2023.08.02.82
Files > Volume 8 > Vol 8 No 2 2023
Use of modern geometric design of fish ponds to increase welfare and blood parameters
1 MSc Water Resource Engineering, Department of Water Resources, College of Agriculture, University of Anbar, Anbar, Ramadi, Iraq.
2 Department of Animal Science, College of Agriculture, University of Anbar, Anbar, Ramadi, Iraq.
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.82
ABSTRACT
Fish currently suffer from a lack of well-being due to the large number of pollutants in the water and the lack of space given to them, which makes them afraid and uncomfortable, which leads to a lack of welfare and, thus, a lack of production and poor quality of meat. This study was designed using a modern geometric design (DGD) of basin design, which is an H-mark. Three hundred fishes were used and distributed in three treatments with four replications; each treatment consisted of 100 fishes, and each replicate included 25. The first treatment was a control treatment (C), represented by raising fish in regular ponds, and the second treatment was fish farming using novelty design ponds in the shape of the H sign (ND). The third treatment was the fish farming treatment in earthen ponds (EP). The statistical analysis findings demonstrated that the ND treatment significantly improved the condition. ( p≤0.05) in the number of red blood cells, over the rest of the treatments, and significantly ( p≤0.05) for HD hemoglobin, was superior to the rest. In PCV, the ND treatment was a significant improvement compared with the rest of the treatments if the highest value was recorded, the results demonstrated significant changes between the experimental treatments at the level (p 0.05). The outcomes revealed that the number of white blood cells significantly increased with ND therapy (p 0.05). Cells, over the rest of the treatments, and a significant improvement ( p≤0.05) for H/L compared with the rest of the treatments; there were no significant differences between the experimental treatments, but there was a significant improvement ( p≤0.05) in ND treatment in the Glucose, cholesterol, triglyceride HDL, LDL, GPT, and GOT, over the rest of the treatments. Using a novelty design in the ponds helped the fish increase their well-being due to their feeling of comfort and lack of fear, which improved their behavior, blood traits, and growth performance.
Keywords: Modern Geometric Design, Fish Ponds, Blood Traits, Behavior Traits, and Growth Performance
INTRODUCTION
Modern geometric design (MGD) is increasingly used in fish ponds to improve welfare behavior and growth performance. The specific geometric design that is best for a particular fish pond will be determined by various elements, including the size of the pond, the species of fish that will be raised, and the intended use of the pond 1. The problem of the study is that the names suffer from a lack of well-being because of their upbringing in unsuitable environments that reduced their production of meat in quantity and quality. Therefore, the research hypothesis was to find a modern design that helps create an environment with high well-being to help fish overcome stress. However, some general principles can be followed to improve fish welfare behavior and growth performance through MGD2. One of the key benefits of MGD is that it can help create a more natural environment for the fish; this is important because fish are naturally attracted to geometric shapes, and they tend to feel more secure in environments similar to their natural habitat3. For example, fish have been shown to prefer rectangular ponds with rounded corners, as these shapes are more reminiscent of the natural water bodies that they would find in the wild4.
Another benefit of MGD is that it can help to improve water quality. This is important because water quality significantly determines fish welfare and growth performance. By carefully designing the shape and orientation of the pond, it is possible to improve the circulation of water and ensure that the fish have access to clean, oxygenated water5. In addition to water quality, MGD can also help to improve fish welfare by providing them with more space and hiding places6. Fish need space to move around and explore their environment and places to hide from predators and rest. By incorporating these factors into the geometric design of the pond, it is possible to create a more stimulating and comfortable environment for the fish7. The MGD can also help to improve fish growth performance8. This is because fish that are stressed or uncomfortable are less likely to eat or grow as quickly; creating a more natural and stimulating environment for the fish can reduce stress and improve their growth performance9. In addition to the benefits of MGD for fish welfare and growth performance, some potential drawbacks should be considered10. For example, some geometric designs may be more difficult to build or maintain than others. Additionally, some geometric designs may not be suitable for all types of fish. Therefore, it is important to carefully consider the specific needs of the fish when designing a fish pond11.The specific examples of how MGD can be used to improve fish’s welfare behavior and growth performance. Rectangular ponds with rounded corners: This type of pond design is more natural for fish and can help to improve water quality12. Ponds with a circular shape have more consistent water quality and may be less stressful for fish. Ponds that are half-round: This type of pond design offers some of the advantages of both rectangular and circular ponds. Ponds with multiple geometric shapes: This type of pond design can help to create a more natural and stimulating environment for fish. Ponds with plants, rocks, and other objects: This type of pond design can enrich fish and help reduce stress13. The relationship between MGD, fish ponds, and blood traits is complex and still being studied. However, some evidence suggests that there may be a link between these factors 14. One study published in the journal Animals in 2022 found that fish raised in rectangular ponds with rounded corners had lower blood cortisol levels than fish raised in square ponds. Cortisol is a stress hormone, so this suggests that the rectangular ponds with rounded corners were less stressful for the fish15. The fish raised in circular ponds had higher levels of some blood proteins, including albumin and immunoglobulin G, than fish raised in rectangular ponds. These proteins are involved in the immune system, so this suggests that the circular ponds may have provided the fish with a more stimulating and healthy environment16.
Some possible mechanisms could explain the link between DGM, fish ponds, and blood traits. The first is water quality: The shape and orientation of the pond can affect the circulation of water, which in turn can affect the levels of dissolved oxygen and other important water quality parameters. This may directly affect the fish’s health, happiness, and blood characteristics. 17. The second, the stress: Stress. The pond’s geometric design can affect the fish’s stress levels and blood traits18. The third is enrichment: The geometric design of the pond can be used to provide enrichment for the fish. This can lessen stress and improve the fish’s welfare. it may also positively impact their blood traits. MGD is increasingly being used in fish farming to improve welfare. The specific geometric design that is best for a specific fish. the pond will depend on several variables, such as the pond’s size and the species it supports. That will be raised and the intended use of the pond20. However, some general principles can be followed to improve fish welfare through MGD. One of the key benefits of MGD is that it can help to create a more natural environment for the fish. This is important because fish are naturally attracted to geometric shapes and tend to feel more secure in environments similar to their natural habitat 21. For example, fish have been shown to prefer rectangular ponds with rounded corners, as these shapes are more reminiscent of the natural water bodies that they would find in the wild22
The objective of this study to create MGD in fish ponds is to create a more natural, stimulating, and comfortable environment for the fish. This can be done by giving the fish food and designing the pond with various geometric shapes. with welfare and growth performance.
MATERIALS AND METHODS
This study was conducted in the Fish Laboratory/ Department of Animal Production/ College of Agriculture/ University of Anbar. The experiment was carried out for 120 days to achieve the highest level of welfare by designing tanks using a modern method that helps create the highest level of welfare. Three hundred common carp fish were randomly distributed with an average weight of 90.5 ± 25 grams per fish in twelve plastic tanks with dimensions of 60 × 25 × 60 cm, with each tank having a capacity of 100 liters. The fish were distributed among three experimental treatments, with four replicates for each treatment and 25 fish per replicate, as follows: the first treatment was a control treatment (C), which was represented by raising fish in regular ponds, and the second treatment was fish farming using novelty design ponds in the shape of the H sign (ND). The third treatment was the fish farming treatment in earthen ponds (EP).
Modern Geometric Design (DGM)
DGM was designed using modern engineering drawing software such as AutoCAD and 3DMAX, as shown in Figure 1. If the dimensions are calculated and evaluated according to the welfare standards for fish, and the suitable water requirements are calculated from the engineering perspective, including water quality and oxygen availability, using the WEAP software.
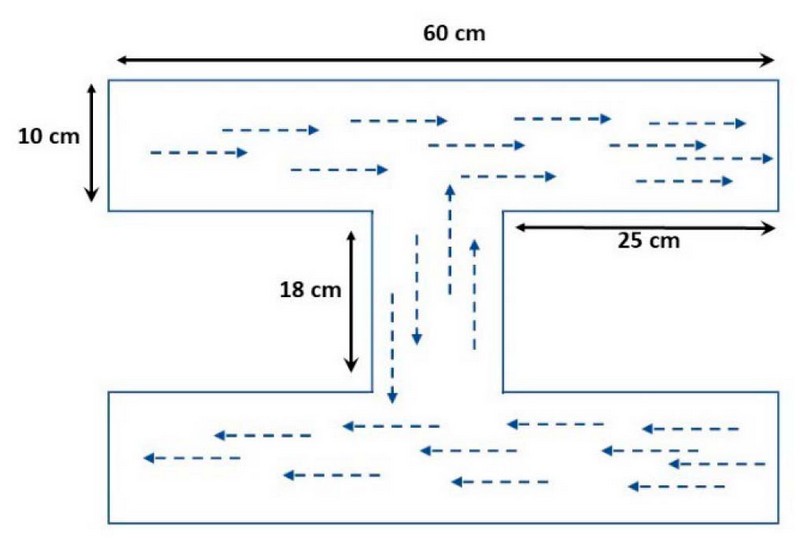
Figure 1. Dimensions of a Modern Geometric Design (MGD)
Water Basin Tests
The results of the water basin tests show the temperature of the water (°C), dissolved oxygen concentration (mg/L), ammonia concentration (mg/L), and pH levels from the beginning to the end of the experiment. The water temperature ranged from 25-20 degrees Celsius throughout the duration of the experiment. Two heaters were used to control the water temperature. The dissolved oxygen concentrations ranged from 5.5-3.5 mg/L during the experiment. The ammonia concentration ranged from 3.00-1.5 mg/L. The pH levels ranged from 7.9-6.7 mg/L, which are within the suitable limits for breeding common carp fish, which range from 8.5-6.0.23
Blood analysis
The blood cellular traits were measured, including red blood cells, hemoglobin, PCV, white blood cells, and the differential count of white blood cells, according to Witeska (2022)24. The fish’s caudal vein was used to draw blood, which was then placed in tiny plastic tubes containing an anticoagulant solution of heparin (0.2 ml/ml blood). Using hemocytometers, the erythrocyte count was calculated for these blood samples. According to Blaxhall and Daisny25, the conversion of hemoglobin (Hb) into red cyanomethaemoglobin under the action of potassium ferricyanide and potassium cyanide was evaluated. Other blood samples were taken and allowed to coagulate for 15 to 20 minutes at 4 degrees Celsius before being centrifuged for 20 minutes at 3,000 rpm to separate the serum. The biochemical blood traits were measured, including Glucose, cholesterol, triglyceride HDL, LDL, GOT, GOT, and LAP, according to Biochemical testing done on the fresh serum. Using test kits provided by (Spectrum Diagnostics), serum glucose (mg/l) was measured. Total protein (g/100 ml) and total fat (g/l) levels were assessed using assay kits provided by Diamond Diagnostics using colorimetric methods. Utilizing assay kits from Spectrum Diagnostics, the activities of aspartate aminotransferase (AST, U/I) and alanine aminotransferase (ALT, U/I) were measured colorimetrically in accordance with the Reitman and Frankel technique (1957). A spectrophotometer (Ultroscopec 3100 Pro) was used to measure the samples.The blood was drawn into micro-capillary and EDTA (anticoagulant) tubes by puncturing the caudal artery at the caudal peduncle. Following the procedure outlined by Blaxhall and Daisny.25
Statistical Analysis
Complete randomization was used for this experiment (CRD). Then, the SAS tool for statistical analysis was used to evaluate the data26. To find significant changes between the averages, Duncan’s polynomial was used to compare the means for each treatment at significance levels of 0.0527.
RESULTS AND DISCUSSION
Table 1 shows the effect of MGD on cellular traits in fish blood; there was a significant improvement in the ND treatment for the RBC, PCV, and HB traits, with values of 3.80, 34.18, and 10.48, respectively, compared to other treatments. Additionally, there was a significant improvement in the WBC and differential count values, indicating progress in the ND treatment compared to the different experimental treatments, with a WBC ratio of 9.1 compared to other experimental therapies. Furthermore, there was a significant improvement in both heterophils and lymphocytes, with values of 66.3 and 23.3, respectively.
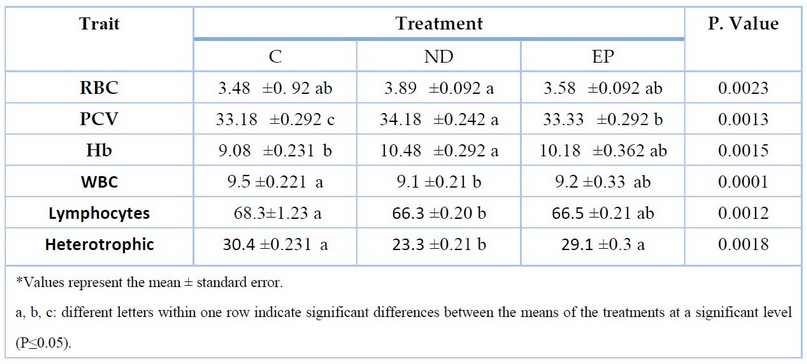
Table 1. The effect of MGD on Blood Cellular traits of fish
Figure 2. shows the effect of the H/L if there is a significant improvement in the ratio of experimental coefficients, as the ND coefficient improved in the H/L ratio value and reached 0.35% compared to other empirical coefficients, which gained 0.44% in C and 0.43% in EP.
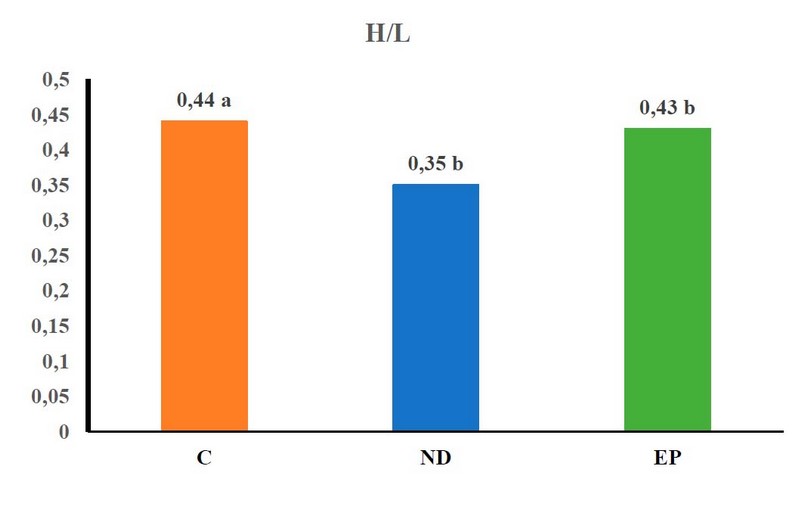
Figure 2. The effect of MGD on H/L traits of fish
Table 2. shows the effect of MGD on biochemical blood traits; there was a significant increase in blood glucose levels for the ND treatment, reaching 129 compared to other experimental therapies. There was also a substantial decrease in cholesterol and triglyceride levels for the ND treatment, getting 132 and 201, respectively. Additionally, there was a significant improvement in HDL levels, reaching 63.1, and a significant decrease in LDL levels, reaching 47.1, compared to other experimental treatments.
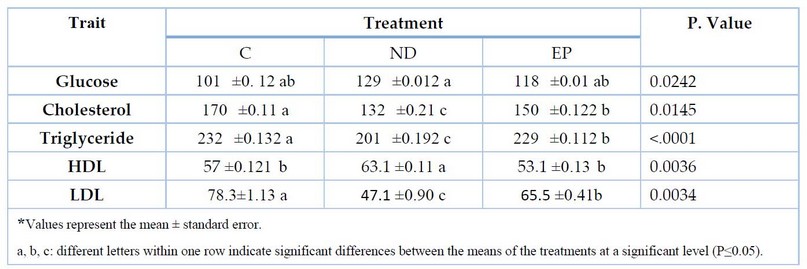
Table 2. The effect of MGD on Blood Biochemical traits of fish
From Figure 3, it is observed that MGD affects liver enzymes GPT and GOT, as there was a significant improvement in the ND treatment in the values of liver enzymes, which reached 53.5 in GPT and 221 in GOT compared to other experimental therapies, which got 68 in GPT for treatment C and 59 in GPT for treatment EP, as well as for GOT, which reached 201, respectively.
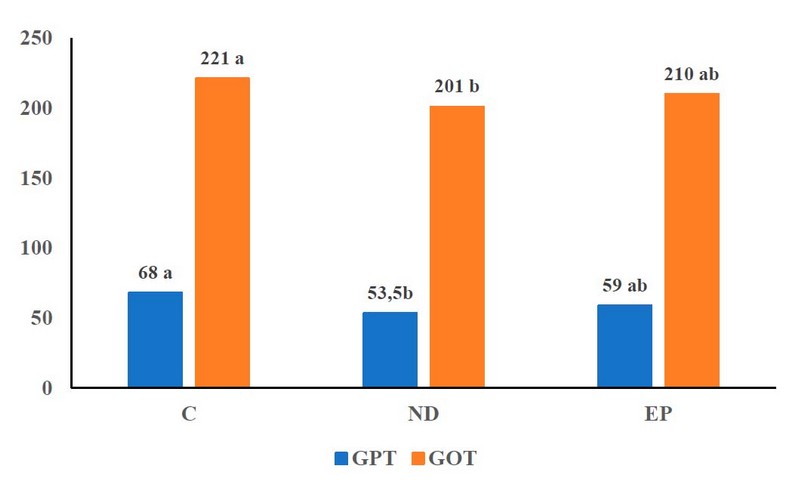
Figure 3. The effect of MGD on Liver enzyme traits of fish
Using a modern and well-studied system with artificial intelligence technology that simulates the external marine environment for fish contributes to providing the highest level of welfare in the fish environment. This is what we observed in our study, as we noticed a significant improvement in the blood characteristics of the fish. The availability of welfare contributes to the improvement of blood characteristics, and this is what has been observed: the welfare of fish can have a significant impact on their red blood cells and the availability of oxygen3. Fish that are stressed or have poor welfare are more likely to have lower levels of red blood cells28, which can lead to problems with oxygen transport. Several factors can contribute to the poor welfare of fish, including low water quality29, high stocking densities, lack of enrichment and handling, and transportation. When fish are stressed, their bodies release hormones that can damage red blood cells. This can lead to a decrease in the number of red blood cells30, as well as a decrease in the amount of oxygen that they can carry. In addition, stressed fish are more likely to develop injuries, which can also lead to blood loss and decreased oxygen levels. Low water quality can also contribute to problems with red blood cells in fish. A rise in parasites and other pathogens brought on by poor water quality can harm red blood cells 31. High stocking densities can also lead to problems with red blood cells in fish. When fish are crowded, they are more likely to compete for food and oxygen30. This can lead to stress and decreased levels of red blood cells. Enrichment is important for fish welfare because it helps to reduce stress and boredom. Fish that are bored or stressed are more likely to develop health issues, such as issues with red blood cells. Fish handling and transportation can be upsetting for them as well. 32. During handling and transportation, fish may be exposed to cold temperatures, rough handling, or low oxygen levels. These stressors can damage red blood cells and lead to problems with oxygen transport. The availability of oxygen is also important for fish welfare13. Fish need a certain amount of oxygen to survive. If the oxygen levels in the water are too low, fish can develop problems with their red blood cells and become stressed. The effect of welfare on glucose metabolism is a complex issue that has not been fully studied. However, there is some evidence to suggest that welfare may have a negative impact on glucose metabolism33. Glucose metabolism is the process by which Glucose is broken down and used for energy. It is essential for the welfare of all cells in the body, including neurons, muscle cells, and red blood cells; additionally crucial to keeping blood sugar levels within a safe range is glucose metabolism 34. One study found that carp fish kept in poor welfare conditions had higher levels of cortisol, a hormone that can impair glucose metabolism35. The study also found that these fish had lower insulin sensitivity levels, meaning they were less able to use Glucose for energy. Another study found that carp fish exposed to stress had lower glycogen levels in their livers.
Glycogen is a form of stored Glucose, and lower glycogen levels can lead to impaired glucose metabolism; these studies suggest that welfare may negatively impact glucose metabolism in carp fish. However, more research is needed to fully understand the mechanisms involved and identify the specific factors contributing to this effect. These studies suggest that welfare may hurt WBC. However, more research is needed to fully understand the mechanisms involved and identify the specific factors contributing to this effect. Some potential mechanisms by which welfare may affect WBC: Stress: Stress can hurt WBC. Fish under stress, such as those living in cramped quarters, may have higher amounts of stress hormones, which might weaken their immune systems. And lead to lower WBC counts37. As well as nutrition, animals not fed a healthy diet may be more likely to develop infections38, which can lead to higher WBC counts39. Also, infection: Animals infected with a disease may have higher WBC counts as the body tries to fight off the infection. Also, Infection: Animals infected with a disease may have higher WBC counts as the body tries to fight off the infection40. It is important to note that these are just potential mechanisms, and more research is needed to confirm their role in the relationship between welfare and WBC. The heterophil-to-lymphocyte (H/L) ratio measures the immune response in animals. A high H/L ratio indicates the animal is under stress or has an infection.
A low H/L ratio indicates that the animal is healthy. There is some evidence to suggest that welfare may have an impact on the H/L ratio. Animals in poor welfare conditions may have higher H/L ratios, indicating they are under stress 37. Stress can depress the immune system, which increases the amount of circulating heterophils 41. Therefore, MGD contributed to providing fish with more welfare due to its comfortable movement and free swimming in the water, which helped reduce their stress hormones and decreased the H/L ratio15. The MGD contributed to improving welfare and promoting a healthy H/L ratio, for example, by providing fish with a clean, comfortable environment and feeding animals a healthy diet. As well as provide fish with opportunities to exercise, and the important role of managing stress levels in fish and monitoring animals for signs of illness or infection42.
There is some evidence to suggest that welfare may have an impact on cholesterol and triglyceride levels in fish. Fish kept in poor welfare conditions may have higher cholesterol and triglyceride levels due to several factors. One factor is stress. Cortisol, a hormone that can increase cholesterol and triglyceride levels, can be produced more frequently due to stress. Another factor is diet. Fish fed a diet high in saturated fat or cholesterol may have more elevated cholesterol and triglyceride levels. Finally, poor water quality can also contribute to high cholesterol levels in fish43. There is some evidence to suggest that welfare may have an impact on HDL (high-density lipoprotein) and LDL (low-density lipoprotein) cholesterol levels in fish. Animals that are kept in poor welfare conditions may have lower HDL levels and higher LDL levels due to some factors. The stress factor is one. Cortisol, a hormone that can cause HDL levels to drop and LDL levels to rise, might be produced more frequently due to stress. Another factor is diet. Animals fed a diet high in saturated fat or cholesterol may have lower HDL and higher LDL levels. Finally, poor water quality can also contribute to low HDL and high LDL levels in fish30.
CONCLUSIONS
In conclusion, the use of a modern system for fish Ponds designed with artificial intelligence and nature-simulating programs has led to an increase in their well-being. This is manifested in their ability to live freely, reducing fear and isolation, resulting in improved physiological characteristics, especially in red and white blood cells, differential blood cell count, H/L ratio, and blood glucose levels. This stress reduction enhances their biochemical traits, mainly blood glucose, which increases oxygen availability. As a result, fish have evolved their biological processes and increased their production rate.
Supplementary Materials:
No Supplementary Materials.
Author Contributions:
M. Y. Khudair designed modern engineering basins that are suitable for the external environment using advanced software. I draw basins to be identical to reality. S. M. Abdulateef, T. Th. Mouhammed; methodology, writing—original draft preparation, S. M. Abdulateef writing—review and editing and paraphrasing, H. S. Alamili manufactured the basins and arranged them to be suitable for the nature and care of fish breeding. All authors have read and agreed to the published version of the manuscript.
Funding:
This research received no external funding.
Institutional Review Board Statement:
The study was conducted in accordance with the protocol authorized by the University of Anbar Ethics Committee.
Informed Consent Statement:
No Informed Consent Statement.
Data Availability Statement:
No Data Availability Statement.
Conflicts of Interest:
The authors declare no conflict of interest.
Acknowledgments:
The authors are thankful for the help of the Animal Resources Field Manager, The College Dean, and the Head of the Animal Production Dept. The College of Agriculture, University of Anbar, Iraq. We would also like to thank the undergraduate students for their valuable help and technical assistance in conducting this research.
REFERENCES
(1) Kord, M. I.; Srour, T. M.; Omar, E. A.; Farag, A. A.; Nour, A. A. M.; Khalil, H. S. The Immunostimulatory Effects of Commercial Feed Additives on Growth Performance, Non-Specific Immune Response, Antioxidants Assay, and Intestinal Morphometry of Nile Tilapia, Oreochromis Niloticus. Front. Physiol. 2021, 12. https://doi.org/10.3389/fphys.2021.627499.
(2) Kord, M. I.; Maulu, S.; Srour, T. M.; Omar, E. A.; Farag, A. A.; Nour, A. A. M.; Hasimuna, O. J.; Abdel-Tawwab, M.; Khalil, H. S. Impacts of Water Additives on Water Quality, Production Efficiency, Intestinal Morphology, Gut Microbiota, and Immunological Responses of Nile Tilapia Fingerlings under a Zero-Water-Exchange System. Aquaculture 2022, 547. https://doi.org/10.1016/j.aquaculture.2021.737503.
(3) Zaidy, A. B.; Eliyani, Y.; Ruchimat, T. Effects of feed reduction on growth performance, water quality, and hematology status of african catfish, clarias gariepinus reared in biofloc pond system. Indones. Aquac. J. 2022, 17 (1). https://doi.org/10.15578/iaj.17.1.2022.37-43.
(4) Yang, X. wen; Liu, L.; Jiang, D. li; Wang, C. li; Sun, A. dong; Shi, Z. dan. Improving Geese Production Performance in “ Goose-Fish” Production System by Competitive Reduction of Pathogenic Bacteria in Pond Water. J. Integr. Agric. 2012, 11 (6). https://doi.org/10.1016/S2095-3119(12)60091-4.
(5) Neori, A.; Krom, M. D.; Ellner, S. P.; Boyd, C. E.; Popper, D.; Rabinovitch, R.; Davison, P. J.; Dvir, O.; Zuber, D.; Ucko, M.; Angel, D.; Gordin, H. Seaweed Biofilters as Regulators of Water Quality in Integrated Fish-Seaweed Culture Units. Aquaculture 1996, 141 (3–4). https://doi.org/10.1016/0044-8486(95)01223-0.
(6) Pitcher, K. A.; Soluk, D. A. Fish Presence and Inter-Patch Connectivity Interactively Alter the Size of Emergent Insects in Experimental Enclosures. Ecosphere 2018, 9 (3). https://doi.org/10.1002/ecs2.2118.
(7) Otieno, N. E.; Shidavi, E. Effectiveness of Physical Barriers and Enhanced Fertilization in Controlling Predation on Tilapia and Catfish Aquaculture Systems by Four Piscivorous Water Bird Families. Front. Sustain. Food Syst. 2022, 6. https://doi.org/10.3389/fsufs.2022.1018064.
(8) Thanh, D. T.; Ty, N. M.; Hien, N. V.; Berg, H.; Nguyen, T. K. O.; Vu, P. T.; Minh, V. Q.; Da, C. T. Effects of Organic Fertilizers Produced from Fish Pond Sediment on Growth Performances and Yield of Malabar and Amaranthus Vegetables. Front. Sustain. Food Syst. 2023, 7. https://doi.org/10.3389/fsufs.2023.1045592.
(9) Zhou, M.; Xu, Y.; Ouyang, P.; Ling, J.; Cai, Q.; Huang, L.; Zhou, X.; Zheng, L. Evolution and Distribution of Resistance Genes and Bacterial Community in Water and Biofilm of a Simulated Fish-Duck Integrated Pond with Stress. Chemosphere 2020, 245. https://doi.org/10.1016/j.chemosphere.2019.125549.
(10) Oron, G.; Appelbaum, S.; Guy, O. Reuse of Brine from Inland Desalination Plants with Duckweed, Fish and Halophytes toward Increased Food Production and Improved Environmental Control. Desalination 2023, 549. https://doi.org/10.1016/j.desal.2022.116317.
(11) Martin, M.; Kalesh, G.; Rajan, A.; V, V. P.; George, F.; Professor, A. Zero Aqua Waste Management; 2019.
(12) Green, B. W.; McEntire, M. E. Comparative Water Quality and Channel Catfish Production in Earthen Ponds and a Biofloc Technology Production System. J. Appl. Aquac. 2017, 29 (1). https://doi.org/10.1080/10454438.2016.1261751.
(13) Lv, M.; Gan, H.; Ruan, Z.; Yang, H.; Wang, R.; Shafique, L.; Naz, H.; Ma, H. Distributions of and Correlations between Cd, Cr, and Hg Concentrations in Suspended Particles and Sediment in Aquaculture Ponds and in Cirrhinus Molitorella Tissues. Pak. J. Zool. 2020, 52 (5). https://doi.org/10.17582/JOURNAL.PJZ/20190317200334.
(14) Seibel, H.; Baßmann, B.; Rebl, A. Blood Will Tell: What Hematological Analyses Can Reveal About Fish Welfare. Frontiers in Veterinary Science. 2021. https://doi.org/10.3389/fvets.2021.616955.
(15) Rickert, D.; Simon, R.; von Fersen, L.; Baumgartner, K.; Bertsch, T.; Kirschbaum, C.; Erhard, M. Saliva and Blood Cortisol Measurement in Bottlenose Dolphins (Tursiops Truncatus): Methodology, Application, and Limitations. Animals 2022, 12 (1). https://doi.org/10.3390/ani12010022.
(16) Carbajal, A.; Soler, P.; Tallo-Parra, O.; Isasa, M.; Echevarria, C.; Lopez-Bejar, M.; Vinyoles, D. Towards Non-Invasive Methods in Measuring Fish Welfare: The Measurement of Cortisol Concentrations in Fish Skin Mucus as a Biomarker of Habitat Quality. Animals 2019, 9 (11). https://doi.org/10.3390/ani9110939.
(17) Ibrahim, L. A.; ElBastamy ElSayed, E. S. The Influence of Water Quality on Fish Tissues and Blood Profile in Arab Al-Ulayqat Lakes, Egypt. Egypt. J. Aquat. Res. 2023, 49 (2). https://doi.org/10.1016/j.ejar.2023.01.006.
(18) McArley, T. J.; Sandblom, E.; Herbert, N. A. Fish and Hyperoxia—From Cardiorespiratory and Biochemical Adjustments to Aquaculture and Ecophysiology Implications. Fish Fish. 2021, 22 (2). https://doi.org/10.1111/faf.12522.
(19) Zhang, Y.; Chua, S. Leptin Function and Regulation. Compr. Physiol. 2018, 8 (1). https://doi.org/10.1002/cphy.c160041.
(20) Susilo, H.; Saleha, Q.; Darmansyah, O.; Oktawati, N. O.; Maryanto, F.; Zulkarnain; Erwiantono. Determinants of Fish Farmers’ Welfare in Brackish Water Pond Culture in Indonesia: Fish Farmer Terms of Trade Index. AACL Bioflux 2021, 14 (2).
(21) Daskalova, A. Farmed Fish Welfare: Stress, Post-Mortem Muscle Metabolism, and Stress-Related Meat Quality Changes. International Aquatic Research. 2019. https://doi.org/10.1007/s40071-019-0230-0.
(22) Barreto, M. O.; Rey Planellas, S.; Yang, Y.; Phillips, C.; Descovich, K. Emerging Indicators of Fish Welfare in Aquaculture. Reviews in Aquaculture. 2022. https://doi.org/10.1111/raq.12601.
(23) Lindholm‐Lehto, P. Water Quality Monitoring in Recirculating Aquaculture Systems. Aquac. Fish Fish. 2023, 3 (2). https://doi.org/10.1002/aff2.102.
(24) Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological Methods in Fish – Not Only for Beginners. Aquaculture. 2022. https://doi.org/10.1016/j.aquaculture.2021.737498.
(25) Blaxhall, P. C.; Daisley, K. W. Routine Haematological Methods for Use with Fish Blood. J. Fish Biol. 1973, 5 (6). https://doi.org/10.1111/j.1095-8649.1973.tb04510.x.
(26) System., S. a S. S. A. SAS User´s Guide; 2004.
(27) Duncan, D. B. Multiple Range and Multiple F Tests. Biometrics 1955, 11 (1). https://doi.org/10.2307/3001478.
(28) Abdul-Lateif, K. M.; Abdulateef, S. M. The Effect of Injecting Hatching Eggs with Different Concentrations of Biotin on the Quality and Physiological Characteristics of the Hatched Chicks. Iraqi J. Vet. Sci. 2012, 26. https://doi.org/10.33899/ijvs.2012.168764.
(29) Stotz, S. A. Academy of Nutrition and Dietetics Complete Food and Nutrition Guide. J. Nutr. Educ. Behav. 2020, 52 (1). https://doi.org/10.1016/j.jneb.2019.11.002.
(30) Abdel-Tawwab, M.; Abdulrahman, N. M.; Ahmad, V. M.; Ramzi, D. O. M.; Hassan, B. R. Effects of Dietary Oak (Quercus Aegilops L.) Acorn on Growth Performance, Somatic Indices, and Hemato-Biochemical Responses of Common Carp, Cyprinus Carpio L., at Different Stocking Densities. J. Appl. Aquac. 2022, 34 (4). https://doi.org/10.1080/10454438.2021.1902450.
(31) Stien, L. H.; Lind, M. B.; Oppedal, F.; Wright, D. W.; Seternes, T. Skirts on Salmon Production Cages Reduced Salmon Lice Infestations without Affecting Fish Welfare. Aquaculture 2018, 490. https://doi.org/10.1016/j.aquaculture.2018.02.045.
(32) Raposo De Magalhães, C.; Schrama, D.; Farinha, A. P.; Revets, D.; Kuehn, A.; Planchon, S.; Rodrigues, P. M.; Cerqueira, M. Protein Changes as Robust Signatures of Fish Chronic Stress: A Proteomics Approach to Fish Welfare Research. BMC Genomics 2020, 21 (1). https://doi.org/10.1186/s12864-020-6728-4.
(33) Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of Stocking Density on the Growth Performance, Physiological Parameters, Redox Status and Lipid Metabolism of Micropterus Salmoides in Integrated Rice–Fish Farming Systems. Antioxidants 2022, 11 (7). https://doi.org/10.3390/antiox11071215.
(34) Baßmann, B.; Brenner, M.; Palm, H. W. Stress and Welfare of African Catfish (Clarias Gariepinus Burchell, 1822) in a Coupled Aquaponic System. Water (Switzerland) 2017, 9 (7). https://doi.org/10.3390/w9070504.
(35) F. I. Al-Bazy; S. M. Abdulateef; B. F. Sulimn. Impact of feeds containing optifeed®, vêo® premium, and oleobiotec® on the lipid peroxidation of male broilers under heat stress. J. Life Sci. Appl. Res. 2022, 3 (2), 25–31.
(36) Jiang, D.; Wu, Y.; Huang, D.; Ren, X.; Wang, Y. Effect of Blood Glucose Level on Acute Stress Response of Grass Carp Ctenopharyngodon Idella. Fish Physiol. Biochem. 2017, 43 (5). https://doi.org/10.1007/s10695-017-0383-y.
(37) Hoseini, S. M.; Abtahi, B.; Yousefi, M. Effects of Fasting on Metabolic and Immunological Responses of Common Carp (Cyprinus Carpio) to a Further Acute Stress. Aquac. Res. 2019, 50 (4). https://doi.org/10.1111/are.13992.
(38) A.R. Alkhateeb; W. I. Ibrahim; A.A. E. Taha. Body conformation with daily milk yield relationship on buffaloes. J. Life Sci. Appl. Res. 2022, 3 (1), 1–3.
(39) Salamanca, N.; Moreno, O.; Giráldez, I.; Morales, E.; de la Rosa, I.; Herrera, M. Effects of Dietary Phenylalanine and Tyrosine Supplements on the Chronic Stress Response in the Seabream (Sparus Aurata). Front. Physiol. 2022, 12. https://doi.org/10.3389/fphys.2021.775771.
(40) Lieke, T.; Meinelt, T.; Hoseinifar, S. H.; Pan, B.; Straus, D. L.; Steinberg, C. E. W. Sustainable Aquaculture Requires Environmental-Friendly Treatment Strategies for Fish Diseases. Reviews in Aquaculture. 2020. https://doi.org/10.1111/raq.12365.
(41) Davis, A. K.; Maney, D. L. The Use of Glucocorticoid Hormones or Leucocyte Profiles to Measure Stress in Vertebrates: What’s the Difference? Methods in Ecology and Evolution. 2018. https://doi.org/10.1111/2041-210X.13020.
(42) Graczyk, S.; Orda, J.; Zawadzki, W.; Baran, M.; Czerski, A. Effect of Protein Sources and Restricted Feeding on Some Blood Parameters and Performance of Chickens. Med. Weter. 2005, 61 (9).
(43) Assem, H.; Khalifa, A.; ELSalhia, M. Physiological and Microbiological Indices as Indicators of Evaluating Dietary Fungi Degraded Date Pits as a Probiotic for Cultured Nile Tilapia Oreochromis Niloticus Fingerling and Its Effect on Fish Welfare. Egypt. J. Aquat. Res. 2014, 40 (4). https://doi.org/10.1016/j.ejar.2014.10.004.
(44) Suárez, M. D.; Trenzado, C. E.; García-Gallego, M.; Furné, M.; García-Mesa, S.; Domezain, A.; Alba, I.; Sanz, A. Interaction of Dietary Energy Levels and Culture Density on Growth Performance and Metabolic and Oxidative Status of Rainbow Trout (Oncorhynchus Mykiss). Aquac. Eng. 2015, 67. https://doi.org/10.1016/j.aquaeng.2015.06.001.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Khudai M Y, Abdulateef S M, Mouhammed T Th, Alamili H S. Use of modern geometric design of fish ponds to increase welfare and blood parameters. Revis Bionatura 2023;8 (2) 82. http://dx.doi.org/10.21931/RB/2023.08.02.82
