2021.06.04.25
Files > Volume 6 > Vol 6 No 4 2021
Determination of the prevalence of blaoxa-like gene and ISAba1 elements among extensiveــdrug resistant (XDR) Acinetobacter boumannii isolates
Salah Mohsin*1 , Wasan Abdul-Elah Bakir2, Majeed Arsheed 3
Available from: http://dx.doi.org/10.21931/RB/2021.06.04.25
ABSTRACT
The capacity of Multiـdrug resistant (MDR) Acinetobacter baumannii to survive in any state of affairs concerning the gaining of various gene types of virulence and antimicrobial agent resistance are the main anxiety in the hospital’s environments. So, it is very crucial to determine the prevalence of insertion sequences in A. baumannii. In the hospitals. Detecting the blaoxa-51 gene through the polymerase chain reaction (PCR) was performed to confirm Acinetobacter baumannii and the search for ISAba1 element. Between October 2020 and February 2021, 540 distinct clinical specimens were gathered from five hospitals in Baghdad. Thirty-eight A. baumannii isolates were obtained from various clinical specimens. The isolates were initially identified phenotypically using standard microbiological techniques and by the Vitek2 compact automated machine. Isolates of A. baumannii were identified genotypically by amplification of the blaoxa-51-like gene. Antimicrobials are studied by Kirby-Bauer (disc diffusion) technique on Muller-Hinton agar as specified by the recent clinical and laboratory standard institute (CLSI) guidelines (2020). The actual results of the current study indicated that from total isolated (38) A.baumannii isolates, 23 isolates (61%) were resistant to meropenem and 25 isolates (66%) were resistant to imipenem. The blaoxa-51 gene was identified in all strains examined, ISAba1 was also present in all A. baumannii isolates. ISAba1 has a high predominance between drug-resistant A. baumannii. Identifying these parameters can assist in the control of infection and decreasing the microorganism’s prevalence rate.
Keywords. Insertion sequence, The blaoxa-51-like gene, Acinetobacter baumannii
INTRODUCTION
An opportunistic infectious agent, A.baumannii encompasses a high rate of occurrence among immunocompromised people, significantly those that have experienced long (more than ninety days) hospital residence 1. Commonly related to aquatic environments 2. It’s been recognized as a “red alert” human infectious agent in recent years, causing concern among medical professionals, owing to its extensive spectrum of antibiotic resistance3.
The development of multidrug-resistant (MDR) pathogens has more and more become a cause for profound importance concerning each healthcare facility and community-acquired infections 4. According to the World Health Organization (WHO), Antimicrobial resistance has recently been recognized in concert as one of the 3 most vital issues facing human health 5. The therapeutic selections are restricted, typically leading to unsuitable medical care and resulting negative consequences on patient 6.
The primary antibiotic resistance mechanisms are enzymatic (production of β-lactamases and enzymatic modification of aminoglycosides) and nonـenzymatic (changing membrane permeability, activating efflux pumps) and altering of the target site) 7.
Insertion sequences (IS) are among the most basic mobile genetic elements (MGEs) and are found across the animal kingdom. To present, more than 4500 IS from 29 families have been discovered 8,9. In Acinetobacter spp., more than thirty different types of Insertion Sequences have been discovered, suggesting that ISs had a significant role in developing this species and contributing to the multidrug-resistant phenotype shown in this genus 10.
Insertion elements have two distinct characteristics: short transposable elements (up to 2500bp) and only code for proteins involved in transposition 11.
In A. baumannii, ISAba1 has been found in conjunction with numerous antibiotic resistance genes 12. ISAba1 has been shown to function in the expression of the Bla ampC gene, the antibiotic resistance gene of A. baumannii, which encodes the naturally occurring cephalosporins enzyme, and the blaOXA-23 gene, which encodes a carbapenem-degrading oxacillinase. Nevertheless, it could also act in the case of other resistance genes 13,14. A composite transposon (defined as Tn2006) formed by two copies of ISAba1 bracketing this ß-lactamase gene, responsible for the movement of blaOXA-23 15.
ISAba1 has been found to regulate the expression of the bla ampC gene, which encodes the naturally occurring cephalosporins enzyme, and the blaOXA-23 gene, which encodes a carbapenem-hydrolyzing oxacillinase enzyme. However, it may also play a role in the development of additional resistance genes. ISAba1 has been demonstrated to produce carbapenem resistance in A. baumannii by causing overexpression of the naturally existing blaoxa-51-like gene 16.
The expression of the blaOXA-23 gene has been linked to ISAba1 and ISAba4 15,17. The expression of the blaOXA-58 gene has been related to the insertion sequences ISAba1, ISAba2, ISAba3, and IS18 18. Therefore, determining the prevalence of insertion sequences genes in A. baumannii in hospitals is very crucial.
MATERIALS AND METHODS
Specimens’ collection
Between October 2020 and February 2021, 540 distinct clinical specimens were collected from five hospitals in Baghdad, including The Burns Hospital, Gazi Al-Hariri Hospital, Baghdad Teaching Hospital, Welfare Teaching Hospital in the Medical City, and Al-Yarmouk teaching hospital. Collected specimens were sputum, blood, fluids such as (cerebrospinal, pleural, and peritoneal), urine, and swabs.
Isolation of Bacteria
In the laboratory (Teaching Laboratories/Medical City, Baghdad) under aseptic conditions, the collected specimens were cultured directly on MacConkey agar and blood agar, incubated for 24 hrs. at 37ºC. The colonies were non-hemolytic opaque creamy on blood agar, while on MacConkey agar were non-lactose fermenting colonies. For obtaining pure, well-isolated colonies, they were subcultured on another MacConkey agar plate and incubated for another 24 hrs. at 37ºC 19.
Bacterial Identification
Microscopical examination
One isolated colony was transferred to a microscopic slide, fixed then stained with Gram stain (GS). Gram reaction, cell shape, and arrangement were recorded. The results were compared with Brooks et al. (2013) 20.
Biochemical tests
To phenotypically identify the isolates as A. baumannii, biochemical tests such as the ability to grow at 42°C, culture on selective medium, negative for oxidase test, absence of lactose fermentation, and others were utilized.
Identification of Acinetobacter baumannii using VITEK® 2 system.
As stated by the manufacturer’s instructions, identification cards of GramــNegative Bacteria (ID-GNB) were used on the VITEK® 2 system to recognize isolates at the species level. The bacterial isolates were inoculated at 37°C on MacConkey agar plates and, after incubated overnight, taken a single colony then suspended. In 0.45 % sodium chloride, the turbidity measurement for the bacterial suspension to meet the McFarland (0.5) standards, The (Gram-Negative Vitek 2 Identity card) then was manually placed into the Vitek-2 system, along with the bacterial suspension tub, the software also prepared according to (BioMerieux, France) the manufacturer’s instructions (21).
Identification of A.baumannii by PCR
Using a primer specific for bla OXA51-like genes, PCR was utilized to amplify bla OXA51-like genes used for A. baumannii isolate recognition.
Test of antimicrobial susceptibility profile
According to the latest clinical and laboratory standard institute (CLSI) criteria (2020), the isolates were tested for antimicrobial susceptibility to 18 antimocrobial agents using the Kirby-Bauer disc diffusion technique on Muller-Hinton agar (Oxoid /England) (22). The antibiotic discs (Mast Group /UK) used throughout the study for A.baumannii isolates are Piperacillin- Tazobactam (100/10 µg/disc), Ampicillin-sulbactam (13/10 µg/disc), Ticarcillin-Clavulanate (55/13 µg/disc), Cefepime(33 µg/disc), Cefotaxime (33 µg/disc), Ceftazidime (33 µg/disc), Ceftriaxone (33 µg/disc), Imipenem (13 µg/disc), Meropenem (13 µg/disc), Colistin sulphate (25 µg/disc), Tobramycin (13 µg/disc), Gentamicin (13 µg/disc), Amikacin (33 µg/disc), Doxycycline(30 µg/disc), Tetracycllin (30 µg/disc), Ciprofloxacin (5µg/disc), Levofloxacin (5µg/disc) and Trimethoprime-Sulphamethoxazole(1.25/ 23.55 µg/disc)
Genomic DNA extraction from bacterial isolates
Extraction of bacterial DNA from isolates under study using a commercial Extraction system (ZR Fungal/Bacterial DNA Miniprep Kit) designed to isolate DNA from Gram-negative bacteria according to the manufacturer’s instructions. For each reaction, a totally of 4 μl of extracted DNA was used.
Molecular recognition of BlaOXA-51-like gene and Insertion sequence elements
To detect XDR A.baumannii isolates, PCR was used to detect the Bla-OXA-51-like gene and ISAba1 elements. Primer sequences for each gene reported above are listed in table (1). These primers (Macrogen, South Korea) were received in a lyophilized state, dissolved in sterile deionized distilled water to a final concentration of 100 picomole/µl, and kept in a deep freezer until use, as advised by the vendor.
The PCR amplification procedure for the genetic level to detecting genes under study by follows steps: Final volume for PCR mixture was 25 μl (12.5 of Green Master Mix 2x, 4 μl extracted template DNA, 1.5 μl from each forward and reverse primer, 5.5 μl nuclease-free water were added in 0.2 ml PCR Eppendorf tubes, mixed for a short time via vortex then been loaded to Veriti™ 96-Well (applied biosystems) Thermal Cycler. The program used for each monoplex PCR reaction was set according to each primer. The best annealing temperature was chosen after the gradient runs through the optimization process of each oligonucleotide primer.
For amplification of the Bla-OXA-51-like gene, the DNA thermal cycler device Veriti™ 96-Well (applied biosystems) was programmed in the following amplification conditions: Following a 5-minute activation at 94°C, 40 cycles of 45 seconds at 94°C (denaturation), 56°C (annealing), and 45 seconds at 72°C (extension) were conducted. In contrary to other genes, the ISAba1 annealing temperature was 50°C. The last cycle was followed by 7 minutes at 72°C (Table 2). Amplified PCR products were examined on 1.5% agarose gel at an electrical current of 7 volt\cm2 in 1X TBE buffer with added Red safe dye (INTRON) has been exposed till the tincture had reached the other side of the gel. The SiZer™-1000 Plus DNA Marker and SiZer™-100 DNA Marker (Intron / Korea) were used as markers during PCR products electrophoresis. After that, the agarose gel was removed from the tank and visualized by a UV transilluminator documentation system (Cleaver scientific /UK) at 336 nm, then photographed using a digital camera.
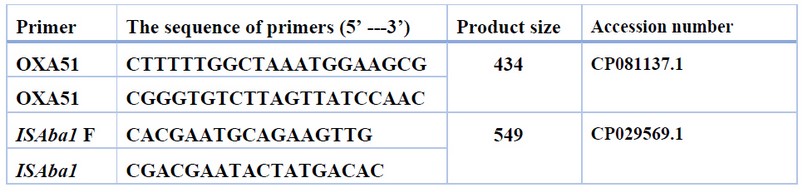
Table 1. Sequences of primers used throughout the study
Sequencing was carried out by Macrogen DNA Sequencing (Seoul, Korea) using 3730xl DNA Analyzer (Applied Biosystems™, Foster City, CA). Two samples from ISAba1 PCR products with forwarding primer (17 pmol/ µl) for each gene were selected and sent to sequencing. Raw reads generated in this study were trimmed or filtered to remove low-quality sequences using (SnapGene software). Once sequencing reads had been obtained, the data analysis process was started, the Basic Local Alignment Search Tool (BLAST) of the National Center for Biotechnology Information (NCBI) was used to analyze DNA sequences and do similarity searches.
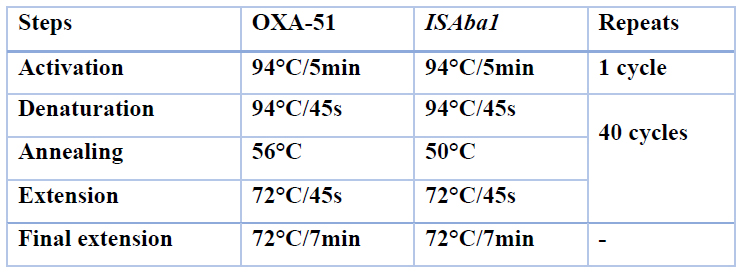
Table 2. Programs were used in the PCR for OXA-51-like gene and ISAba1 element.
RESULTS
540 clinical specimens were collected between October 2020 and February 2021 from some hospitals in Baghdad, including Baghdad medical city and Al-Yarmouk hospitals. Thirty-eight bacterial isolates were obtained, which differentiated to A.baumannii. Following conventional identification techniques, antimicrobial sensitivity testing was carried out by examining the findings in (Figure 1), revealing a high degree of resistance of A.baumannii clinical isolates to the majority of the antimicrobial agents under test.
In this study, antibiotic resistance profile of Acinetobacter baumannii for 18 antibiotics as following: Trimethoprime/sulphamethoxazole 32 (84%), Ciprofloxacin 27 (71%), Piperacillin/ tazobactam 26(68%), Ticarcillin/clavulanate 26 (68%),Ceftazidime 26(68%), Ceftriaxone26(68%), Cefotaxime 25 (66%), Imipenem 25(66%), Doxycycline 26(68%), Levofloxacin 25 (66%),Cefepime 24(63%),Tetracycline 24(63%), Meropenem 23(61%), Gentamicin 22 (58%), Tobramycin 15(39%), Amikacin 17(45%), Ampicillin/sulbactam 12 (32%), Colistin 0(0%). The exact and important results of current study indicated that from total isolated (38) A.baumannii isolates, 23 isolates (61%) and 25 isolates (66%) were resistant to meropenem and imipenem respectively.
All the A. baumannii isolates were positive for blaoxa-51-like genes and ISAba1 genes. Figures 1 and 2 demonstrate agarose gel electrophoresis of PCR products of blaoxa-51-like genes and ISAba1 elements, respectively.
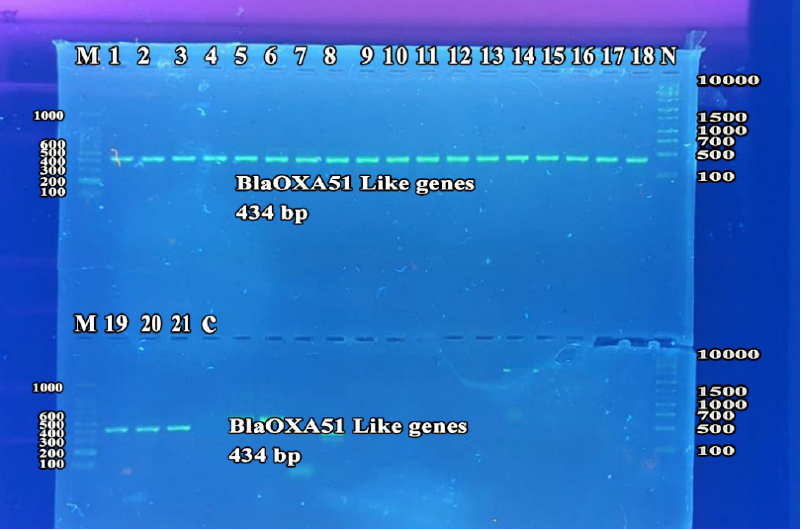
Figure 1. Detection of blaOXA51 like gene by monoplex PCR for A.baumannii isolates. lanes 1-21, XDR A.baumannii; Lane C, Negative control. Lane M, 100 bp DNA marker. Lane N, 1000 bp plus DNA marker. Detection was done on agarose gel (1.5%) at 5 Volt/cm for 1.5 hours, stained by Red Safe dye, and imagined on a UV transilluminator documentation system.
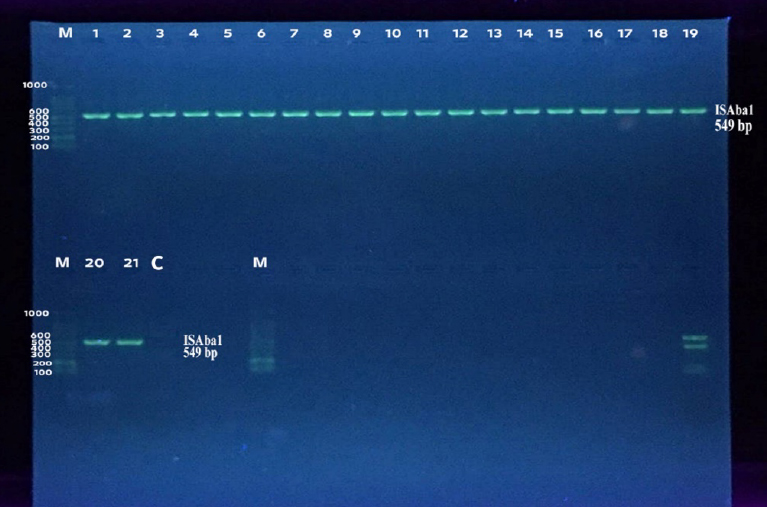
Figure 2. Detection of ISAba1 gene by monoplex PCR of isolates. Lanes 1-21: XDR A.baumannii isolates. Lane C: Negative control. Lane M: 100 bp DNA marker. Detection was done on agarose gel (1.5%) at 5 Volt/cm for 1.5 hours, stained by Red Safe dye, and imagined on a UV transilluminator documentation system.
The sequence was analyzed by BLAST software at NCBI. Figure (4) shows the alignment result with the USA: San Diego isolates (Accession number CP053098.1). The sequence was found to share 99% nucleotide homology with the reference isolates. Tables 3 and 4 show nucleotide changes and Features of ISAba1(Forward) from Acinetobacter baumannii (X9 isolate) with Acinetobacter baumannii ATCC 17978 chromosome from the USA: San Diego.
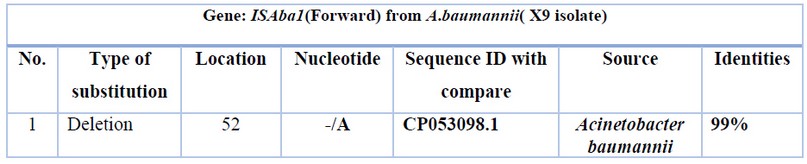
Table 3. Nucleotide changes of ISAba1(Forward) from Acinetobacter baumannii (X9 isolate) with Acinetobacter baumannii ATCC 17978 chromosome from the USA: San Diego.

Table 4. ISAba1(Forward) features from Acinetobacter baumannii (X9 isolate) with Acinetobacter baumannii ATCC 17978 chromosome from the USA: San Diego.
The sequence was analyzed by BLAST software at NCBI. Figure (4) shows the alignment result with USA: San Diego isolates (Accession number CP050388.1). The sequence was found to share 99% nucleotide homology with the reference isolates. Tables 5 and 6 show the nucleotide changes and features of ISAba1(Forward) from Acinetobacter baumannii (X10 isolate) with Acinetobacter baumannii strain VB473 chromosome from India.
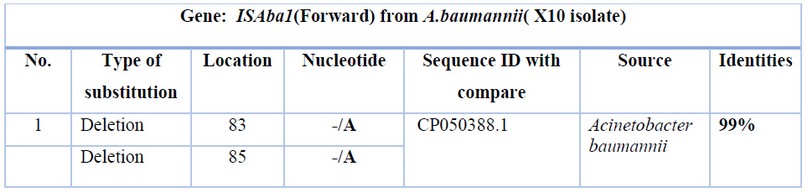
Table 5. Nucleotide changes of ISAba1(Forward) from Acinetobacter baumannii (X10 isolate) with Acinetobacter baumannii strain VB473 chromosome from India.
Table 6. Features of ISAba1(Forward) from Acinetobacter baumannii (X10 isolate) with Acinetobacter baumannii strain VB473 chromosome from India.
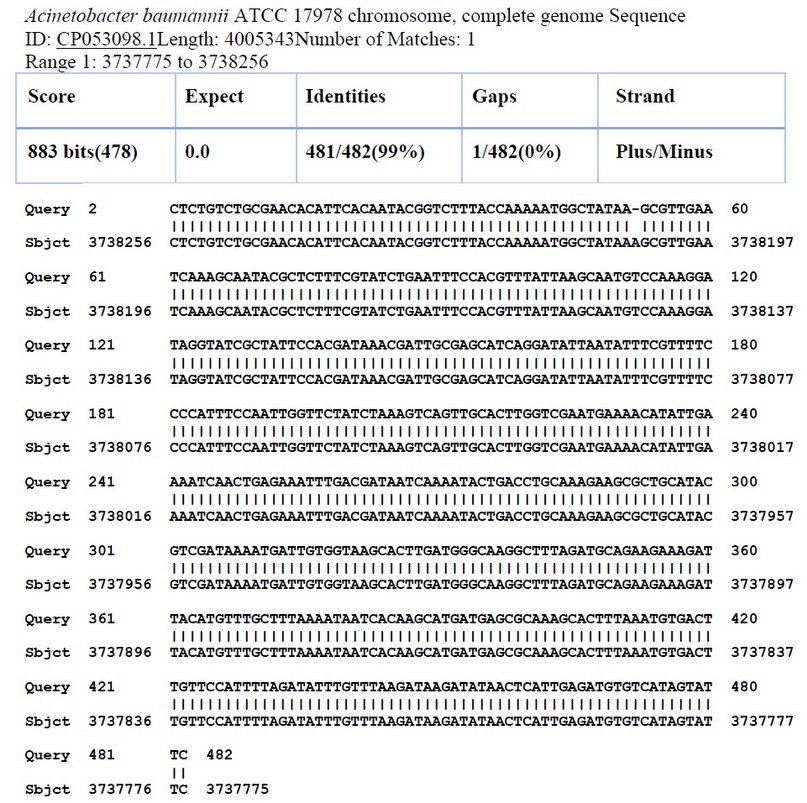
Figure 3. Sequence alignment of ISAba1(Forward) of A.baumannii( X9 isolate) with Acinetobacter baumannii ATCC 17978 chromosome from USA: San Diego.
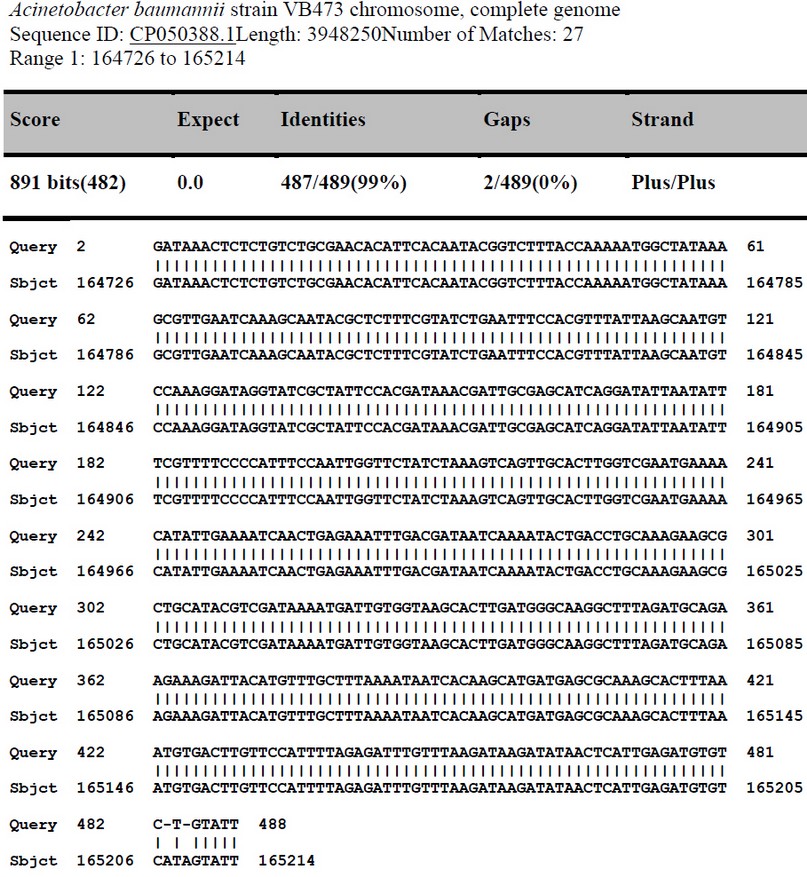
Figure 4. Sequence alignment of ISAba1(Forward)of A.baumannii( X10 isolate) with Acinetobacter baumannii strain VB473 chromosome, from India .
DISCUSSION
A. baumannii has developed as a well-established nosocomial pathogen with a high level of antibiotic resistance. Various medical facilities frequently report extensively drug-resistant, and pan drug-resistant isolates 23. By 2007, up to 70% of isolates in specific locations had evolved multidrug resistance, particularly resistance to carbapenems, which were formerly thought to be the gold standard for treating MDR A. baumannii infections 24.
The antibiotic of choice for treating A. baumannii is carbapenems. Due to rising resistance rates, A. baumannii infections are becoming increasingly ineffectual. Resistance to the newer antibiotic tigecycline is also quickly developing. Colistin, a previously abandoned antibiotic, is now used as a last option, yet resistance to this medication is increasing at an alarming pace throughout the world 23.
Aside from its proclivity for the critically ill in intensive care units, A. baumannii has lately been linked to a slew of infectious diseases among military troops injured in the Iraq and Afghanistan wars 4.
Resistance of Carbapenem is frequently connected to the making of oxacillinase enzymes. Metallo β-lactamases (MBL), on the other hand, can cause carbapenem resistance in A. baumannii 25.
The actual results of the current study indicated that of 38 A.baumannii isolates, 23 isolates (61%) and 25 isolates (66%) were resistant to meropenem and imipenem, respectively. The antibiotic resistance results of imipenem and meropenem are less than that found by al Al-Saadi (2018) 26. From 162 A.baumannii isolates, 112 isolates (88.19%) and 107 (84.25%) were resistant to meropenem and imipenem, respectively. Prior investigators in Iran found that the resistance rates to imipenem and meropenem were (95.23%) and (98.09%) respectively 27, indicating that these bacteria have a wide range of resistance mechanisms. This would imply significant risks among hospitalized patients, mainly where this antibiotic class was previously considered the standard therapy for A.baumannii infections 28.
The recognition of the Bla OXA-51-like gene can be utilized to identify A.baumannii reliably and straightforwardly 29,30. Furthermore, because this gene was controlled by insertion sequences such as ISAba1, the presence of intrinsic chromosomally placed genes of the bla OXA-51-like gene did not correlate with the amount of carbapenem resistance of A.baumannii isolates 31.
Additionally, all isolates of A.baumannii carried the Bla OXA-51-like gene and attributed the imipenem resistance state to the presence of ISAba1 upstream of the blaoxa-51-like gene serves as a promoter for gene expression as one of these isolates’ resistance methods 32,33.
From 21 XDR A. baumannii isolates all have blaoxa-51 like genes. This corroborated those of previous local investigations 26,34,35.
A study in Egypt revealed that genes encoding blaoxa-51 (belonging to class D carbapenemases) were found in 100% of the studied isolates 36. According to research by Bahador et al., all 62 CRAB isolates tested positive for blaoxa-51-like genes 37.
The present data could affirm that all A.baumannii isolates were positive for the ISAba1 gene. It was found that the prevalence of ISAba1 was 100% 36,27. ISAba1 was the most common insertion element (90.6%) 38. The prevalence of ISAba1 is equal to that seen in 59 Spanish isolates (93.2%) 39. While Taiwan (36%) 40 and India (33%) 41 have lower prevalence rates than the rest of the world. The presence of various insertion sequences in A. baumannii makes it resistant to carbapenems 42.
These insertion sequences are found near genes that code for several OXA-type carbapenemases and are implicated in their overexpression 13.
CONCLUSIONS
In conclusion, A. baumannii is a significant pathogen in several nations. According to the findings of this study, it has a high resistance rate against most antibiotics, threatening in patients as a red alarm bacterium in hospitals, producing a high rate of death and morbidity due to its numerous mechanisms of resistance and the fact that it is not or only rarely treated with conventional antibiotics.
This bacterium can cause dangerous and long-term infections, especially in youngsters and people with immunological deficiencies. Our research focused on specific mobile components transported between species to change the antimicrobial pattern and enhance antimicrobial resistance. ISAba1 has a high prevalence among extreme drug-resistant A. baumannii isolated from several Baghdad hospitals. Identifying these parameters can aid in controlling infection and reducing the microorganism’s prevalence rate.
REFERENCES
1. Montefour K, Frieden J, Hurst S, Helmich C, Headley D, Martin M, et al. Acinetobacter baumannii: an emerging multidrug-resistant pathogen in critical care. Crit Care Nurse. 2008;28(1):15–25.
2. Turton JF, Woodford N, Glover J, Yarde S, Kaufmann ME, Pitt TL. Identification of Acinetobacter baumannii by detection of the bla OXA-51-like carbapenemase gene intrinsic to this species. J Clin Microbiol. 2006;44(8):2974–6.
3. Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63:1055–60. doi: 10.1002/iub.533.
4. Peleg AY, Seifert H ,Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. 2008; 21(3): 538–582.
5. Bassetti M, Ginocchio F, Mikulska M. New treatment options against gram-negative organisms. Annu Updat Intensive Care Emerg Med 2011. 2011;501–15.
6. Rice LB: Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis 2008, 197: 1079-1081.
7. Vrancianu CO, Gheorghe I, Czobor IB, Chifiriuc MC. Antibiotic Resistance Profiles, Molecular Mechanisms and Innovative Treatment Strategies of Acinetobacter baumannii. Microorganisms. 2020;8(6):935. Published 2020 21 June. doi:10.3390/microorganisms8060935
8. Siguier P, Filée J, Chandler M. Insertion sequences in prokaryotic genomes. Curr Opin Microbiol. 2006;9(5):526–31.
9. Siguier P, Gourbeyre E, Chandler M. Bacterial insertion sequences: their genomic impact and diversity. FEMS Microbiol Rev. 2014;38(5):865–91.
10. Montaña S, Almuzara M, Pennini M, et al. IS CR2 and IS 26: two insertion sequences highly dispersed among Acinetobacter spp. clinical strains. J Bacteriol Mycol Open Access. 2017;4(1):33-36.
11. Campbell N A, and J B. Reece. Biology (6th ed.) Benjamin Cummings, San Francisco. 2002; pp. 345-346
12. Naas T, Namdari F, Réglier-Poupet H, Poyart C, Nordmann P. Panresistant extended-spectrum β-lactamase SHV-5-producing Acinetobacter baumannii from New York City. J Antimicrob Chemother. 2007;60(5):1174–6.
13. Corvec S, Caroff N, Espaze E, Giraudeau C, Drugeon H, Reynaud A. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J Antimicrob Chemother .2003; 52:629–635
14. Héritier C, L. Poirel, P. Nordmann. Cephalosporinase over-expresssion resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 2006;12123-130.
15. Corvec S, Poirel L, Naas T, Drugeon H, Nordmann P. Genetics and expression of the carbapenem-hydrolyzing oxacillinase gene bla OXA-23 in Acinetobacter baumannii . Antimicrob Agents Chemother. 2007;51(4):1530–3.
16. Turton J F ,Woodford N , Glover J , Yarde S , Kaufmann M E ,Pitt T L. Identification of Acinetobacter baumannii by detection of the blaOXA 51-like carbapenemase gene intrinsic to this species. J. Clin. Microbiol. 2006a; 44(8): 2974-2976.
17. Bogaerts P, G. Cuzon, T. Naas, C. Bauraing, A. Deplano, B. Lissoir, P. Nordmann, and Y. Glupczynski. Carbapenem-resistant Acinetobacter baumannii isolates expressing the blaOXA-23 gene associated with ISAba4 in Belgium. Antimicrob Agents Chemother. 2008; 52: 4205-4206.
18. Poirel L, Nordmann P. Genetic structures at the origin of acquisition and expression of the carbapenem-hydrolyzing oxacillinase gene bla OXA-58 in Acinetobacter baumannii. Antimicrob Agents Chemother. 2006;50(4):1442–8
19. Forbes BA, Sahm DF, Weissfeld AS. Diagnostic microbiology. Mosby St Louis; 2007.
20. Brooks G F, Carroll KC, Butel JS , Morse S A , Mietzner,T.A. Medical Microbiology. Jawetz , Melnick and adelberg’s. McGraw-Hill Companies, Inc. USA 2013; 26th ed.
21. Ling T K , Liu Z K, Cheng A F. Evaluation of the vitek 2 system for rapid direct identification and susceptibility testing of gram-negative bacilli from positive blood cultures. Journal of clinical microbiology.2003; 41(10): 4705-4707.
22. CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100.Wayne, PA: Clinical and Laboratory Standards Institute; 2020.
23. Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii : pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist. 2018;11:1249-1260
24. Kempf M, Rolain JM .Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: clinical impact and therapeutic options. Int J Antimicrob Agents .2012; 39: 105–114. pmid:22113193].
25. Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii . J Antimicrob Chemother. 2010;65(2):233-8.
26. Fatima S . Assessment correlation between biofilm formation and type II toxin-antitoxin system in imipenem-resistant Acinetobacter baumannii. Submitted to the Council of College of Science / Mustansiriyah University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy (Ph.D.) in Biology/ Microbiology. 2018
27. Owrang M, Fallah F, Irani S, Rahbar M, Eslami G. Identification and Isolation of Insertion Sequences, in Carbapenem Resistant Clinical Isolates of Acinetobacter baumannii from Tehran Hospitals, Jundishapur J Microbiol. 2018 ; 11(6):e58251. doi: 10.5812/jjm.58251
28. McConnell MJ, Domínguez-Herrera J, Smani Y, López-Rojas R, Docobo-Pérez F, Pachón J. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii . Infect Immun. 2011;79:518–26.
29. Turton JF , Ward M E , Woodford N , Kaufmann M E , Pike R , Livermore D M ,Pitt T L. The role of ISAba1 in expression of OXA carbapen emase genes in Acinetobacter baumannii. FEMS Microbiol. Lett. 2006b; 258(1):72-77.
30. Evans B A , Hamouda A, Towner K J ,Amyes S G. OXA-51-like beta-lactamases and their association with particular epidemic lineages of Acinetobacter baumannii. Clin. Microbiol. Infect. 2008;14(3): 268-275.
31. Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27(4):351–3.
32. Alexandre P Zavascki, Cecília G Carvalhaes, Renata C Picão & Ana C Gales. Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii: resistance mechanisms and implications for therapy, Expert Review of Anti-infective Therapy.2010; 8:1, 71-93.
33. Sohrabi B, Vanani IR, Tahmasebipur K, Fazli S. An exploratory analysis of hotel selection factors: A comprehensive survey of Tehran hotels. Int J Hosp Manag. 2012;31(1):96–106.
34. Ghaima K K , Saadedin S M K ,Jassim K A. Isolation, molecular identification and antimicrobial susceptibility ofAcinetobacter baumannii isolated from Baghdad. Int. J. Sci. Res. Publ. 2016; 6(5): 351-356.
35. Hussein N H, Al-Mathkhury H J F, Sabbah M A. Imipenem-Resist ant Acinetobacter baumannii isolated from patients and hospitals environment in Baghdad. Iraqi J. Sci. 2013; 54(4): 803-812
36. Abouelfetouh A, Torky AS, Aboulmagd E. Phenotypic and genotypic characterization of carbapenem-resistant Acinetobacter baumannii isolates from Egypt. Antimicrob Resist Infect Control. 2019;8(1):1–9
37. Bahador A, Raoo An, R Farshadzadeh Z, Beitollahi L, Khaledi A, Rahimi S, Mokhtaran M, Mehrabi Tavana A, Esmaeili D. The Prevalence of IS Aba 1 and IS Aba 4 in Acinetobacter baumannii Species of Different International Clone Lineages Among Patients With Burning in Tehran, Iran. Jundishapur journal of microbiology.2015; 8(7), e17167.
38. Alsultan AA, Aboulmagd E, Evans BA, Amyes SG. Clonal diversity of Acinetobacter baumannii from diabetic patients in Saudi Arabian hospitals. J Med Microbiol. 2014;63(11):1460–6.
39. Villalón P., Valdezate S., Medina-Pascual M.J., Carrasco G., Vindel A., Saez-Nieto J. A. 2013; Epidemiology of the Acinetobacter-derived cephalosporinase, carbapenem-hydrolysing oxacillinase and metallo-β-lactamase genes, and of common insertion sequences, in epidemic clones of Acinetobacter baumannii from Spain. J Antimicrob Chemother 68:550–553
40. Lu P L, Doumith M, Livermore D M, Chen T P, Woodford N.Diversity of carbapenem resistance mechanisms in Acinetobacter baumannii from a Taiwan hospital: spread of plasmid-borne OXA-72 carbapenemase. J Antimicrob Chemother .2009; 63:641–647
41. Karunasagar A, Maiti B, Shekar M, Shenoy M S, Karunasagar I. Prevalence of OXA‐type carbapenemase genes and genetic heterogeneity in clinical isolates of Acinetobacter spp. from Mangalore, India. Microbiol Immunol. 2011;55(4):239–46.
42. Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011;52(6):879–91.
Received: 7 August 2021
Accepted: 21 September 2021
Salah Mohsin*1 , Wasan Abdul-Elah Bakir2, Majeed Arsheed 3
1,2Department of Microbiology, College of Medicine, Mustansiriyah University. Baghdad. Iraq
3Gene bank Department/ Forensic DNA for research and training Centre/ Al-nahrain University, Baghdad. Iraq
*Corresponding author email: [email protected]
https://orcid.org/0000-0003-3391-7483
