2023.08.02.55
Files > Volume 8 > Vol 8 No 2 2023
Neutral red dye as a novel reagent in spectrophotometric determination of Doxycycline via oxidation and bleaching color of the dye
Nameer Mouyed Khalaf 1,*, Nabeel Sabeeh Othman2
1 Chemistry Department / College of Science / University of Mosul / Mosul, Iraq
2 Chemistry Department / College of Science / University of Mosul / Mosul, Iraq [email protected].
* Correspondance: [email protected],
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.55
ABSTRACT
A simple and accrued spectrophotometric method was developed to estimate Doxycycline as pure and in its formulation. The method included using a novel reagent neutral red dye the present method. The method was based on the oxidation of Doxycycline with an excess amount of N-bromosucinimide in an acid medium, then the residual amount of N-bromosucinimide oxidized the neutral red dye and caused bleaching of the color of the dye. The absorbance of the dye's color did not bleach measured at wavelength 525 nm. The absorbance is proportional to the concentration of Doxycycline in the solution. The linearity was from 1 to 15 µg/ ml, and the higher concentration above 15 µg/ ml gave deviation from Beer's law. The important analytical parameter included molar absorptivity and Sandell's sensitivity index, which were calculated and equal to 3.67 x104 l/mol.cm, 0.01261µg/cm2, respectively. An application part included estimating Doxycycline in its dosage form (capsule) from different manufacturers with satisfactory results.
Keywords: Doxycycline, Neutral red, Bleaching Color, Oxidation,
INTRODUCTION
Doxycycline is a medicinal preparation of the tetracycline class, a group of broad-spectrum antibiotics. Doxycycline structure contains four hydrocarbon rings and is used in the treatment of chlamydia, infections of the respiratory tract, urinary tract infections, tonsillitis and sinusitis, malaria, acne and dermatitis 1.

Figure 1. Chemical structure of Doxycycline
Neutral Red Dye (NRD) is a dye used for coloring tissues. It is dissolved in water, gives a red solution in the middle acidic, and is used as an indicator in neutralizing (acid-base) due to the change in the color of the dye from red to yellow when the pH changes from 6.8 to 8.0. NRD can bleach when oxidizing agents are added to its aqueous solution. The dye is Neutral Red) or what is called toluene red (3-Amino-7-dimethylamino-2-methylphenazine hydrochloride).Figure 2 3-4.

Figure 2. The chemical structure of NRD.
According to our information and through a literary survey, the NRD was not used in any analytical method to estimate one of the drug compounds, so it was used in the current research to estimate the Doxycycline after it was oxidized with an excess amount of the oxidizing agent. The remaining oxidizing agent inserts in an oxidation reaction to bleach the color of NRD. The absorbance of residual NRD is directly proportional to the concentration of the Doxycycline in the solution.
MATERIALS AND METHODS
Apparatus
Spectral measurements and absorbance readings were carried out using a JASCOV-630 spectrometer, and glass cells with a light path of 1 cm were used. The acidity of the solutions was measured using TRANSE BP3001 professional pH meter were used in present investigation.
Chemicals
All chemicals used were of a high degree of purity.
Doxycycline solution (100µg/ml). In a volumetric flask, this solution was prepared by dissolving 0.0100 g of Doxycycline (Doxyc) in 100 ml of distilled water.
N-bromosuccinamide solution (1 x 10-3 M). In a volumetric flask, this solution was prepared by dissolving 0.0177g of N-bromosuccinimide(NBS) in 100 ml of distilled water.
Neutral Red dye (100 µg/ml). In a volumetric flask, this solution was prepared by dissolving 0.0100g of neutral red dye(NRD) in 100 ml of distilled water.
Hydrochloric acid solution (approximate 1M). They were prepared by dilution of 8.4 ml of concentrated acid. The concentrate hydrochloric acid was added to a volumetric flask of 100 ml containing 20 ml of distilled water, and the volume was completed with distilled water to mark the limit.
Preparation of pharmaceutical preparation, Five capsules of formulation capsules were carefully weighed and mixed well; the amount of the powder equivalent to 0.0100 g of pure Doxcyc was weighed and dissolved in distilled water, then filtered into a volumetric bottle of 100 ml and supplemented with distilled water up to the mark.
The principle of the method
The principle of the present method is based on the oxidation of Doxcyc using an excess of the oxidizing agent NBS in an acidic medium 21, as shown in the following equation:

Then, unreacted NBS bleaches the color of NRD 22. The color of the unbleached NRD is measured at the maximum wavelength of the dye 525 nm ( absorbance increased with the concentration of Doxcyc), as shown in the below equation:

Procedure and standard curve
The standard curve was prepared for the determination of Doxycycline by adding increasing amounts of doxycycline solution(1 to 15 µg/ml of Doxycycline) to a series of 10 ml volumetric flasks, then 0.5 ml of hydrochloric acid, and 1.2 ml of 1 x10-3 M NBS solution, then leave the solutions for 10 minutes at room temperature, then adding 1 ml (0.01%) of NRD, leave the flasks for 10 minutes, and complete the volume to the mark with distilled water. The absorbance is measured against the blank solution at a wavelength of 525 nm. Figure 2 represents the curve standard for determining Doxycycline via the present method. Beer's law obeys from 1 to 15 µg/mL and the determination coefficient value0.9996, the molar absorptivity is 3.67x104 l/mol.cm, and Sandell's sensitivity index is 0.01261 µg/cm2.

Figure 3. The standard curve for Doxycycline. Estimation according to the proposed method
RESULTS
Spectrum of NRD
The dye spectrum was taken to determine the maximum wavelength (λmax) that will be used in subsequent measurements by taking 1 ml of the NRD solution and adding 0.5 ml of 1MHCl the volume was completed with distilled water to 10 ml, and the spectrum against the black solution has been taken Figure 3.

Figure 4. NRD absorption spectrum.
Figure 4 shows the highest absorption of NRD at a wavelength of 525 nm. Thus, it was recommended in the subsequent experiments.
Chosen the optimal volume of NR dye
Different volumes of NRD solution ranging from 0.1- 1.2 ml were studied, and the absorbance was measured at wavelength 525 nm against the blank solution, and the results are shown in Figure 5.

Figure 5. Standard curve for NRD.
Figure 5 shows that the linearity continues to 1.2 ml with a determination coefficient of 0.9996, and 1 ml was chosen from the dye to give it acceptable absorbance and that it is within the standard curve.
Selection of the oxidizing agent
The effect of oxidizing agents on bleaching the color of NRD was studied by testing several oxidizing agents (1x10-3M of NBS, N-chlorosuccinamide, potassium iodate and sodium iodate). The experiment included adding 1 ml of each oxidizing agent to serous 10-ml volumetric flasks containing 1 ml of NRD and 0.5 ml of hydrochloric acid. Then completes, the volume with distilled water to the mark, and the solutions are left for 5 minutes; then, the absorption is measured at the range of wavelength from 400 to 700 nm vs. blank solution Figure 6.

Figure 6. Effect of oxidizing agents on bleaching the color of NRD.
Figure 6 shows that the oxidizing agent NBS gives the best bleaching process, which was chosen in subsequent experiments.
Effect of the amount of oxidizing agent on the dye
The effect of the amount of NBS on bleaching the dye color in an acidic medium was studied by adding different volumes of oxidizing agent(0.2-1.2 ml), and the results are cited in Figure 7.

Figure 7. The effect of NBS on bleaching color of NRD.
Chosen acid type
The effect of different types of acids on the oxidation of doxy was studied by adding 0.5 ml of different acids to 1 ml of doxy. Then add 1.2 ml of NBS, wait 10 minutes, add 1 ml of NRD, and then leave for five minutes before completing the volume to the mark with distilled water. The absorbance was measured at a wavelength of 525 nm Table 1.

Table 1. Choosing the appropriate acid for the Doxcyc oxidation process.
Hydrochloric acid was chosen to give it the highest absorbance, which shows that the most significant amount of Doxyc suffered from oxidation.
The effect of the amount of acid
The effect of the amount of hydrochloric acid required to complete the oxidation process of Doxyc was studied, as shown In Table 2.

Table 2. Choosing the best volume of hydrochloric acid for the oxidation process
Table 2 shows that the volume of 0.5 ml of hydrochloric acid gave the highest absorbance of the residual dye, so this volume is the adoption of in subsequent experiments.
Oxidation time and dye bleaching
The time required for the oxidation of Doxycycline was studied by adding 1.2 ml of NBS to 1 ml of Doxyc with 0.5 ml of hydrochloric acid and then the volumetric flasks left for different periods up to20 minutes and then added 1ml of the NRD, then diluted to the mark and measure the absorbance at wavelength 525 nm, results as in Table 3.
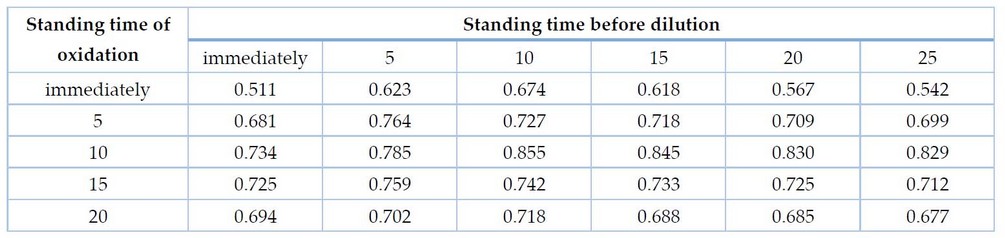
Table 3. Choosing the oxidation time of Doxyc and the bleaching time.
The results in Table 3 show that the best time for Doxyc oxidation is 10 minutes, and the best time for bleaching the color of the NRD is 10 minutes, so they were adopted in subsequent experiments.
The effect of temperature and time on the oxidation of Doxyc.
The effect of temperature on the oxidation of Doxyc was studied. It was noted that there is a slowdown or weakness in the oxidation process at the 0-5 oc temperature, and the results are close when using temperatures from 25 to 40 oc. Still, it was the adoption of room temperature for ease of measurement.
The order of additions to the reaction components Several experiments were conducted by changing the sequence to add the oxidizing agent to obtain the best oxidation process, and the results are shown in Table 4.

Table 4. Effect of the addition sequence on the oxidation of Doxyc.
From the above results in Table 4, sequence I was adopted in subsequent experiments, which indicates the oxidation of the most significant amount of Doxycycline.
Effect of surfactants
The addition of some of the surface active substances (positive, negative and neutral) has been studied, as shown in Table 5.

Table 5. Effect of some surfactants on the absorption of residual dye.
From the above results, there is no increase in absorbance, so adding it in subsequent experiments was not recommended.
Effect of time on the absorbance of residual NR dye
The color stability of the remaining NRD was studied by taking two different amounts of Doxyc (5 and 10 µg/ml) and applying the suggested. The absorbance with different times ranging after dilution immediately - 60 minutes, and the results are as in Table 6.

Table 6. The stability of residual NRD.
From the results listed in Table 6, the remaining dye is stable for 50 minutes. Therefore, subsequent experiments adopted the absorption reading after 5 minutes of dilution.
The effect of different solvents
In addition to the water, several organic solvents were used in diluting to the mark. The results indicate that methanol gave the highest absorption compared to other solvents, and acetone and ethanol also gave higher absorption than water. Still, in subsequent experiments, we remain on the use of water because it is available, safe and cheap Figure 8 and Table 7.

Figure 8. Effect of different solvents on the absorption spectrum of residual dye.
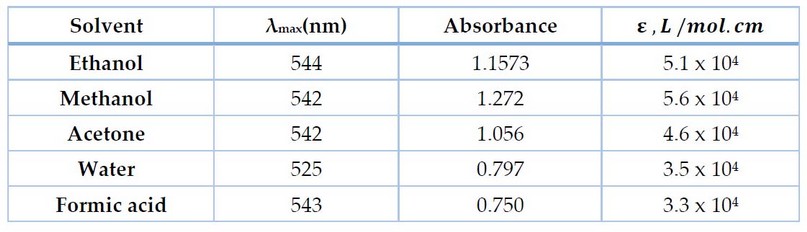
Table 7. Effect of different solvents on residual dye absorption spectrum
Application of the proposed method to pharmaceutical preparations
The proposed method has been applied for the determination of Doxycycline in the pharmaceutical
Preparation (capsule) via
Apply the method and calculate using the straight line equation (in Figure 3) to find the concentration. The proposed method was applied to pharmaceutical preparations for Doxycycline from two companies, which were a capsule.
Doxycycline capsule analysis The method was applied by taking different volumes of the standard solution 100 μg / ml (solution of a pharmaceutical preparation) to obtain concentrations of 3, 5 and 10 µg/ml and treated according to the suggested method described before. The concentration of Doxcyc in the capsule was found using the straight line equation of the curve for the Doxcyc compound in its pure form, and the obtained results are summarized in Table 8.

Table 8. Application in the estimation of Doxcycin pharmaceutical preparation.
Standard addition method
To prove that the developed method is successful in estimation and its freedom from additive interference, the standard addition method was applied in the determination of Doxycycline in the pharmaceutical preparation, as shown in Figures 9 and 10

Figure 9. Standard addition curve for the estimation of Doxyc in the pharmaceutical Preparation of (Saudi Arabia / Tabuk) Company

Figure 10. This Standard addition curve for the determination of (Doxyc) in the pharmaceutical preparation of (India / Ajanta pharma limited)
Results taken out from Figures 9 and 10 are listed in Table 9.

Table 9. Results from applying standard addition method for estimation of Doxcyc in capsules.
We conclude that the proposed method has proven its success and credibility in estimating Doxycycline in its pharmaceutical preparations. (capsules) for two different companies, and the estimate has no additive overlap.
Method comparison
Several analytical variables for the proposed method were compared with the same variables for two spectrophotometric methods. The results as in Table 10.

Table 10. Compare the analytical variables selected for the proposed method with other methods.
DISCUSSION
Doxycycline has an anti-inflammatory effect by decreasing the expression of cytokines. Therefore, it was used as an antiviral (Covid-19) to interfere with the virus upon or after its entry 2. A literary survey of the modern methods used to estimate the compound under study(Doxycycline) showed that the chromatographic 5-12 and electrical methods 13-14 are characterized by high sensitivity. The most common spectrophotometric 14-18 methods remain due to the availability and cheapness of spectrophotometer devices, the availability of many reagents and the high accuracy of the different methods, and many other techniques such as flow injection 19 and Fluorometric 20.
CONCLUSION
A sensitive spectrophotometric method has been developed for the determination of Doxycycline by its oxidation by an oxidizing agent -N-bromosuccinimide, then the determination of the unreacted oxidizing agent by bleaching the color of the neutral red dye, and the measurement is done at the wavelength 525 nm for the residual NRD color which is proportional to the concentration of Doxycycline. The proposed method has been successfully applied to determine Doxycycline in the pharmaceutical preparation (capsule).
REFERENCES
1. British Pharmacopoeia Commission, British pharmacopoeia in: M.a. Healthcare, P.R. Agency (Eds.)London, 2016.
2. Malek ,A.E.; Granwehr B.P.; Kontoyiannis D.P. Doxycycline as a potential partner of COVID-19 therapies, IDCases 2020, 21,e00864.
3. Tommy, R. Newly emergent 2019-nCoV and new uses of an old medicine, doxycycline; a hypothesis, infectious disorders-drug targets (formerly current drug targets-infectious disorders) 2020, pp. 351-351.
4. Reppetto, G.; Del Peso, A., ; Zurita, J. L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity, Nature Protocols 2008, 3(7), 1125–1131.
5. Ghidini, L.; Koga, W.; Ana, S.; Hérida, R. N. Eco-friendly green liquid chromatographic for determination of Doxycycline in tablets and in the presence of its degradation products, Drug Anal.Res.,2018, 2(2), 49-55.
6. Mileva, R. Determination of free doxycycline concentrations in the plasma and milk of sheep and in the plasma of rabbits by using the HPLC method. Maced. Veter. Rev. 2019, 42(2), 123-130.
7. Mashru, R.; Koshti, N. Development and validation of UV-Spectrophotometric and RP-HPLC method for simultaneous estimation of Metformin and Doxycycline in bulk and synthetic mixture. J.Drug Del. and Thera. 2021, 11(4-S), 26-35.
8. Abdul-Lateif, K. M., & Abdulateef, S. M. (2012). The effect of injecting hatching eggs with different concentrations of biotin on the quality and physiological characteristics of the hatched chicks. Iraqi Journal of Veterinary Sciences, 26, 391-397.Permana, A. D.; Tekko, I. A.; McCarthy, H. O. ; Donnelly, R. F. New HPLC–MS method for rapid and simultaneous quantification of doxycycline, diethylcarbamazine and albendazole metabolites in rat plasma and organs after concomitant oral administration. J. Pharma. and Biomedical Anal. 2019, 170, 243-253.
9. H. Esaa, F. .; Kassim, J. K. . Identification And Distribution Of Minerals In Soils From Al-Ahrar Area, Waist Province, Iraq. JLSAR 2021, 2, 14-20..
10. Kumssa, L.;Layloff, T.; Hymete, A. ; Ashenef, A. High performance thin layer chromatography (HPTLC) method development and validation for determination of doxycycline hyclate in capsule and tablet formulations. Acta Chroma.., 2021,1-9.
11. Ali, T. A.; Mohamed, G. G.; El-Sonbati, A. Z.; Diab, M. A.; Elkfass, A. M. A potentiometric sensor for determination of doxycycline hydrochloride in pharmaceutical Preparation and biological fluids. Ru. J. Electr. 2018, 54(12), 1081-1095.
12. Tian, X., ; Fan, Z. Novel ratiometric probe based on the use of rare earth-carbon dots nanocomposite for the visual determination of Doxycycline. Spectrochimica Acta Part A: Mol. and Biomol. Spectr. 2021, 260, 119925.
13. Awad, F. H., ; Taki, A. G. Spectrophotometric Determination of Doxycycline Via Oxidation Reduction Reactions. Egy. J.Chem. 2021, 64(11), 5-6.
14. Al-Maathedy, M. H., Mohammed, Th. T. & Al-Asha'ab, M. H. The effect of vitamin e supplementation and different levels of dried tomato pomace on common carp diets (cyprinus carpio l.) on productive performance. Biochemical and Cellular Archives, 2020; 20(2): 5371-5377.Gholse, Y. N.; Chaple, D. R. ; Kasliwal, R. H. Development and Validation of Novel Analytical Simultaneous Estimation Based UV Spectrophotometric Method for Doxycycline and Levofloxacin Determination. Bioint. Res.in App. Chem. 2022, 12(4) , 5458 – 5478
15. Patil, M. S.; Khatal, S. T.; Ranpise, A. S.; Thorve, J. P.; Naik, S. S.; Jain, A. S. Development and validation of UV spectrometeric method for estimation of doxycycline hyclate. World J. Pharm. Res 2020, 9, 2037-2050.
16. Abbas, R. F.;Waheb, A. A.; Hami, H. K.; Mahdi, N. I. Smartphone digital image using for determination of dch by a diazotization reaction. Curr. Anal.Chem., 2020, 16(8), 988-995.
17. Tawfeeq, A. H.; Qassim, B. B. A green method for assay of doxycycline hyclate using continuous flow injection/merging zones technique via coupling with azo metol in aqueous medium. Vop. Khimii I Khimiche. Tekhnol., 2020, 4, 31-37.
18. Sun, Y. Fluorometric determination of Doxycycline based on the use of carbon quantum dots incorporated into a molecularly imprinted polymer. Microchimica Acta, 2018, 185(11), 1-9.
19. Ramachandrappa, R.; Mayanna, S. M.; Made Gowda, N. M. Kinetics and mechanism of oxidation of aspirin by bromamine-T, N-bromosuccinimide, and N-bromophthalimide. Inter. J. Chemi. Kinet., 1998, 30(6), 407-414.
20. El Hamd, M. A.;Derayea, S. M. ; Abdelmageed, O. H.; Askal, H. F. Spectrophotometric method for determination of five 1, 4-dihydropyridine drugs using N-bromosuccinimide and indigo carmine dye. Int. J. Spectroscopy. 2013, D 243059, 1-7.
21. Abbas, R. F.; Wheeb, A. A. Spectrophotometric determination of doxycycline hyclate in pure and capsule using diazotization reaction. Al-Must. J.Sci., 2016,27(5), 50–54.
22. Mahmoud, K. M. ; Abdurahman, M. T. Spectrophotometric Determination of Doxycyline hyclate Using Oxidative Coupling Reaction. Inter. J. Curr. Res., 2017,9(01), 45416–45421.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Khalaf, Nameer M.; Othman, Nabeel S. Neutral red dye as a novel reagent in spectrophotometric determination of Doxycycline via oxidation and bleaching color of the dye. Revis Bionatura 2023;8 (2) 55. http://dx.doi.org/10.21931/RB/2023.08.02.55
