2023.08.03.98
Files > Volume 8 > Vol 8 No 3 2023
Incidence of COVID-19 in vaccinated patients in Almuthanna province
1 College of Science / Al-Muthanna University/ Al-Muthanna/Iraq ; [email protected]
2 College of Science / Al-Muthanna University/ Al-Muthanna/Iraq; dhayali [email protected]
3 College of Science / Al-Muthanna University/ Al-Muthanna/Iraq; Sheereehan A.M @mu.edu.iq
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.98
ABSTRACT
In recent years, the world has been in a crisis of COVID-19, and there has been a decrease in research and studies in Al-Muthanna Governorate on this pandemic. Since these vaccines are new, many people are afraid of taking the vaccine, so this study decided to shed light on this field. The coronavirus that causes severe acute respiratory syndrome first comes into contact with mucosal surfaces (SARS-CoV-2). Most SARS-CoV-2 vaccinations produce targeted IgG responses but very moderate mucosal protection. Since the SARS-CoV-2 virus is transmitted through the respiratory tract and almost all COVID-19 vaccines are administered intramuscularly, it is challenging to successfully establish mucosal immunity to the antiviral through these means of administration. This study showed that (39) vaccinated individuals out of (74) patients were infected with COVID-19 and revealed positive results for qualitative IgM fluorescence immunoassay (FIA). Keywords: COVID-19 vaccines; Pfizer; Sinopharm; systemic vaccine; Mucosal Vaccine.
INTRODUCTION
Global efforts to find suitable vaccine candidates began after the coronavirus (COVID-19) started rising and spreading globally in December 2019. mRNA, inactivated viruses, and vector-based vaccines have all received licenses. For national health services and the clinical management of the pandemic, it is crucial to understand the kinetics of SARS-CoV-2 antibody generation and the persistence of humeral immunity in vaccination recipients 1.
The COVID-19 vaccine aids in lowering the likelihood of hospitalization, severe illness, and death. Individuals who have been vaccinated are still vulnerable to COVID-19. The chance of experiencing severe symptoms is significantly lower in COVID-19 vaccination recipients than in non-recipients. When someone who has gotten either a first episode or a first series plus a booster shot catches the virus that causes COVID-19, it is referred to as a "vaccine breakthrough infection." Individuals who have had the COVID-19 vaccine are much less likely compared to people who have not had it to experience severe symptoms. After obtaining a vaccination, those contracting COVID-19 infections can spread the disease to others. More Infections with COVID-19 in a community signal that the virus is spreading more widely. Even with high vaccination rates, more breakthrough infections will happen when more virus is circulating 2.
In comparison to COVID-19 vaccinations that lower the risk of infection, which prevent diseases while reducing symptoms but do not prevent infection, There will be fewer infections prevented by vaccines that protect against COVID-19 sickness but not against SARS-CoV-2 infection, necessitating more significant and quicker vaccination rollouts in order to have a significant population influence. Expected immunization in Spring 2021 will only have a limited impact if unchecked transmission persists throughout the United States 3. The COVID-19 vaccination might not shield against nasal. Systemic, respiratory vaccinations often offer poor protection against viral multiplication and shedding within the airway since this demands a localized mucosal secretory IgA response 4: SARS-CoV-2 infection and undetected transmission. Patients who have received a systemic vaccination may nevertheless get an infection and spread a live virus from the upper airway, according to Bleier et al. It is known that COVID-19 spreads through aerosols and respiratory droplets. Furthermore, many medical and surgical endonasal operations have been demonstrated to produce aerosols 4.
MATERIALS AND METHODS
Using AFIAS COVID-19 Ab is fluorescence immunoassay (FIA) for the qualitative determination of IgM antibodies against novel coronavirus (SARS-COV-2) in human serum for screening early mild, undetected or acute patients for coronavirus infection identification in Alsammawah Specialized Laboratory, (74) patients revealed positive IgM. There were (35) unvaccinated and (39) vaccinated with different types of covid-19 vaccines: Pfizer (32), Sinopharm (2), AstraZeneca (3) and (2) were vaccinated by both: Pfizer and Sinopharm.
Serum was isolated from blood samples, and 100μl of the serum was dispensed into the specimen well on the cassette using a pipette. The findings are presented on the monitor after 10 minutes of putting the cartridge in the holder and the tip in the cartridge's tip hole and tapping the START symbol (AFIAS COVID-19).
RESULTS
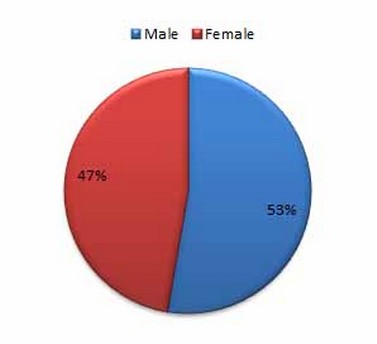
Figure 1 . Patient percentages according to gender
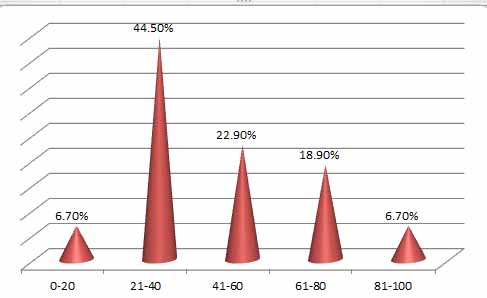
Figure 2. Age groups of Patients
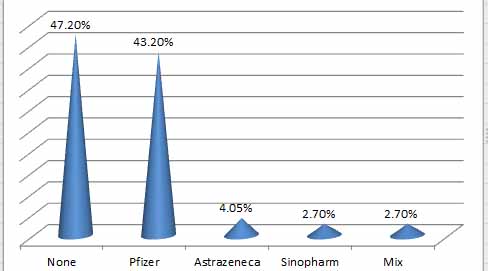
Figure 3. Percentage of vaccinated and unvaccinated patients
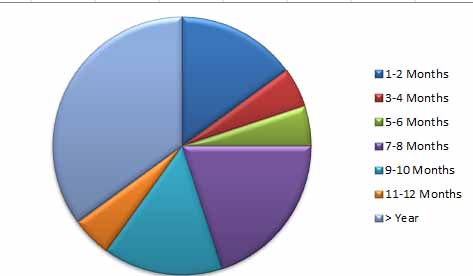
Figure 4. The period between vaccination and infection
DISCUSSION
Seventy-four patients were infected with COVID-19, as represented in Table (1). (52.7 %) were men, while (47.2 %) were female. The humeral and cell-mediated immune responses are affected by sex. When young adults respond, females frequently do so more vigorously because their T and B cell counts are higher than males 5. There were significant differences (p < 0.05) between age groups; the far more infected age group (21–40) had a 44.5% infection rate. The more excellent rates were likely brought about by changes in social behaviors, including the inadequacy of masking and the absence of social isolation among adolescents and young adults. The underrepresentation of COVID-19 in these age groups is due to older persons feeling vulnerable and adhering to masking and social distancing norms, as well as the fact that teenagers and young people are less likely to exhibit symptoms and get tested. Even though NK cells play a vital role in protecting against viral infection, it is interesting that newborn NK cell blood counts are significantly higher than adult ones. However, these levels quickly drop by 2-3 times in newborns' blood a few days after delivery. They then gradually decline throughout childhood, approaching adult levels (mean of 200 NK cells/mm 3) around age 5 years 6.
There were no significant variations between unvaccinated patients (47.2 %) and vaccinated (43.2 %) with the three types (Pfizer, Sinopharm and AstraZeneca), which indicates that both groups were susceptible to infection. The three vaccination types are all given through injection and are designed to trigger particular IgG defenses, which can clear the virus and prevent further damage from COVID-19 and viremia. However, they cannot induce mucosal immunity [4]. These findings concur with A survey in Washington state that compiled information that included more roughly 4 million participants who had completed their entire course of vaccines. The statistics show that between January 17 and August 21, 2021, 1 in 5,000 individuals experienced a breakthrough infection 7. Breakout COVID-19 cases occur in fully immunized individuals, and they appear to be more common now that the delta type is widely distributed and that immunity may be diminishing in individuals who received the vaccine several months earlier. Although none of the three coronavirus vaccines on the market is 100% effective in avoiding infection, they are all quite good at defending you against the severe effects of COVID-19. The more contagious delta version of the coronavirus, which is capable of causing breakthrough COVID-19. 8
The study showed that the most recorded vaccinated patients were infected after 6 months from immunization. The efficacy of the COVID-19 vaccine against severe disease remained strong; however, it did drop slightly 6 months after complete vaccination. Vaccine efficacy or efficiency against infection and symptomatic disease, on the other hand, declined by roughly (20-30) % points after 6 months. The decline in vaccine efficacy or effectiveness is likely caused by fading immunity, while bias cannot be excluded. It will be critical to update the COVID-19 vaccine policy by evaluating vaccine efficacy or effectiveness after 6 months 9.
There may be several additional biases when estimating the longevity of vaccine efficacy or effectiveness over time. The following are some significant biases that could lead to an overestimation of decreases in vaccine efficacy or effectiveness over time: people who receive vaccinations earlier in life have a sustained increased risk of infection compared to those who receive vaccinations later; people who receive vaccinations change their behavior and testing frequency over time, which increases the likelihood of contracting an infection or being detected as infected, especially with increased mobility for those who receive vaccinations later 10. However, ongoing observation is necessary to evaluate the COVID-19 vaccines' ability to protect for prolonged periods. To make informed decisions about vaccine policy, such as the necessity, timing, and target populations for booster doses, it is imperative to understand whether and how vaccine effectiveness declines 9. Early after the vaccine launch, the Pfizer-BioNTech and Moderna mRNA vaccines effectively avoided SARS-CoV-2 infection in nursing facility residents. However, in recent months, this population's effectiveness has dramatically decreased. These findings emphasize the vital necessity of COVID-19 immunization of employees, inhabitants, and attendees and adherence to robust COVID-19 prevention techniques in preventing SARS-CoV-2 transmission in nursing homes. For nursing home and long-term care facility residents, an additional COVID-19 vaccination may be considered to maximize the immune system's defenses 11.
Since COVID-19 vaccines are still relatively new, more time is required to research the duration of immunity. Studies that are currently available demonstrate that vaccine recipients exhibited robust COVID-19 protection. Although it appears that immunity will persist for some time, researchers must monitor immune levels over time 12. Therefore, creating a vaccine that is efficacious and directly administered through the (mucosal route) improves and triggers the regional mucosal defense against SARS-CoV-2, which is crucial for stopping earlier viral multiplication and respiratory spilling. Since the respiratory system comes into touch directly with the outside environment, there is a chance that it will come into contact with microbes that can actually cause respiratory diseases and sometimes even turn into fatal diseases, mainly when those microbes cause a robust immune cell recruitment and activation response that can increase airway inflammation 13. Alturaiki presumes that perhaps a relatively new mucosa vaccination SARS-CoV-2 mRNA employing BAFF (B-cell activation factor) / APRIL (A proliferation-inducing Ligand) or CXCL13 (C-X-C motif chemokine ligand 13) as elicitors in combination with the mRNA encoding the spike protein could improve the effectiveness of the regional immune defense and preclude the initial phases of SARS-CoV-2 infection and prompt viral removal from the air passages 14.
CONCLUSIONS
Mucosal immunity following systemic vaccination ensures adequate protection against re-infection. Although the number of people infected with the virus after vaccination is a lot in our study, the period between the vaccine and infection was nearly more than 6 months for most of them. Since COVID-19 vaccines are still relatively new, more time is required to research the duration of immunity. The world needs more research to highlight the infection period after the vaccine and the possibility of producing vaccines to induce mucosal immunity.
Funding: This research received no external funding.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Acknowledgments: We are highly indebted to Al- Samawah laboratory staff.
REFERENCES
1. Nickel, O. Rockstroh, A. Wolf, J. et al. Evaluation of the systemic and mucosal immune response induced by COVID-19 and the BNT162b2 mRNA vaccine for SARS-CoV-2. PLOS ONE. (2022). 17(10).
2. CDC, June 23, 2022 Content source: National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases
3. Swan, D. Bracis C. Janes, H. Moore, M. Matrajt, L. Reeves, D. et al., COVID-19 vaccines that reduce symptoms but do not block infection need higher coverage and faster rollout to achieve population impact. (2020).; doi: https://doi.org/10.1101/2020.12.13.20248142
4. Bleier, B.S.; Ramanathan, M.; Lane, A.P. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol. Head Neck Surg. 2021, 164, 305–307.
5. Gubbels Bupp, M.R.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, 1269
6. De Vries E, Bruin-Versteeg S, Comans-Bitter WM, de Groot R, Hop WC, et al.: Neonatal blood lymphocyte subpopulations: a different perspective when using absolute counts. Biol Neonate. 2000;77:230–235
7. Disease Control and Health Statistics. SARS-CoV-2 Vaccine Breakthrough Surveillance and Case Information Resource.Health. Washington State Department of Health October 05, 2022.
8. Maragakis, L. and Kelen,G. Breakthrough Infections: Coronavirus After Vaccination. Health.Updated on November 23, 2021.
9. Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O'Brien KL, Smith PG, Wilder-Smith A, Zeger S, Deloria Knoll M, Patel MK. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022 Mar 5;399(10328):924-944. doi: 10.1016/S0140-6736(22)00152-0. Epub 2022 February 23. Erratum in: Lancet. 2022 Apr 4;: Erratum in: Lancet. 2023 Feb 25;401(10377):644.
10. Kahn R, Schrag S, Verani J, Lipsitch M. Identifying and alleviating bias due to differential depletion of susceptible people in post-marketing evaluations of COVID-19 vaccine. medRxiv. 2021 doi: 10.1101/2021.07.15.21260595.
11. Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, Li Q, Bagchi S, Dubendris H, Link-Gelles R, Jernigan JA, Budnitz D, Bell J, Benin A, Shang N, Edwards JR, Verani JR, Schrag SJ. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1163-1166.
12. Ucdavis Health. How the COVID-19 vaccine works, potential side effects and more. .( 2022). Updated October 28, 2022.
13. Holt, P.G.; Strickland, D.H.; Wikström, ME; Jahnsen, FL Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 2008, 8, 142–152.
14. Alturaiki, W.(2022). Considerations for Novel COVID-19 Mucosal Vaccine Development. Vaccines 2022, 10, 1173. https://doi.org/10.3390/vaccines10081173
Received: 25 June 2023/ Accepted: 26 August 2023 / Published:15 September 2023
Citation: Al-Nuaimy W A, Azeez D A and Albyati S A M. Incidence of COVID-19 in vaccinated patients in Almuthanna province. Revis Bionatura 2023;8 (3) 98 http://dx.doi.org/10.21931/RB/2023.08.03.98
