2023.08.01.81
Files > Volume 8 > Vol 8 No 1 2023
Investigation of the Fusarium oxysporum-caused watermelon wilt infection and testing of some hybrids' susceptibility to infection
Mohammed Abd Saleh 1,* and Dr. Jasim Mahmood Abed 2
1 Department of Plant Protection, College of Agriculture, University of Anbar, Iraq. [email protected].
2 Department of Plant Protection, College of Agriculture, University of Anbar, Iraq.
* Correspondence: [email protected];
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.81
ABSTRACT
This study was conducted in the College of Agriculture, the University of Anbar, which aimed to diagnose the Fusarium oxysporium. The field survey results showed the spread of the disease in all areas for the seasons 2020-2021 and 2021-2022 in the governorates of Anbar, Baghdad, Salah al-Din, and Babylon. The infection rate ranged from 20-66%. The infection rate ranged from 20-66%, and the results of the phenotypic and molecular diagnosis showed the presence of the Fusarium oxysporium in the isolated samples. The results of the hybrid sensitivity test ( Jocker, Natasha, Pasha) for the three most pathogenic isolates, as all the hybrids were sensitive to highly pathogenic fungi, and the isolates were All of them are highly pathogenic.
Keywords: Fusarium wilt, watermelon, hybrids, sensitivity
INTRODUCTION
Watermelon ( Citrull lanatus), which belongs to the Cucurbitaceae family, is considered one of the most crucial summer vegetable crops from an economical and nutritional point of view. Its fruits are rich in carbohydrates, especially sugars, in addition to fiber and mineral salts potassium, iron, and potassium. It also contains lycopene, carotene pigments, and vitamins such as vitamin B6. C, A 1, The cultivated area of the Watermelon crop in Iraq is estimated at 16,891 hectares, with a production of 222,595 tons 2. The watermelon is infected with many pathogens, including those that affect the roots in the soil 3. The fusarium wilt disease caused by the fungus Fusarium oxysporum is considered one of the most common diseases of the rhizome plant. It causes significant losses in economic terms all over the world 4–6 (Hao et al., 2010). Smith is the first researcher to describe the Fusarium oxysporum and conducted studies on infection and transmission within the plant tissue and the formation of tyloses in plant vessels 7. Fusarium sp spread in many countries such as Pakistan, Egypt, South Africa, Brazil, Central America, and Mexico 8,9. The Fusarium oxysporum is the main cause of wilt diseases in more than a hundred species of important economic plants. Plants of the cucurbit family are widely infected with vascular wilt pathogens caused by different physiological strains of F. oxysporum 10. Despite the occurrence of early infection of the plant, the symptoms of infection with the disease can only be seen at the stage of plant stress in the stage of flowering and contracting, as the growth of the plant is normal at the beginning of the infection, but when the plant reaches the stage of fruit formation, the symptoms appear on the leaves as they change to the color yellow, then one of the plant branches withers, and then the infection develops into complete wilt of the plant 10,11. The wilt disease caused by the fungus Fusarium oxysporum is a determining factor for cultivating sodomy in large areas of Iraq. According to unpublished data, field surveys over the past ten years indicated the spread of the disease significantly. Several methods were used to combat the vascular wilt disease caused by Fusarium oxysporum, including Chemical pesticides and agricultural rotations in addition to the use of resistant varieties and solar pasteurization as well as a biological control to control vascular wilt disease 12. The study aims to show the spread of diseases in areas of central Iraq in the governorates of (Anbar - Salah al-Din - Baghdad - Babil) and to diagnose the caused (Fusarium oxysporum) pathogen phenotypically and by using molecular methods and to test the sensitivity of some Watermelon hybrids to infection with this disease.
MATERIALS AND METHODS
Sample Collection
Samples of Watermelon plants that showed symptoms of fusarium wilt were collected from different fields in four governorates: Baghdad, Anbar, Salah al-Din, and Babil. The collected samples were placed in polyethylene bags, and the date of collection, the region's name and the governorate were recorded on them. The percentage of injury was calculated according to the following equation:
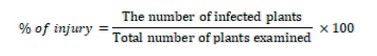
Isolation and identification of fungi from roots and stems of watermelon plants
The samples were brought to the laboratory, washed with tap water for 30 minutes, and thoroughly cleaned of the suspended soil. Parts of the roots, the bases of the stems and the stem were cut with scissors into small pieces 0.5 cm in length and superficially sterilized by placing them in a petri dish containing a solution of sodium hypochlorite at a concentration of 1% free chlorine for two minutes after that. Those pieces were washed with sterile water to remove the effect of sterilization; then they were placed on sterile filter paper to dry, then those pieces were transferred to Petri dishes with a diameter of 9 cm, where four pieces were placed for each dish using sterile forceps inside the luminizer. These dishes contain medium PDA (Potato dextrose agar). The medium was prepared by dissolving 40 g of prepared medium PDA per 1000 ml of water, then placed inside the purifier for sterilization at a temperature of 121 °C and a pressure of 1.5 kg / cm2 for 20 minutes. The medium was left to cool slightly, then the antibiotic amoxicillin (at a concentration of 200 mg / l) was added before it solidified. Then, the medium was poured into the dishes and left to solidify. Petri dishes were closed with parafilm tape, and the date of the day and the area's name was recorded. Those dishes were placed in the incubator at 25 ± 2 ° C. The dishes were left for 3 days. After that, a part of the developing fungal colony was taken from the plant part and examined under a light microscope. The Fusarium oxysporum was identified through its phenotypic characteristics at the genus level and species 13; these fungi were purified by taking a handful of sterile needles and grown on new plates to obtain pure fungi. Isolates preserved at 4c.
Molecular diagnosis of Fusarium oxysporum
Three isolates that proved to be highly pathogenic were selected for molecular diagnosis and to confirmation of the phenotypic diagnosis process. The DNA extraction process for isolates of the fungus Fusarium oxysporum was carried out in the Molecular Diagnostics Laboratory belonging to Al-Musayyab Bridge Company (Baghdad - Iraq) using the Master Mix extraction tool manufactured by Bioneer Company. Korean according to the method recommended by the manufacturer. Using a pair of primers, ITS1 as a forward (F: 5′- TCCGTAGGTGAACCTGCGG-3′) and ITS4 as a reverse (R: 5′TCCTCCGCTTATTGATATGC-3′), the target region of the fusarium oxysporum was amplified using a polymerase chain reaction (PCR) polymerase chain reaction (PCR) device 14.
Pathogenicity test of Fusarium oxysporum isolates
The pathogenicity was tested according to the method of Bolkan and Butler (1974) for 12 fungal isolates in 9 mm Petri dishes containing PDA culture media and with 3 replicates for each isolate. The colony of Fusarium is 6 days old, and the dishes were left in the incubator for 3 days at 25 ± 2 °C. Then the local radish seeds were planted in the dishes after being washed with tup water to remove dust. Then they were superficially sterilized with sodium hypochlorite solution (1%) free chlorine for two minutes, then placed in distilled water for 2 minutes. They were placed on a sterile filter paper two minutes later to dry. The seeds were planted 1 cm from the edge of the fungal colony by 10 seeds per plate, and three dishes were left without fusarium oxysporium for comparison as control. After that, the percentage of germination of seeds was calculated with the treatments and compared with the control to determine the most severe and influential fungi on seed germination.
Citrull lanatus sensitivity to Fusarium oxysporum
A sensitivity test experiment for the pathogenic Fusarium oxysporum isolates was carried out on seedlings of three hybrids: Pasha(Charleston Gray), Natasha( Crimson Sweet), and Joker (Charlee) in the greenhouse of the Plant Protection Department, College of Agriculture, University of Anbar, according to a complete random design (CRD).( Mixture + Petmos) at a ratio of 1:1 sterilized using the sterilizer device at a temperature of 121 °C and a pressure of 1.5 kg / cm2 for 60 minutes in two phases separated by one day. The inoculum of fusarium oxysporum was added to the three most pathogenic isolates at the rate of 10 g / kg of fusarium oxysporum pollen grown on millet seeds after three days. The seeds of the three hybrids were planted in pots (three seeds per pot) with three replications for each isolate. After 10 weeks of planting, the severity of the infection was calculated. The experiment was carried out according to the CRD with three replications for each treatment, and the pots were irrigated regularly and with time. The plants were uprooted, and the severity of the disease was calculated according to formula 15.
RESULTS
Field survey of fusarium wilt diseases on watermelon
The results of the field survey are shown in table 1, where the prevalence of fusarium wilt disease in all areas covered by the study for the agricultural season 2020-2021 and 2021-2022 in the governorates of Anbar - Baghdad - Salah al-Din - Babil, and the infection rate ranged between 20-66%. This percentage cannot be ignored as it causes economic losses to plants, as well as their use of large amounts of organic fertilizers. The sensitivity of the cultivated varieties may have a role in the spread of the disease, perhaps because the cultivated varieties of sodomy may be resistant to some strains of the pathogen. The deterioration of the land and agricultural cycles With other crops reduced the accumulation of pathogenic fungus pollen in the cultivated lands, as well as the solar Sterilization process.
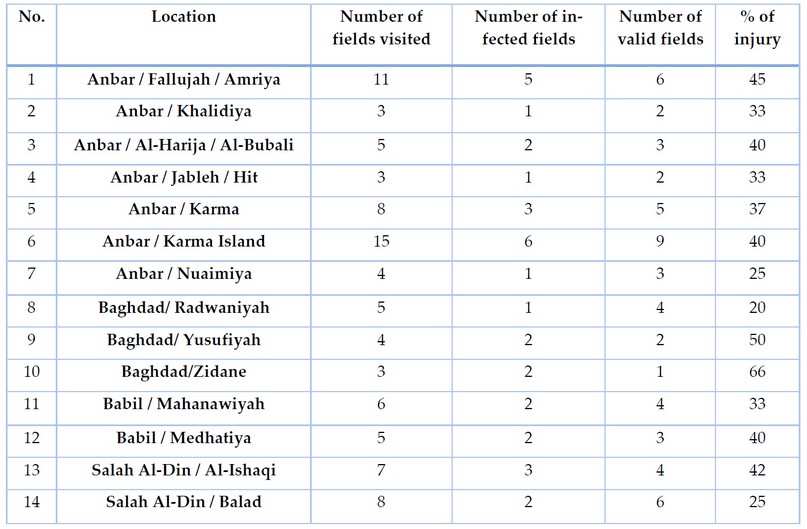
Table 1. A field survey of Fusarium wilts disease on the ascendant in some governorates of Iraq for the agricultural season.
The results of isolation and diagnosis showed in Table (2), and the presence of the fungus F. oxysporum in all samples collected from the watermelon plants showed symptoms of the disease..
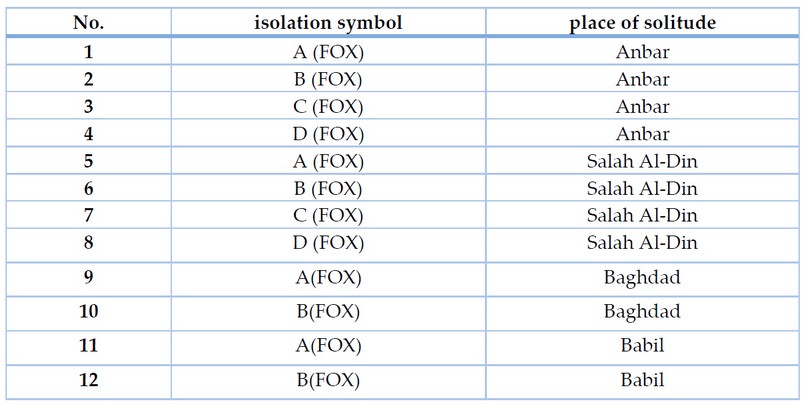
Table 2. Shows the distribution of F. oxysporum was isolates
Molecular diagnosis of F. oxysporum using Polymerase Chain Reaction (PCR) technique.
After DNA extraction from the conidial mycelium spores, a polymerase chain reaction was carried out to amplify the DNA of the ITS (Internal Transcribed Spacer) using the primer pair (ITS1-ITS4), and the results of the electrophoresis using ultraviolet rays showed the presence of A clear band. The size of the amplicon was 550 base pairs with the ladder of DNA on gel electrophoresis.
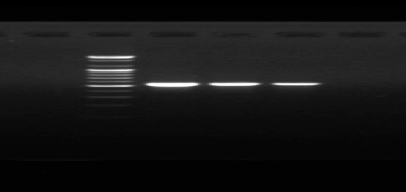
Figure 1. Showing the electrophoresis on agarose gel of the products of DNA replication
Pathogenicity test of isolates of the pathogenic fungus F. oxysporum using radish seeds
The results of the test showed that all isolates of the F. oxysporum induced a significant reduction in the percentage of radish seeds germination in the laboratory (Table 4), as the percentage of germination was 0.00-63.33%. Compared to the control treatment in which the germination rate was 100%. Where isolate Anbar D achieved the highest percentage of a significant reduction, as it completely inhibited the germination of radish seeds, followed by isolates Anbar C, Anbar A, Salah al-Din D, Anbar B, Salah al-Din A, Baghdad B, Babel B, Babel A, Baghdad A, Salah al-Din C and Salah al-Din B, where the germination rate reached 10, 13.33, 16.66, 20, 20, 20, 23.33, 33.33, 43.33, 56.66, 63.33%, respectively. The reason for the difference in the rates of inhibition may be due to the genetic variation between members of the species, as they are isolated from different regions, in addition to the difference in the production of enzymes that degrade pectin and cellulose, as well as the difference in the production of secondary metabolites that lead to rotting seeds and preventing them from germination.
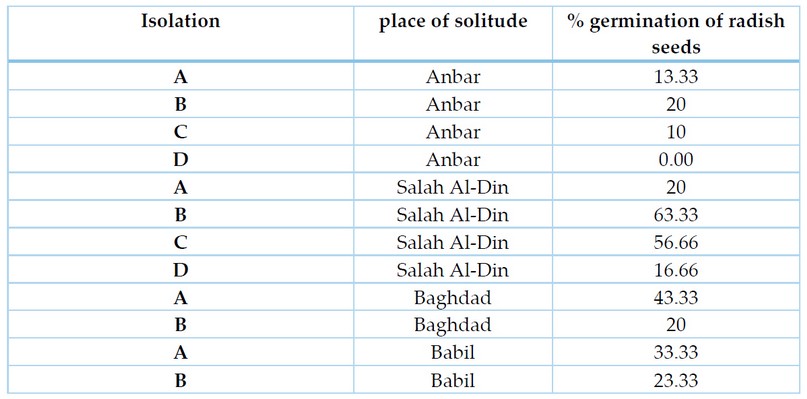
Table 3. shows the pathogenicity of isolates of F. oxysporum on radish seeds
Sensitivity test hybrids to F. oxysporum
This study showed that all the tested Watermelon hybrids were sensitive to infection with isolates (D, C, A) of the F. oxysporum but varying degrees between them (Table 4). 0.362, while the highest degree of sensitivity to infection with F. oxysporum isolates was the Pasha hybrid, with a severity of infection of 0.383, while the hybrid Natasha showed a moderate disease severity of 0.362 compared to the Joker and Pasha hybrids, and these differences in varieties may be attributed to infection with pathogens in general as a result of the difference in composition Plant cell walls or the degree to which they contain another type of incompatibility as mentioned in previous studies 17(Lagrimini et al., 1993). Al-Anbar isolates D achieved the highest infection severity of the tested Watermelon hybrids. It reached 0.538. Also, Al-Anbar A and Al-Anbar C isolates showed infection severity of 0.464 and 0.417, respectively.
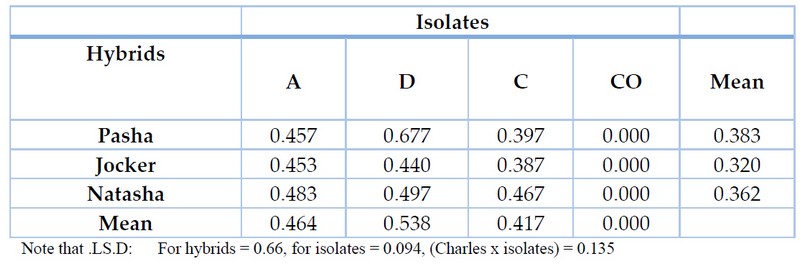
Table 4. shows the sensitivity of Watermelon hybrids to F. oxysporum
DISCUSSION
The colors of the fungal isolates grown on the PDA culture medium varied from white to pinkish red, where the thread was observed. Divided fungi and conidia of their three types: Microconidia, Macroconidia, and Chlamydospores. These traits are identical to F. oxysporum 16. Furthermore, the hybrid Natasha showed a moderate disease severity of 0.362 compared to the Jocker and Pasha hybrids, and these differences in varieties may be attributed to infection with pathogens in general as a result of the difference in composition Plant cell walls or the degree to which they contain another type of incompatibility as mentioned in previous studies 17. Moreover, the reason for the difference in this pathogenicity may be due to the different abilities of F. oxysporum isolates to secrete different enzymes, including chitin and pectinase enzyme, which cause the decomposition of the plant cell and allow the hyphae Fungi to enter through the crust into the carrier vessels, which leads to the closure of those vessels as a result of the presence of the mycelium and its reproductive parts in them, causing water stress on the plants, offset by the formation of tyloses as a defense method from the plant, which contributes to closing these vessels to prevent the progression of pathogenic fungi inside the transporting vessels 18. In addition, some types of Fusarium secrete phytotoxins such as Fusaric acid. Studies have shown that Fusaric acid can cause damage to the membranes of host cells, in addition to increasing the oxygen content in mitochondria and inhibiting the production of ATP energy, causing cell death, so the accumulation of pathogens In the root zone and microorganism imbalance causes wilting of foliar plants 5. The differences in the degree of sensitivity or resistance of hybrids to infection with fungi or microorganisms, in general, are due to the difference in the composition of plant cell walls in the degree to which they contain active substances in stimulating plant defenses, such as containing different percentages of Cellulose, Callose, Lignin and Subrin, which play an important role in preventing Or reduce the penetration of plant cells from microorganisms, so the sensitivity of any type of plant to pathogenic fungi or resistance is related to the mechanism of action of the biochemical defense system to a greater degree than its structural defenses 17.
CONCLUSIONS
The results of the field survey showed the spread of Fusarium wilts on watermelons in all the areas covered by the survey. The F. oxysporum was diagnosed in all watermelon samples that showed symptoms of fusarium wilt. The fungus was diagnosed microscopically, depending on the shape of the conidia, the conidial carrier and the structures it forms. The experiment of cross-susceptibility of plants to F.oxysporum showed that all of them were sensitive to infection with the F.oxysporum The sensitivity different depens on the strain of watermelon.
REFERENCES
1. Schaefer H, Renner SS. Cucurbitaceae. In: Flowering plants eudicots. Springer; 2010. p. 112–74.
2. Book FAOSY. World food and agriculture, Food and Agriculture Organization of the United Nations, Economic and Soc. Dev Dept, Roma. 2017;
3. Noaman A I, Khalaf R M, Emad GH, Al-Abbasy, Mohammed Th. T. Effect of flaxseed oil dosing on fertility, growth characteristics and some physical, biochemical, and hormonal blood parameters during the early pregnancy of Awassi ewes. Revis Bionatura. 2022;7(4) 5. http://dx.doi.org/10.21931/RB/2022.07.04.5.
4. Abed JM, Farhan TA, Kadhum AA, Abed MM, Zaki NA, Edbeib MF. Effect of some biocontrol factors and their efficacy in resistance to Fusarium wilt disease caused by Fusarium oxysporum f. sp. cucumerinum on cucumber plant under open field conditions. In: AIP Conference Proceedings. AIP Publishing LLC; 2019. p. 20031.
5. Wu H, Yin X, Liu D, Ling N, Bao W, Ying R, et al. Effect of fungal fusaric acid on the root and leaf physiology of watermelon (Citrullus lanatus) seedlings. Plant Soil. 2008;308(1):255–66.
6. Turóczi G, Posta K, Badenszky L, Bán R. Fusarium wilt of water melon caused by Fusarium solani in Hungary. Plant Breed Seed Sci. 2011;63:23–8.
7. Martyn RD, Vakalounakis DJ. Fusarium wilts of greenhouse cucurbits: melon, watermelon, and cucumber. Fusarium wilts Greenh Veg Ornam Crop APS Press St Paul, MN. 2012;298–305.
8. Akbar A, Hussain S, Ullah K, Fahim M, Ali GS. Detection, virulence and genetic diversity of Fusarium species infecting tomato in Northern Pakistan. PLoS One. 2018;13(9):e0203613.
9. Zheng S-J, García-Bastidas FA, Li X, Zeng L, Bai T, Xu S, et al. New geographical insights of the latest expansion of Fusarium oxysporum f. sp. cubense tropical race 4 into the greater Mekong subregion. Front Plant Sci. 2018;9:457.
10. Egel DS, Martyn RD. Fusarium wilt of watermelon and other cucurbits. Plant Heal Instr. 2007;10:1094.
11. Al-Maathedy, M. H., Mohammed, Th. T. & Al-Asha'ab, M. H. The effect of vitamin e supplementation and different levels of dried tomato pomace on common carp diets (cyprinus carpio l.) on productive performance. Biochemical and Cellular Archives. 2020, 20(2): 5371-5377.
12. Hussien, S. .; Doosh, K. S. . Production And Characterization Of Β-Galactosidase Enzyme In The Plant Extract From (Ziziphus Spina-Christi) And Its Application In Milk. ). Journal of Life Science and Applied Research. 2021, 2, 1-8..
13. Booth C. Fusarium. Laboratory guide to the identification of the major species. Commonwealth Mycological Institute.; 1977.
14. F. T. Al-Rawi, Y. T. Abdul-Rahaman , Abdullah I.Noaman , Th. T. Mohammed, S. M Abdulateef, Nadia Jebril and KI. Mahmud. Role of ascorbic acid and appetite stimulants on a few blood serum biochemical characteristics in pregnant Iraqi ewes under heat stress. Al-Rawi F T, Abdul-Rahaman Y T, Noaman Revis Bionatura 2022;7(4) 6. http://dx.doi.org/10.21931/RB/2022.07.04.6..
15. McKinney H. INFLUENCE OF SOIL TEMPERATURE AND MOISTURE ON INFECTION OF WHEAT SEEDLINGS BY HELMIN. J Agric Res. 1923;26:195.
16. Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007;111(5):509–47.
17. Walters D, Walsh D, Newton A, Lyon G. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology. 2005;95(12):1368–73.
18. Ortiz E, Cruz M, Melgarejo LM, Marquínez X, Hoyos-Carvajal L. Histopathological features of infections caused by Fusarium oxysporum and F. solani in purple passionfruit plants (Passiflora edulis Sims). Summa Phytopathol. 2014;40:134–40.
Received: January 15, 2023 / Accepted: February 25, 2023 / Published:15 March 2023
Citation: Saleh, M.; Abed, J. Investigation of the Fusarium oxysporum-caused watermelon wilt infection and testing of some hybrids' susceptibility to infection. Revis Bionatura 2023;8 (1) 81. http://dx.doi.org/10.21931/RB/2023.08.01.81
