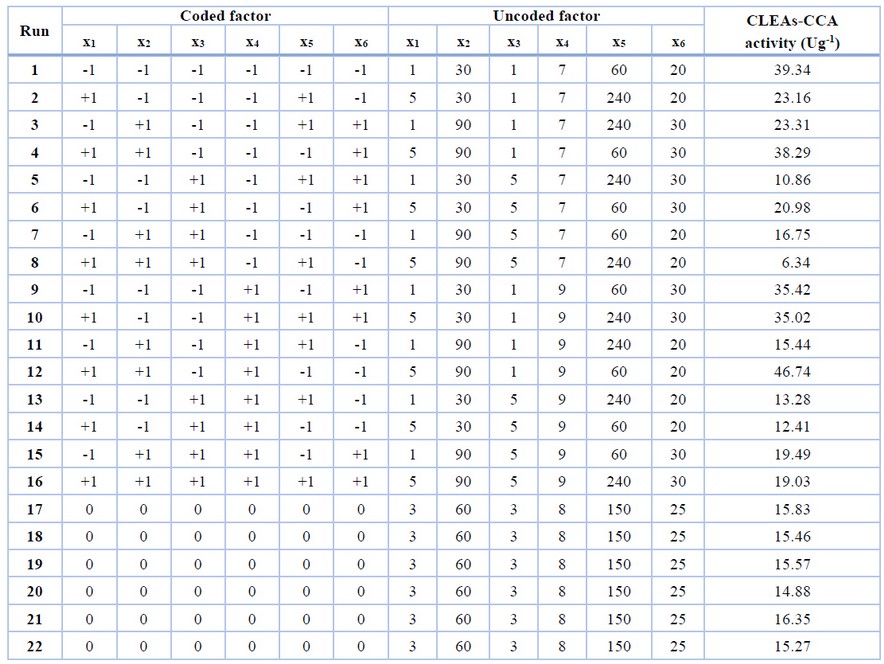
Table 2. Experimental FFD for factor screening and the result of CLEAs-CCA activity.
Steepest ascent method
The level of significant factors obtained from the FFD experiment was initially optimized using the steepest ascent method to determine the center point of each factor. Sequential experiments were carried out by applying various influencing factors along the direction of the maximum increase in the response as a rapid and efficient method for approaching the optimum neighborhood.24 Two points flanking the maximum response point of each factor are selected for the next optimization step.
2.2.8. Optimization immobilization conditions
The CCD with RSM was employed to optimize the two independent significant factors (glutaraldehyde concentration and crosslinking time). The other factors which have an insignificant effect were kept constant at the minimum level. The CCD and the result of the experiments are presented in Table 3.
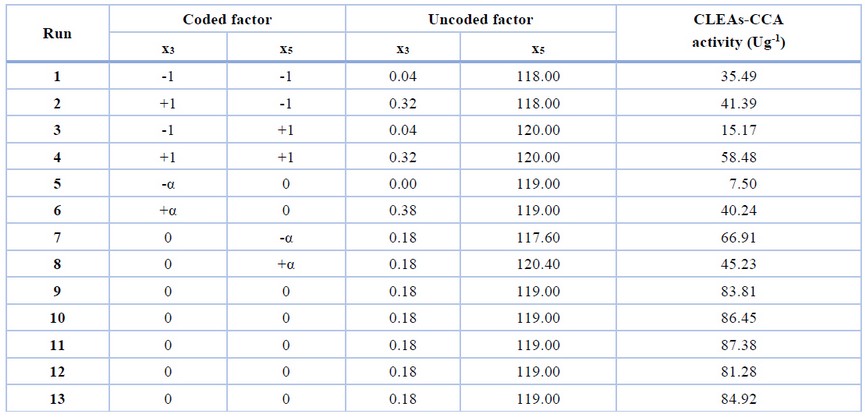
Table 3. CCD and experimental optimization result of CCA immobilization.
The effect of significant factors was modeled with a second-order polynomial equation as given by equation (2).
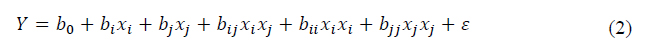
where Y represents the response predicted value, b0 is a constant, bi is the linear coefficient, bij is the interaction coefficient, bii and bjj is the quadratic coefficient, and xixi, xjxj are the coded level of the factors. Validation of optimum conditions obtained by the model was carried out by experiment with three replications. The relative error between predicted and experimental values was calculated by equation (3):
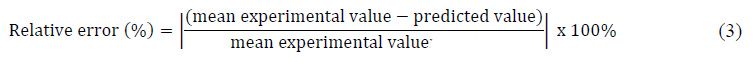
Statistical analysis
The effect of significant factors on CLEAs-CCA activity was analyzed using ANOVA and measured its relative significance based on an F-test with a confidence level of 95% (p-value < 0.050 is significant). The statistical analysis was carried out using Design-Expert version 12.0.11.0 software.
The immobilization yield and activity yield of CLEAs-CCA was determined according to equations (4) and (5):25

Characterization of CLEAs-CCA
· pH stability
The pH stability assay was conducted by incubating CLEAs-CCA and free CCA at 4oC for 30 minutes in phosphate buffer solution (pH 7.5 and 6.5) and acetate buffer solution (pH 5.5 and 4.5). The pH of buffer solutions was adjusted to the desired value by adding HCl 1 N or NaOH 1 N. After the incubation, the sample was washed three times with a working buffer and measured the activity. The residual activity was expressed as a percentage of its maximum activity (equation 6).

· Thermal stability
The thermal stability was evaluated by incubating CLEAs-CCA and free CCA at a temperature range of 35 - 65°C for 30 minutes. After incubation, the samples were cooled at 4oC, and the residual activities were determined.
RESULTS
Screening of the influencing factors
The result of the screening experiment using FFD is shown in Table 2. The ANOVA of the first-order model and regression analysis of the variables (Table 4) revealed that the model and the two factors (i.e., glutaraldehyde concentration and crosslinking time) have a significant effect (p-value < 0.050) on CLEAs-CCA activity. Other factors, namely, chitosan concentration, co-aggregation time, suspension pH, and crosslinking temperature, did not significantly affect the experimental range applied.
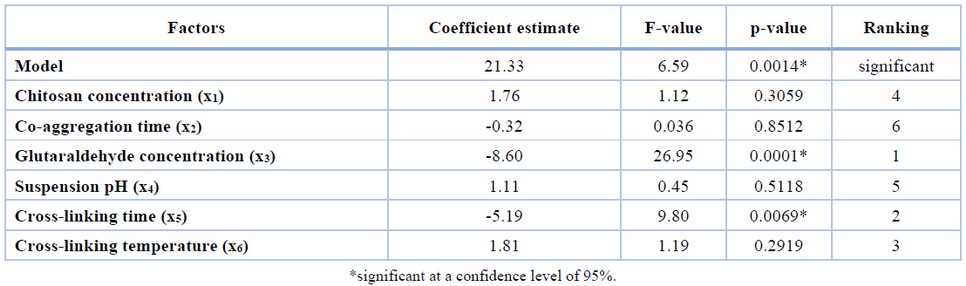
Table 4. ANOVA of the FFD screening experiments.
The linear model based on the significant factors was given in equation (7):
The model showed that glutaraldehyde concentration (x3) and crosslinking time (x5) have a negative value, which means both factors affect CLEAs-CCA activity inversely. Lower glutaraldehyde concentration and shorter crosslinking time would increase CLEAs-CCA activity. In addition, the FFD result showed a considerable variation in CLEAs activity, indicating that optimization of the significant factors would result in higher CLEAs-CCA activity.
Level determination of the significant factors for optimization
The two significant factors obtained from the FFD were applied simultaneously in the steepest ascent to determine their level used in optimization. Because the screening experiment still uses a wide range of factor levels, the significant factor level is still far from its optimum point. When predicted using response surface methodology, they need to be redefined to obtain an accurate optimum point. The movement direction of the factors towards the area where the response increases rapidly can be assisted using the steepest ascent experiment. The experiment results showed that the maximum value of CLEAs-CCA activity was approximated to be in the range of the glutaraldehyde concentration 0.04-0.32% (v/v) and the crosslinking time 118-120 minutes (Fig.1). The insignificant factors, namely chitosan concentration, co-aggregation time, pH suspension, and crosslinking temperature, were kept constant at a minimum level (i.e., 1 mgmL-1, 30 minutes, 7, and 20oC, respectively) during optimization.
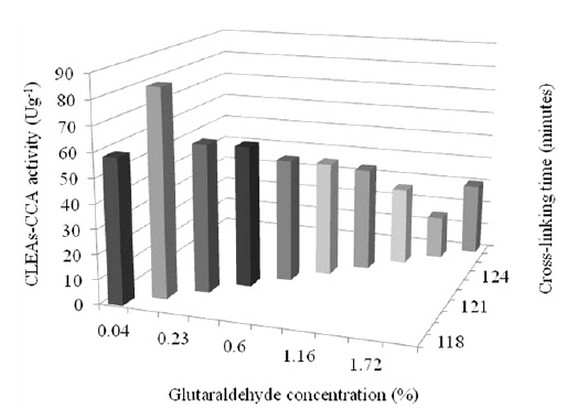
Figure 1. The steepest ascent experiment to determine the new level of significant factors is used in optimization.
Optimization of the immobilization conditions
The result of the CCD experiments is shown in Table 3. The ANOVA of the quadratic model (Table 5) demonstrated that the model was highly significant with a good coefficient determination, R2 (0.986). It showed that the model fit with experimental data. The p-value of lack of fit, the variation of the model due to the model inadequacy, is 0.055, which implies that the lack of fit is insignificant. The value of ‘Adequate Precision’ 27.097 indicates an adequate signal. A value of ‘Adequate Precision’> 4 is desirable.
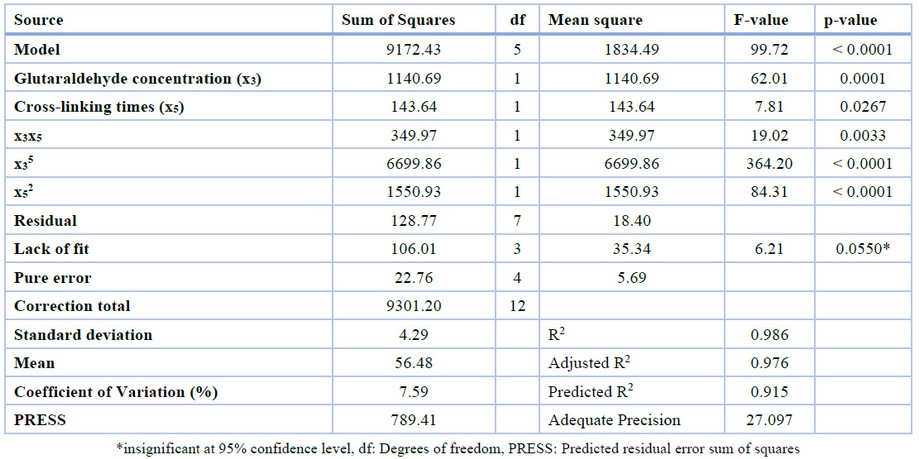
Table 5. ANOVA for response surface quadratic model.
A quadratic model equation fitted to the experimental data, given as coded factors, was shown in equation (8):
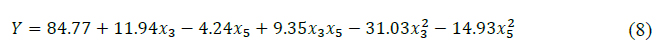
The 3D surface plot of the quadratic model helps to investigate the desired response value and find the optimal operating conditions (Fig. 2). The peak of the curve is the maximum response obtained from the effect of significant factors and their interactions under optimal conditions. According to the quadratic model, the optimum condition for CLEAs-CCA immobilization was x3 = 0.18 and x5 = -0.09, or in actual value, glutaraldehyde concentration was 0.20 % (v/v) and crosslinking time was 119 minutes, with maximum predicted CLEA activity was 86.02 Ug-1.
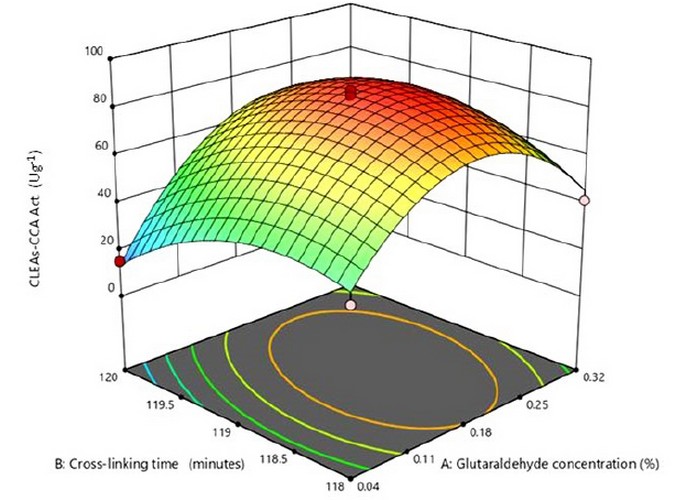
Figure 2. The 3D surface plot of the quadratic model.
Validation of the model
A three-replicate experiment validated the prediction of CLEAs-CCA activity obtained under optimum conditions. As shown in Table 6, the experimental data were in good agreement with the predicted value in CLEAs-CCA activity. The relative error between predicted and experimental values fell at 0.13%. It means that the results are within the confidence interval of 95%, confirming that the model is valid.
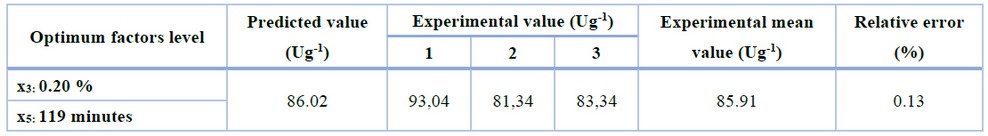
Table 6. Experimental data at optimum conditions for model validation.
Stability of CLEAs-CCA
The thermal stability tests showed that free CCA had a slight decrease of activity (2.82%) after incubation at 45oC compared to 35oC. However, at 55oC, its activity decreased sharply and only retained 4.57% activity. CLEAs-CCA showed better thermal stability and even showed an increase after incubation at 45oC. However, the CLEAs-CCA activity decreased after incubation at 55oC and retained only 25.87% activity. The experimental results are depicted in Figure 3a.
The pH stability tests also showed that CLEAs-CCA had better stability than free CCA. After incubation at pH 4.5 for 30 minutes, the CLEAs-CCA activity retained was 62.87%, while free CCA was 45.87 %. The experimental results are presented in Figure 3b.
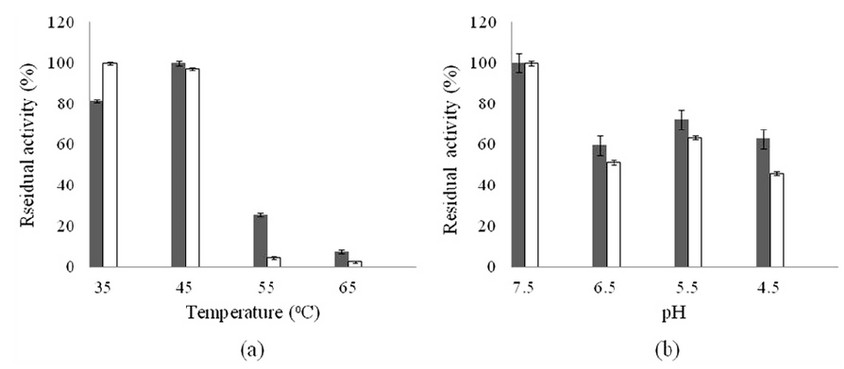
Figure 3. (a) Thermal stability test results; (b) pH stability test results; ■: optimized CLEAs-CCA; □: free CCA. Data are presented as mean values ± S.E.
DISCUSSION
A simple immobilization technique and high enzyme stability are critical for enzymes in their production process. Immobilizing enzymes using the CLEAs technique is a potential method to fulfill those demands. This technique involves only a simple preparation and does not require high purity of enzymes. In comparison, immobilization of CCA on the epoxy matrix was preceded by enzyme purification using immobilized metal affinity chromatography to obtain high purity enzyme.4,26 On the other hand, immobilization by the CLEAs technique does not require pure enzyme. The effect of enzyme purity on the CLEAs-penicillin G acylase (CLEAs-PGA) was studied by Rajendhran and Gunasekaran.27 The CLEAs-PGA generated from partially purified enzymes (enzyme purity 10 times higher than the initial crude enzymes) had 1.5 times higher activity than purified by the Ni-NTA chromatography technique (purity 31 times higher than the initial crude enzyme).27 Therefore, complex, high-cost, and time-consuming enzyme purification steps are unnecessary for CLEAs preparation.
In general, CLEAs formation involves two critical steps, i.e., enzyme precipitation to induce the physical enzyme aggregates formation and aggregates crosslinking to form permanently insoluble enzymes through covalent coupling of the aggregates while preserving their globular structure.7,28 The physical enzyme aggregates formation could be carried out by adding salts, organic solvents, or non-ionic polymers to enzyme solutions, as commonly used in protein purification. Partial purification of CCA using the salting-out method with the addition of AS was used in this study due to it being a simple method and relatively small effect on enzyme activity.8 The active fraction of 20-60% saturation was used for CCA immobilization. The partially CCA purification aimed to remove the contaminant protein in the lysate, such as esterase and β-lactamase, which are co-expressed when E. coli was used as an enzyme expression system.29 Both enzymes could hydrolyze CPC resulted in decreasing in yield of 7-ACA.3
The number of lysine residues on the enzyme surface influences the effectiveness of crosslinking between crosslinkers and enzyme molecules. The amount of lysine residues also affects protein functionalization and enzyme immobilization.30,31 Lysine is the amino acid with an NH2 (amino) side chain, the most reactive residue on the enzyme surface. Luo et al.32 reported that CCA contains only low (nine) superficial lysine residues. Therefore, a co-aggregate compound rich in free amino groups, such as Chitosan, must be added before crosslinking. Chitosan as a co-aggregate can increase the effectiveness of crosslinking of CLEAs formation for various enzymes.14,15 In this study, the Chitosan with high DD (>75%) was mixed with the enzyme to provide an adequate number of free amino groups for crosslinking. DD is the ratio of N-amino-D-glucosamine to N-acetyl-D-glucosamine.
Glutaraldehyde is a widely applied bi-functional reagent for many CLEAs preparations since it is an efficient crosslinker with low cost and high availability.33 In this study, glutaraldehyde crosslinking was carried out at pH > 6.5. Under these conditions, the deprotonated amino group of Chitosan becomes more reactive as a nucleophilic agent.6 Glutaraldehyde acts as an amino fixing arm to form an imine bond between the primary amino group of Chitosan and the enzyme, creating networks among them.14 The reaction of primary amino groups of superficial lysine residues from neighboring enzyme molecules to glutaraldehyde can form inter-and intra-molecular aldol condensates, thereby increasing the stability of biocatalyst activity.9,34,35 However, the CLEAs formation using excessive glutaraldehyde would lead to enzyme inactivation due to its high reactivity and small size, allowing it to penetrate the enzyme’s active site and access all essential amino acids involved in catalytic activity,36 leading the changes in intrinsic enzyme properties.6,37,38 In addition, more severe inactivation may occur for enzymes that have reactive amino acid residue in the vicinity of their active site.36 CCA has an essential N-terminal serine in its active site, which is susceptible to reacting with glutaraldehyde.38 He et al.38 reported that crosslinking CCA with glutaraldehyde causes activity loss by 18% due to its excessive crosslinking. Therefore, adding Chitosan is necessary for crosslinking when glutaraldehyde is used. It could form reticulated covalent bonds between enzyme-enzyme, enzyme-polymer, and polymer-polymer, thereby increasing the number of crosslinks and reducing enzyme inactivation as well as increasing aggregate size. In this study, the particle size of CLEAs-CCA without chitosan addition was tiny and increased along with chitosan addition. The particle size of CLEAs is essential to facilitate mass transfer rate, product separation as well as its reusability after enzymatic processing.12
Chitosan could also act as a coating agent for enzymes.15,17 As a poly-ionic biopolymer, Chitosan can interact with enzymes, preventing enzyme dissociation. Nevertheless, under certain conditions, such as low pH or high temperature, the biopolymer can be desorbed and lose its function as a protective layer. As a crosslinker agent, glutaraldehyde stabilizes CLEAs structure by crosslinking the biopolymer to the enzyme surface and among the biopolymers, avoiding the enzyme being re-dissolved during the buffer rinsing and their application.14,33,34
This study used CCD for response surface optimization with two process variables (glutaraldehyde concentration and crosslinking time). It revealed that the optimized conditions were achieved at glutaraldehyde concentration 0.20% (v/v) and crosslinking time 119 minutes. The other factors were set at a minimum value: chitosan concentration 1 mgmL-1, co-aggregation time 30 minutes, pH suspension 7, and crosslinking temperature 20oC. The maximum CLEAs-CCA activity achieved was 85.91 Ug-1 (Table 6). Theoretically, at low glutaraldehyde concentration, one glutaraldehyde molecule reacts with a few amino groups of enzymes.33 Excessive interaction between residual amino acids on protein surface with glutaraldehyde must be avoided to maintain the catalytic activity of the immobilized enzyme. In addition, relatively low crosslinking temperatures were used in this study to avoid CCA degradation. Within the temperature range used, maximum CLEAs activity was achieved in 2 hours.
The maximum activity of CLEAs-CCA obtained from this experiment was comparable and even exhibited slightly higher than that of immobilized CCA with matrix-binding obtained by Zhu et al.4 and Wei et al.26, i.e., 81 Ug-1 and 60 Ug-1, respectively. However, the immobilization yield of CLEAs-CCA was 60.31%, and the activity yield was only 13.89%. Enzyme activity can be partially lost upon the precipitation and glutaraldehyde crosslinking step caused by the changes in intrinsic enzyme properties.6,37,38 Besides, the issues of diffusion and transport would arise since the pore sizes of the CLEAs may be small.39 However, the immobilization processes could promote enzyme resistance to pH or temperature changes and its repetitive use, making it more efficient than a free enzyme.7,28
The poly-ionic biopolymer chitosan is essential to form larger particles and contributes to a stronger bond between the enzyme aggregates to obtain stable CLEAs.12,33,34 However, it becomes a part of the CLEAs non-catalytic structure, resulting in diluting the enzyme activity.9,34 Immobilized enzyme resulting from the origin CLEAs (without co-aggregate addition) was claimed as almost 100% undiluted, containing only enzyme and a tiny fraction of crosslinker.7 Therefore, the dilution of CLEAs-CCA could be defined as the ratio of the bound enzyme and the quantity of Chitosan used. The addition of Chitosan as co-aggregate contributed to the enzyme dilution of 44.85%. However, this dilution effect is much lower than that of the matrix-bound immobilized enzyme, which causes the enzyme dilution up to 99%.7,28
The thermal stability of the immobilized enzyme influences its industrial application.40 The rigid structure of CLEAs obtained by crosslinking is the reason for their increased stability.7,8,14,18 In addition, the thermal stability of the CLEAs can be generated by the effect of enzyme coating by Chitosan.17 In this study, chitosan solution was prepared at pH 5.5 (pKa of Chitosan is around 6.5); therefore, Chitosan has a low negative charge. Electrostatic interaction and/or hydrogen bonding between the N-acetyl-D-glucosamine hydroxyl group of Chitosan and positively charged amino acid residues on the enzyme surface may occur, thereby increasing the thermal stability of CLEAs.15 In addition, during the conversion CPC to 7-ACA, the pH of the solution tends to decrease due to the by-products accumulation, resulting in a decrease in the hydrolysis rate and enzyme stability.41 The CLEAs-CCA showed good stability at low pH up to 4.5; therefore, product inhibition related to low pH could be minimized.
CONCLUSIONS
The maximum CLEAs-CCA activity of 85.91 26 Ug-1 was obtained with the optimized immobilization conditions: glutaraldehyde concentration 0.2% (v/v) and crosslinking time 119 minutes. Chitosan as co-aggregate increases the number of crosslinks, which is indicated by the formation of larger particles size and increasing CLEAs-CCA stability. CLEAs-CCA immobilization also succeeded in reducing enzyme dilution up to 55.15%.
Author Contributions: Conceptualization and methodology, J.J. and A.W.; performing experiments and analyzing data, J.J.; writing—original draft preparation, J.J.; writing—review and editing, A.W. and K.S.; supervision, A.W. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Acknowledgments: The author thanks BRIN, Indonesia, to facilitate these experiments.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Selvi, A.; Das, D.; Das, N. Potentiality of yeast Candida sp. SMN04 for degradation of cefdinir, a cephalosporin antibiotic: kinetics, enzyme analysis and biodegradation pathway. Environ. Technol 2015, 36, 3112-24. doi: 10.1080/09593330.2015.1054318.
2. Collignon, P.J.; Conly, J.M.; Andremont, A.; McEwen, S.A.; Aidara-Kane, A. World Health Organization ranking of antimicrobials according to their importance in human medicine: A critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clin. Infect. Dis 2016, 63, 1087-93. doi: 10.1093/cid/ciw475.
3. Pollegioni, L.; Rosini, E.; Molla, G. Cephalosporin C acylase: dream and(/or) reality. Appl. Microbiol. Biotechnol 2013, 97, 2341-55. doi: 10.1007/s00253-013-4741-0.
4. Zhu, X.; Luo, H.; Chang, Y.; Su, H.; Li, Q.; Yu, H.; Shen, Z. Characteristic of immobilized cephalosporin C acylase and its application in one-step enzymatic conversion of cephalosporin C to 7-aminocephalosporanic acid. World J. Microb. Biot 2011, 27, 823-9. doi: 10.1007/s11274-010-0523-3.
5. Gröger, H.; Pieper, M.; König, B.; Bayer, T.; Schleich, H. Industrial landmarks in the development of sustainable production processes for the β-lactam antibiotic key intermediate 7-aminocephalosporanic acid (7-ACA). Sustain. Chem. Pharm 2017, 5, 72-9. doi: 10.1016/j.scp.2016.08.001.
6. Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol 2007, 40, 1451-63. doi: 10.1016/j.enzmictec.2007.01.018.
7. Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: carrier-bound or carrier-free? Curr. Opin. Biotechnol 2003, 14, 387-94. doi: 10.1016/s0958-1669(03)00096-x.
8. Velasco-Lozano, S.; López-Gallego, F.; Mateos-Díaz, J.C.; Favela-Torres, E. Cross-linked enzyme aggregates (CLEA) in enzyme improvement – a review. Biocatalysis 2016, 1, 166-77. doi: 10.1515/boca-2015-0012.
9. Wilson, L.; Illanes, A.; Soler, L.; Henríquez, M.J. Effect of the degree of crosslinking on the properties of different CLEAs of penicillin acylase. Process Biochem 2009, 44, 322-6. doi: 10.1016/j.procbio.2008.11.010.
10. Razib, M.S.M.; Rahman, R.N.Z.R.A.; Shariff, F.M.; Ali, M.S.M. Biochemical and structural characterization of crosslinked enzyme aggregates (CLEAs) of organic solvent tolerant protease. Catalysts 2020, 10, 55-70. doi: 10.3390/catal10010055.
11. Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485. doi: 10.1039/c3ra40818c.
12. Ye, J.; Li, A.; Chu, T.; Pan, X.; He, B. Poly-lysine supported crosslinked enzyme aggregates of penicillin G acylase and its application in synthesis of beta-lactam antibiotics. Int. J. Biol. Macromol. 2019, 140, 423-8. doi: 10.1016/j.ijbiomac.2019.08.021.
13. Shah, S.; Sharma, A.; Gupta, M.N. Preparation of crosslinked enzyme aggregates by using bovine serum albumin as a proteic feeder. Anal. Biochem 2006, 351, 207-13. doi: 10.1016/j.ab.2006.01.028.
14. Mageed, H.; Ezz, N.; Radwan, R. Bio-inspired trypsin-chitosan crosslinked enzyme aggregates: a versatile approach for stabilization through carrier-free immobilization. BioTechnologia 2019, 100, 301-9. doi: 10.5114/bta.2019.87589.
15. Grajales-Hernandez, D.; Armendariz-Ruiz, M.; Velasco-Lozano, S.; Lopez-Gallego, F.; Mateos-Diaz, J.C. Chitosan-based CLEAs from Aspergillus niger type A feruloyl esterase: high-productivity biocatalyst for alkyl ferulate synthesis. Appl. Microbiol. Biotechnol 2020, 104, 10033-45. doi: 10.1007/s00253-020-10907-2.
16. Wibisana, A.; Sumaryono, W.; Mirawati, S.T.; Pudjilestari, S.P. Optimization of surfactin production by Bacillus amyloliquefaciens MD4-12 using response surface methodology. Microbiol. Indones 2015, 9, 120-8. doi: 10.5454/mi.9.3.4.
17. Arsenault, A.; Cabana, H.; Jones, J.P. Laccase-based CLEAs: Chitosan as a novel crosslinking agent. Enzyme Res 2011, 2011, 376015. doi: 10.4061/2011/376015.
18. Mahmod, S.S.; Yusof, F.; Jami, M.S.; Khanahmadi, S. Optimizing the preparation conditions and characterization of a stable and recyclable crosslinked enzyme aggregate (CLEA)-protease. Bioresour. Bioprocess 2016, 3, 3-13. doi: 10.1186/s40643-015-0081-5.
19. Martius, E.; Wibisana, A.; Ardiani, Y. The optimization of soluble cephalosporin C acylase expression in E. coli. Int. J. Eng. Sci 2018, 7, 29-34. doi: 10.9790/1813-0703012934.
20. Wingfield, P.T. Preparation of soluble proteins from Escherichia coli. Curr. Protoc. Protein Sci 2015, 78, 6.2.1–6.2.22. doi: 10.1002/0471140864.ps0602s78.
21. Fernandez-Arrojo, L.; Santos-Moriano, P.; Rodriguez-Colinas, B.; Ballesteros, A.O.; Plou, F.J. Micro-scale procedure for enzyme immobilization screening and operational stability assays. Biotechnol. Lett 2015, 37, 1593-600. doi: 10.1007/s10529-015-1835-z.
22. Li, R.; Lin, D.K.J. Variable selection for screening experiments. Qual. Technol. Quant. Manag 2009, 6, 271–80. doi: 10.1080/16843703.2009.11673199.
23. Gunst, R.F.; Mason, R.L. Fractional factorial design. WIREs Comp. Stats 2009, 1, 234-44. doi: 10.1002/wics.027.
24. Chen, H.; Zhang, J.; Dang, Y.; Shu, G. Optimization for immobilization of β-galactosidase using plackett-burman and steepest ascent. J. Chem. Pharm 2014, 6, 612-6. doi:
25. Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: why, what and how. Chem. Soc. Rev 2013, 42, 6223-35. doi: 10.1039/c3cs60075k.
26. Wei, Y.; Luo, H.; Chang, Y.; Yu, H.; Shen, Z. Reversible immobilization of cephalosporin C acylase on epoxy supports coated with polyethyleneimine. Biocatal. Biotransformation 2016, 33, 250-9. doi: 10.3109/10242422.2016.1168814.
27. Rajendhran, J.; Gunasekaran, P. Application of crosslinked enzyme aggregates of Bacillus badius penicillin G acylase for the production of 6-aminopenicillanic acid. Lett. Appl. Microbiol 2007, 44, 43-9. doi: 10.1111/j.1472-765X.2006.02043.x.
28. Sheldon, R.A. CLEAs, combi-CLEAs and ‘smart’ magnetic CLEAs: Biocatalysis in a bio-based economy. Catalysts 2019, 9, 261-91. doi: 10.3390/catal9030261.
29. Sonawane, V.C. Enzymatic modifications of cephalosporins by cephalosporin acylase and other enzymes. Crit. Rev. Biotechnol 2006, 26, 95-120. doi: 10.1080/07388550600718630.
30. Campanella, B.; Bramanti, E. Detection of proteins by hyphenated techniques with endogenous metal tags and metal chemical labelling. Analyst 2014, 139, 4124-53. doi: 10.1039/c4an00722k.
31. Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal 2007, 349, 1289-307. doi: 10.1002/adsc.200700082.
32. Luo, H.; Zhao, H.; Chang, Y.; Wang, Q.; Yu, H.; Shen, Z. Oriented immobilization and characterization of a poly-lysine-tagged cephalosporin C acylase on glyoxyl agarose support. Appl. Biochem. Biotechnol 2015, 175, 2114-23. doi: 10.1007/s12010-014-1411-3.
33. Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv 2014, 4, 1583-600. doi: 10.1039/c3ra45991h.
34. Wahab, M.K.H.A.; El-Enshasy, H.A.; Bakar, F.D.A.; Murad, A.M.A.; Jahim, J.M.; Illias, R.M. Improvement of crosslinking and stability on crosslinked enzyme aggregate (CLEA)-xylanase by protein surface engineering. Process Biochem 2019, 86, 40-9. doi: 10.1016/j.procbio.2019.07.017.
35. Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution reaction with protein, and application to enzyme crosslinking. BioTechniques 2004, 37, 790-802. doi: 10.2144/04375RV01.
36. Mateo, C.; Palomo, J.M.; van Langen, L.M.; van Rantwijk, F.; Sheldon, R.A. A new, mild crosslinking methodology to prepare crosslinked enzyme aggregates. Biotechnol. Bioeng 2004, 86, 273-6. doi: 10.1002/bit.20033.
37. Velasco-Lozano, S.; Lopez-Gallego, F.; Vazquez-Duhalt, R.; Mateos-Diaz, J.C.; Guisan, J.M.; Favela-Torres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated crosslinking. Biomacromolecules 2014, 15, 1896-903. doi: 10.1021/bm500333v.
38. He, H.; Wei, Y.; Luo, H.; Li, X.; Wang, X.; Liang, C.; Chang, Y.; Yu, H.; Shen, Z. Immobilization and stabilization of cephalosporin C acylase on aminated support by crosslinking with glutaraldehyde and further modifying with aminated macromolecules. Biotechnol. Prog 2015, 31, 387-95. doi: 10.1002/btpr.2044.
39. Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal 2011, 353, 2885-904. doi: 10.1002/adsc.201100534.
40. Chapman, J.; Ismail, A.; Dinu, C. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238-65. doi: 10.3390/catal8060238.
41. Xiao, Y.; Huo, X.; Qian, Y.; Zhang, Y.; Chen, G.; Ouyang, P.; Lin, Z. Engineering of a CPC acylase using a facile pH indicator assay. J. Ind. Microbiol. Biotechnol 2014, 41, 1617-25. doi: 10.1007/s10295-014-1501-9.
Received: 13 June2021 / Accepted: 10 August 2021 / Published: date. 15 February 2022
Citation: Julkipli J, Syamsu K and Wibisana A. Optimization of cephalosporin C acylase immobilization using crosslinked enzyme aggregates technique. Revis Bionatura 2022;7(1). 9. http://dx.doi.org/10.21931/RB/2022.07.01.9
