2022.07.01.5
Files > Volume 7 > Vol 7 No 1 2022
Study the relationship between Helicobacter pylori and bladder cancer
1 Department of Pediatric Nephrology, School of Medicine, Qom University of Medical Sciences, Qom, Iran.
2 Department of Surgery, Qom University of Medical Sciences, Qom, Iran
3 Student Research Committee, Qom University of Medical Sciences, Qom, Iran
* Corresponding author: [email protected].
Available from: http://dx.doi.org/10.21931/RB/2022.07.01.5
ABSTRACT
Given that bladder cancer is one of the most common cancers, and Helicobacter pylori infection also has 30-80% prevalence in different communities, this study investigates the role of H. pylori in developing bladder cancer; From December 2013 to February 2020, 200 patients with bladder tumors who underwent bladder tumor resection through the urethra in Kamkar-Arabnia Hospital were included in this study. H. pylori Ab, IgA, and IgG tests were first requested from all patients. If their antibodies were positive, other periodic tests including creatinine-sodium-potassium, Prothrombin Time (PT), Prothrombin Time Test (PTT), and International Normalized Ratio (INR), urinalysis, and culture were taken. The obtained results were analyzed using SPSS software version 25, and in the chi-square test, P <0.05 was considered a significant level; (3) Results: Based on laboratory findings, 66.5% of patients were H. pylori + (p <0.05). The result of the PCR test was positive in 4% of all patients. Besides, 6% of patients who tested positive for H. pylori Ab also showed positive PCR tests. Further studies are needed to investigate the association between H. pylori infection and bladder tumors to evaluate the proper role of H. pylori in tumors of the urinary system, especially the bladder and prostate, which have not been treated or reduced by treating H. pylori.
Keywords. Helicobacter pylori, Bladder cancer, Urinary system, Polymerase Chain Reaction.
INTRODUCTION
Most published research points to several factors that cause cancer, such as toxins, drugs, smoking, and obesity. However, there are few studies on cancer development through bacterial infections. Besides, the mechanisms of cancer through bacterial infections are not well understood. H. pylori are the first known bacterium to cause stomach cancer and may also be associated with cancer out of the human stomach1. Therefore, there is a lot of focus and attention on H. pylori infection nowadays. This bacterium, which lives in the upper gastrointestinal tract, is found in half of the world’s population. Its prevalence in geography, ethnicity, age, and socio-economic factors is very high in developing countries and less in developed countries2,3. H. pylori is a gram-negative flagellate, microaerophilic, and helical bacterium that causes gastritis and can eventually cause stomach cancer4-7. Studies have recently shown that this bacterium also causes organs outside the digestive system6,8,9. Other studies suggest that H. pylori may cause bladder and prostate inflammation or involve other organs10. On the other hand, vitamin D3 deficiency may also cause prostate cell proliferation and cancer11. H. pylori infection causes chronic inflammation that can lead to metaplasia, dysplasia, and cancer6,10,12. H. pylori infection is one of the risk factors in cancer development. However, its presence does not mean the definitive development of cancer13. The World Health Organization identifies it as a class 1 carcinogen because H. pylori in the stomach increase cancer risk by six times5,14. Given that bladder cancer is one of the most common cancers and H. pylori infection also has 30-80% prevalence in different communities5, this study investigates the role of H. pylori in developing bladder cancer.
MATERIALS AND METHODS
Ethical consideration
The ethical committee approved this research’s Qom University of Medical Sciences principles, Qom, Iran (Ethical number: IR.MUG.REC.1395.69). Additionally, Written consent was obtained from all patients included in the study.
Study design and patients
In a dissertation study from December 2013 to February 2020, 200 patients with bladder tumors referred to Kamkar-Arabnia Hospital, Qom, Iran, were assessed. After confirming the bladder tumor by cystoscopy15, patients underwent resection of the bladder tumor through the duct16.
Antibody detection
First, all patients underwent H. pylori antibody (Ab), Immunoglobulin A (IgA), and IgG tests17. For this purpose, peripheral blood was collected to determine anti-H pylori IgG and IgA serum levels. ELISA method (Accubind®, USA) was used to determine serum anti-H pylori IgG and IgA levels. The serum samples were diluted to 1/100. Other steps were performed according to the instructions of the manufacturer.
Complementary tests
After antibody detection, other periodic tests, including creatinine-sodium-potassium PT, A partial thromboplastin time (PTT), Prothrombin Time Test and INR (PT/INR), urinalysis, and culture were performed on patients with positive antibodies. Furthermore, Polymerase Chain Reaction (PCR) test was performed after the surgery18.
DNA extraction
The removed bladder tissue was immediately frozen in liquid nitrogen and stored at -80 °C until the experiment. A maximum of 25 mg of tissue was divided into small pieces and placed in a 1.5 mL microcentrifuge tube. Genomic DNA was extracted from the bladder tissues using the DNA extraction and purification kit (Qiagen, Valencia, CA). DNA was extracted directly from each tissue sample and used as a template to identify the specific H. pylori 16S rRNA gene. The quality and purity of extracted DNA samples were checked by gel electrophoresis and NanoDrop device (MA, USA), respectively19-21.
PCR procedure
H. pylori was identified using the 16S rRNA-based PCR (primers: HP-F: 5'-CTGGAGAGACTAAGCCCTCC-3' and HP-R: 5'-ATTACTGACGCTGATTGTGC-3')22. PCR circumstances and volumes were done according to described method22. Briefly, PCR was performed in of 50 µL volume. Ingredients were amplified in a device (Eppendorf Co., Germany) at several temperatures, including 1 cycle at 94 °C for 2 min, 30 cycles of 30 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C and another one cycle of 8 min at 72 °C. Positive control was H. pylori 26695. The negative control was PCR-grade water (Thermo Fisher Scientific). Electrophoresis was performed by agarose gel (2.5%) at 120 V for 30 min. UVI doc system was applied for gel visualization23, 24.
Data analysis
Obtained data were analyzed by SPPS.V.25 according to the Chi-square test. P <0.05 was level of significancy25,26.
RESULTS
Table 1 shows the demographic characters of the studied patients and the distribution of H. pylori. The distribution of male and female patients amongst the examined population was 65% and 35%. One-hundred and thirty-three out of 200 (66.5%) examined samples were positive for H. pylori. Findings revealed that 85% of samples had positive homogeneity. Additionally, 87.5% of patients were cytologically positive. Besides, 82.5% of patients were diagnosed using ultrasonic technology. The majority of examined patients were educators (29%), followed by the farmer (28%) and labor (18.5%). Of the 200 patients in the study, 89 patients had high blood pressure (p <0.05) and were taking aspirin (ASA). There were 120 smokers (P <0.05). However, 25% of patients had no risk factor.
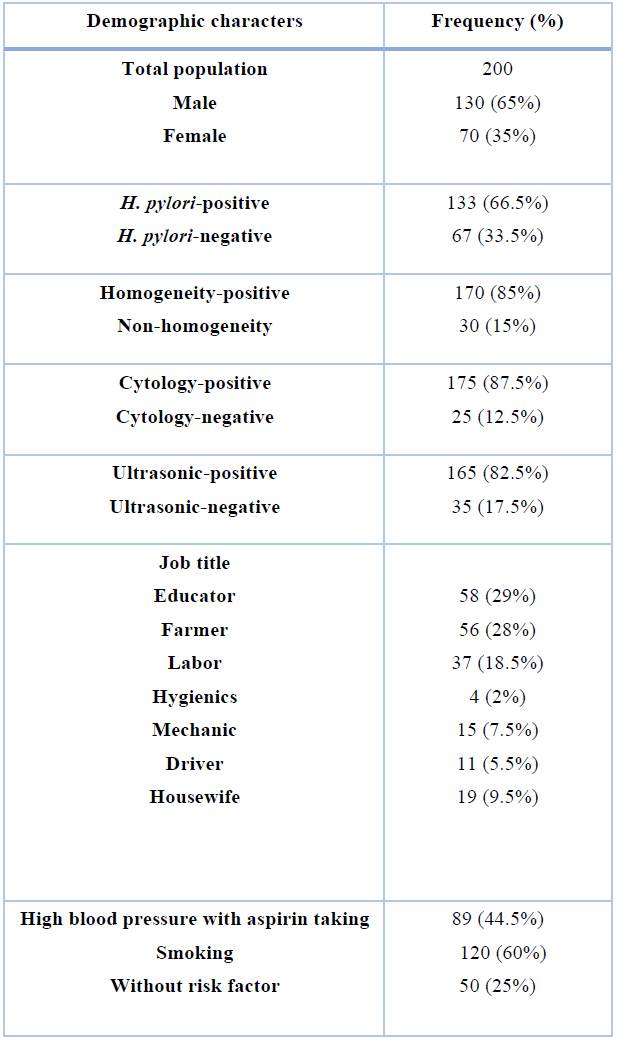
Table 1. Demographic characters of the studied patients and distribution of H. pylori.
Figure 1 shows the age distribution of patients examined in the present study. The mean age was 67 years, and the ratio of men to women was 1.9. The prevalence of bladder cancer was significantly higher in men than in women (P <0.05). 61-80 years old men have the highest cancer incidence (P <0.05).
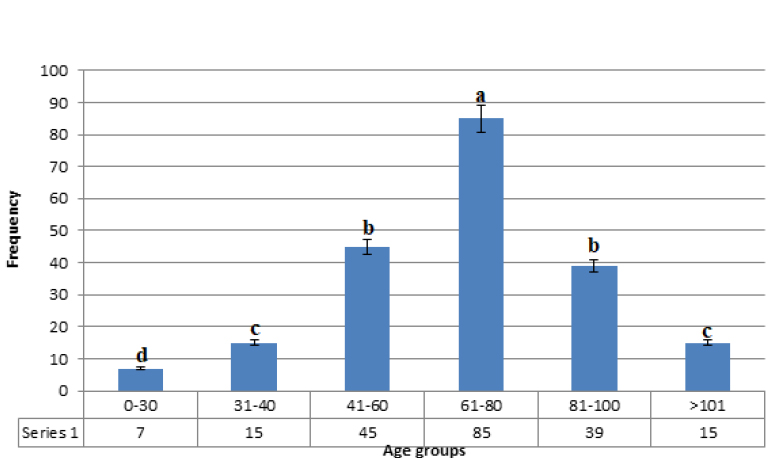
Figure 1. Age distribution of patients with bladder carcinoma. Dissimilar letters in each column show statistically significant differences (P <0.05).
Figure 2 shows the PCR electrophoresis.
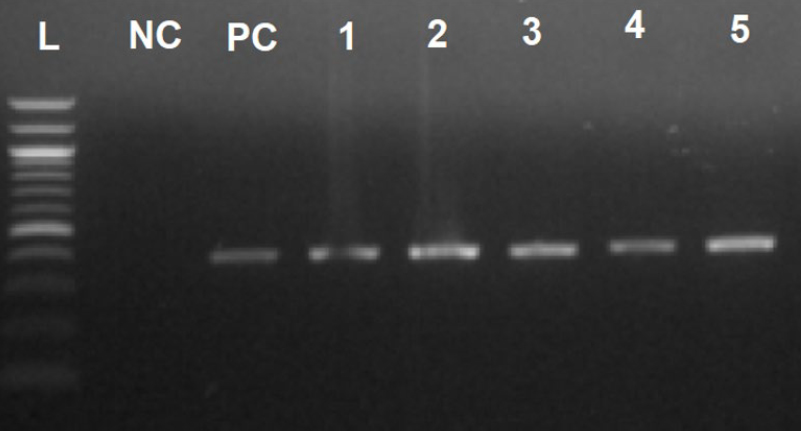
Figure 2. PCR electrophoresis. L: Ladder (100 bp), NC: Negative control, PC: Positive control, 1-5: Positive samples for H. pylori (446 bp).
Table 2 shows the histopathological features of the bladder cancer examined in the present research. Transitional cell cancer (TCC)was the most commonly identified cancer type (87.5%), while adenocarcinoma (2.5%) was the less commonly identified.
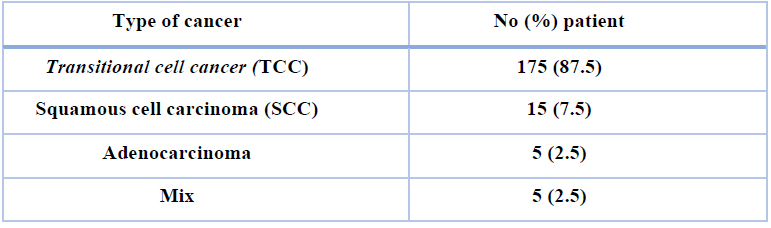
Table 2. Histological features of bladder cancer:
Table 3 shows the German immune lab test findings for the detection of H. pylori amongst examined samples. A total of 133 out of 200 (66.5%) cases were recognized as H. pylori-positive using the German immune lab test (P <0.05).
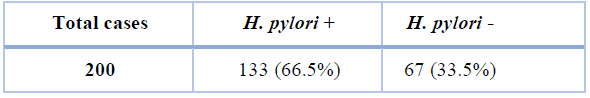
Table 3. Findings of the German immune lab test.
Table 4 shows the PCR results for the detection of H. pylori in diverse kinds of bladder cancers. The result of the PCR test was positive in 4% of all patients. Besides, 6% of patients who tested positive for H. pylori Ab also showed positive PCR results.

Table 4. PCR results for the detection of H. pylori in diverse bladder cancers.
DISCUSSION
Infectious diseases have been considered health-threatening issues in the last centuries27-40. Studies have shown that common organisms in saliva include three specimens of H. pylori, Campylobacter, and Neisseria cinerea, which are also present in the gastrointestinal tract. In vitro, these samples can catalyze many drugs and cause long-term gastrointestinal infections by causing nitrosamine compounds and gastric cancer41. However, in a study conducted by Heidari in Qom (2020), it was found that vitamin D3 deficiency plays a role in causing Benign prostatic hyperplasia (BPH) and possibly cancer11. Also, in the study of Alireza Abdollahi et al. on 126 patients, 33.3% had prostatitis with pelvic pain, and 84 patients in the control group had no symptoms. All were positive for H. pylori and antibodies, although they had no prostatitis symptoms, detrimental to H. pylori in inflammatory prostate disease13. In this study conducted in Iran, many of these bacteria were identified in the prostate, BPH, and prostate cancer. H. pylori were examined by immunohistochemistry (IHC) and PCR, and the results were determined by DNA sequencing. However, H. pylori have been reported positively in one case immunohistochemistry13. In the study by Michaud et al. (2004), men with gastric tumors had a higher prevalence of bladder cancer, but cancer risk was not higher in patients with a duodenal ulcer42. Gastric ulcers were significantly more common among patients with gastric cancer than renal cancer. The gastric/duodenal ulcers proportion in the gastric group was 6.5, and the renal cancer group was 0.33 42. In this study, H. pylori were identified as a risk factor for gastric and duodenal cancer. This bacterium causes stomach ulcers in the duodenum due to high acidity, but in the stomach, low acid production, gastritis, and ulcer disorders cause poor absorption of antioxidants, oxidative stress, and high levels of nitrates. Nitrates and nitrosamine compounds are also known as bladder carcinogens42. Due to this condition, it also occurs in the bladder as it does in the stomach. In this study, the condition closest to H. pylori was not correlated with gastric cancer, which seems to include a direct carcinogenic effect of the bacterium in the other studies was associated with H. pylori in 70-80% cases was positively correlated to gastric cancer42. Oral sex is one of the most common sex practices in the world. H. pylori transmitted via the act of sex through the urethra may lead to infection42. Several studies have shown that the transfer of bacterial metabolites of H. pylori can play a role in developing urinary tract cancers43. Matsumoto’s study showed that H. pylori infection caused Hodgkin’s lymphoma, regressed by H. pylori treatment. Finally, further studies are needed to prove the role of H. pylori infection directly or indirectly in various tumors, including the urinary syste44.
Infection and chronic inflammation have been recognized as essential predisposing factors for carcinogenesis and tumors. International agency for cancer (IARC) research has estimated that approximately 11% of cancers are related to infectious diseases like bacteria, viruses, and parasites. Human cancer is caused by infectious agents such as H. pylori, Human papillomavirus (HPV), Epstein-Barr virus (EBV), Sickle hemoglobin (HbS), and Hepatitis C virus (HCV), and human immunodeficiency viruses (HIV)14. Chronic inflammations are accountable for about 25% of cancer cases. Environmental issues, including HCV, HPV, HBV, and H. pylori infections, may be accountable for around 65-80% of gastric cancers, 80% of hepatocellular cancers, and 90% of cervical cancers14. Under inflammation conditions, reactive oxygen species (ROS) and reactive nitrogen species (RNS) are made from inflammatory and epithelial cells. These agents are capable of causing damage to various cellulars such as nucleic acids, proteins, and lipids. In this regard, H. pylori infection could affect the chronic bladder inflammation related to releasing large amounts of pro-inflammatory and vasoactive substances such as IL-1, IL-7, IL-1, and TNF-a or eicosanoids (leukotrienes, prostaglandins) and acute-phase proteins involved in the number of inflammatory diseases10. Besides, H. pylori-induced cytotoxin promotes intracellular survival of bacterium, modulates host immune responses, and induces autophagy because H. pylori as an intracellular microorganism invade and replicate in the cells. Compared to the translocation of H. pylori from the oral cavity to the stomach, H. pylori may reach the bladder through to the urethra, contaminated by saliva, and so on45.
In the study by Heidari et al.11, H. pylori infection was present in the urinary secretions of 8-hydroxy-2-deoxyguanosine, which causes DNA damage. In the 24-hour urine study of the subjects, 8ohdG was significantly higher in H. pylori-infected individuals than in the control group. This study found that 8ohdG is one of the most abundant lesions in DNA. The 8ohdG generated by ROS is caused by a radical oxygen attack on DNA and interferes with DNA repair43. This bacterium is significantly involved in developing bladder cancer and causes inflammation of the stomach, duodenum, and gall bladder 4. H. pylori’s role in developing lower gastric lymphoma has also been identified44. Recently, the association of this bacterium with urinary tract infection has been confirmed13. In a pilot study in patients with chronic prostatitis and pelvic pain, more people had a positive H. pylori antibody than in the control group45, 46. However, it is not clear exactly how H. pylori are transmitted and why some individuals become symptomatic, and some do not4. Bacteria are likely to be transmitted through feces, mouth, saliva, and contaminated water and food47. H. pylori is present in several locations, including adhesions to epithelial cells or inside vacuoles in epithelial cells. This bacterium stays in the lipid tissue and carbohydrates around the membrane by creating adhesio45. The reason that suggests the role of H. pylori in the development of bladder disease is the observation of H. pylori in other organs associated with other cancers, and this case has been identified for a long time48.
Bladder malt lymphoma also resolves after H. pylori treatment45, 49. Because H. pylori can cause infection into the bladder and prostate mucosa through the urethra (oral sex, anal sex, etc.) it causes chronic inflammation and the process of prostate and bladder cancer45. Al-Marhoon, in various studies that have examined the association between H. pylori and urological diseases, has stated that the most crucial reason for the role of H. pylori in causing chronic inflammation is that it leads to lymphoma. In a study by Shria Kumar et al. on 371,813 people diagnosed with Helicobacter pylori infection, treatment of H. pylori infection reduced gastric cancer risk only if eradication was successful50. As shown in the study of Heidari et al., BCG injection effectively treated interstitial cystitis51. Bacillus Calmette-Guerin (BCG) injection can also be used to treat high-grade bladder cancers52. In this study, we had eight positive PCR cases of bladder tumors. Considering that there were 133 positive antibody tests in the study, the small number of patients due to infection is not conclusive. Of course, laboratory and individual disorders may play a role. Although, laboratory and individual disorders may play a role in this regard.
CONCLUSIONS
The current research showed that H. pylori infections might predispose factors for bladder cancer. In this regard, a total of 133 out of 200 (66.5%) cases were recognized as H. pylori-positive using the German immune lab test (P <0.05). Additionally, 6% of patients who tested positive for H. pylori Ab showed positive PCR results. Further studies are needed to investigate the association between H. pylori infection and bladder tumors. These studies should investigate the proper role of H. pylori in tumors of the urinary system, especially the bladder and prostate, which have not been treated or reduced by treating H. pylori.
REFERENCES
1. Yahaghi E, Khamesipour F, Mashayekhi F, Safarpoor Dehkordi F, Sakhaei MH, Masoudimanesh M, Khameneie MK. Helicobacter pylori in vegetables and salads: genotyping and antimicrobial resistance properties. BioMed Res Int. 2014;2014.
2. Atapoor S, Dehkordi FS, Rahimi E. Detection of Helicobacter pylori in various types of vegetables and salads. J J Microbiol. 2014;7(5).
3. Ranjbar R, Farsani FY, Dehkordi FS. Phenotypic analysis of antibiotic resistance and genotypic study of the vacA, cagA, iceA, oipA and babA genotypes of the Helicobacter pylori strains isolated from raw milk. Antimicrob Res Infect Control. 2018;7(1):1-4.
4. Mousavi S, Dehkordi FS. Virulence factors and antibiotic resistance of Helicobacter pylori isolated from raw milk and unpasteurized dairy products in Iran. J Venom Anim Tox Incl Trop Dis. 2015;20:1-7.
5. Mashak Z, Jafariaskari S, Alavi I, Shahreza MS, Dehkordi FS. Phenotypic and genotypic assessment of antibiotic resistance and genotyping of vacA, cagA, iceA, oipA, cagE, and babA2 alleles of Helicobacter pylori bacteria isolated from raw meat. Infect Drug Res. 2020;13:257.
6. Ranjbar R, Yadollahi Farsani F, Safarpoor Dehkordi F. Antimicrobial resistance and genotyping of vacA, cagA, and iceA alleles of the Helicobacter pylori strains isolated from traditional dairy products. J Food Safety. 2019;39(2):e12594.
7. Mousavi S, Safarpoor Dehkordi F, Valizadeh Y. Genotyping of Helicobacter pylori strains isolated from raw milk and dairy products. J Food Microbiol. 2017;4(3):41-53.
8. Kountouras J, Tsiaousi E, Trigonis S, Zavos C, Kouklakis G. Helicobacter pylori infection in a Greek cohort with biliary disease. Br J Biomed Sci. 2014;71(4):178-179. doi:10.1080/09674845.2014.11669984
9. Houben GM, Hooi J, Hameeteman W, Stockbrugger RW. The frequency of Helicobacter pylori associated peptic ulcer disease and of autoimmune-associated conditions in gastric and renal cancer: a retrospective comparison in 267 patients. Eur J Cancer Prev. 1994 Dec;3 Suppl 2:75-9. doi: 10.1097/00008469-199412002-00014. PMID: 7735052.
10. Witherell HL, Hiatt RA, Replogle M, Parsonnet J. Helicobacter pylori infection and urinary excretion of 8-hydroxy-2-deoxyguanosine, an oxidative DNA adduct. Cancer Epidemiol Biomarkers Prev. 1998;7(2):91-96.
11. Heidari M, Hadi A. Relationship between Serum Level of Vitamin D in patients with BPH compared to healthy people in age group over 45. Pak. J. Med. Health Sci. 2020;14(2):1376-80.
12. Brüggemann, H., & Al‐Zeer, M. A. (2020). Bacterial signatures and their inflammatory potentials associated with prostate cancer. Apmis, 128(2), 80-91.
13. Abdollahi A, Etemadian M, Shoar S, Nozarian Z. Is Helicobacter pylori Infection a Risk Factor for Prostatitis? A Case-Control Study in a Referring Tertiary Care Center. Iran J Pathol. 2016;11(4):323-327.
14. Sarker, K. K., Kabir, M. J., uddin Bhuyian, A. M., Alam, M. S., Chowdhury, F. R., Ahad, M. A., ... & Rahman, M. M. (2017). H. pylori infection and gastric cancer in Bangladesh: a case-control study. International journal of surgery. Oncology, 2(10), e44.
15. Herr, H. W. (1990). Outpatient flexible cystoscopy and fulguration of recurrent superficial bladder tumors. The Journal of urology, 144(6), 1365-1366.
16. Hall, R. R. (1992). Transurethral resection for transitional cell carcinoma. Prob Urol, 6, 460-471.
17. Hirschl, A. M., & Makristathis, A. (2007). Methods to detect Helicobacter pylori: from culture to molecular biology. Helicobacter, 12, 6-11.
18. Ho, S. A., Hoyle, J. A., Lewis, F. A., Secker, A. D., Cross, D., Mapstone, N. P., Dixon, M. F., Wyatt, J. I., Tompkins, D. S., & Taylor, G. R. (1991). Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. Journal of clinical microbiology, 29(11), 2543–2549. https://doi.org/10.1128/JCM.29.11.2543- 2549.1991.
19. Dehkordi FS, Valizadeh Y, Birgani TA, Dehkordi KG. Prevalence study of Brucella melitensis and Brucella abortus in cow’s milk using dot enzyme-linked immunosorbent assay and duplex polymerase chain reaction. J Pure Appl Microbiol. 2014;8(2):1065-9.
20. Abdolmaleki Z, Mashak Z, Dehkordi FS. Phenotypic and genotypic characterization of antibiotic resistance in the methicillin-resistant Staphylococcus aureus strains isolated from hospital cockroaches. Antimicrobial Resistance & Infection Control. 2019;8(1):1-4.
21. Dehkordi FS, Momtaz H, Doosti A. Application of Real-Time PCR for detection of Aspergillus species in aborted ruminant foetuses. BulgaJ Vet Med. 2012;15(1):30-6.
22. Ho SA, Hoyle JA, Lewis FA, Secker AD, Cross D, Mapstone NP, Dixon MF, Wyatt JI, Tompkins DS, Taylor GR. Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. J Clin Microbiol 1991; 29: 2543-2549.
23. Rahi A, Kazemeini H, Jafariaskari S, Seif A, Hosseini S, Dehkordi FS. Genotypic and phenotypic-based assessment of antibiotic resistance and profile of staphylococcal cassette chromosome mec in the methicillin-resistant Staphylococcus aureus recovered from raw milk. Infection and drug resistance. 2020;13:273.
24. Dehkordi, F.S., Saberian, S., Momtaz, H. Detection and segregation of Brucella abortus and Brucella melitensis in Aborted Bovine, Ovine, Caprine, Buffaloes and Camelid Fetuses by application of conventional and real-time polymerase chain reaction. The Thai J Vet Med. 2012;42(1):13.
25. Dehkordi FS, Khamesipour F, Momeni M. Brucella abortus and Brucella melitensis in Iranian bovine and buffalo semen samples: The first clinical trial on seasonal, Senile and geographical distribution using culture, conventional and real-time polymerase chain reaction assays. Kafkas Univ Vet Fak Dergisi. 2014;20(6):821-.
26. Dehkordi FS. Prevalence study of Coxiella burnetii in aborted ovine and caprine fetuses by evaluation of nested and real-time PCR assays. American J Anim Vet Sci. 2011;6(4):180-6.
27. Dehkordi FS, Tirgir F, Valizadeh Y. Effects of Guajol® ointment synthesized from medicinal smoke condensate of jennet feces on burn wound healing on Wistar rat. Vet Res Forum. 2017; 8(3):215.
28. Dehkordi FS, Tavakoli-Far B, Jafariaskari S, Momtaz H, Esmaeilzadeh S, Ranjbar R, Rabiei M. Uropathogenic Escherichia coli in the high vaginal swab samples of fertile and infertile women: virulence factors, O-serogroups, and phenotyping and genotyping characterization of antibiotic resistance. New Microb New Infect. 2020;38:100824.
29. Dehkordi FS, Haghighi N, Momtaz H, Rafsanjani MS, Momeni M. Conventional vs real-time PCR for detection of bovine herpes virus type 1 in aborted bovine, buffalo and camel foetuses. Bulgar J Vet Med. 2013;16(2):102-12.
30. Dehkordi FS, Yazdani F, Mozafari J, Valizadeh Y. Virulence factors, serogroups and antimicrobial resistance properties of Escherichia coli strains in fermented dairy products. BMC Res Notes. 2014;7(1):1-8.
31. Dehkordi FS, Barati S, Momtaz H, Ahari SN, Dehkordi SN. Comparison of shedding, and antibiotic resistance properties of Listeria monocytogenes isolated from milk, feces, urine, and vaginal secretion of bovine, ovine, caprine, buffalo, and camel species in Iran. Jundishapur J Microbiol. 2013;6(3):284.
32. Ghorbani F, Gheisari E, Dehkordi FS. Genotyping of vacA alleles of Helicobacter pylori strains recovered from some Iranian food items. Trop J Pharm Res. 2016;15(8):1631-6.
33. Dehkordi FS, Gandomi H, Basti AA, Misaghi A, Rahimi E. Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob Res Infect Control. 2017;6(1):1-1.
34. Dehkordi FS. Prevalence study of Bovine viral diarrhea virus by evaluation of antigen capture ELISA and RT-PCR assay in Bovine, Ovine, Caprine, Buffalo and Camel aborted fetuses in Iran. AMB Express. 2011;1(1):1-6.
35. Dehkordi FS, Parsaei P, Saberian S, Moshkelani S, Hajshafiei P, Hoseini SR, Babaei M, Ghorbani MN. Prevalence study of Theileria annulata by comparison of four diagnostic Techniques in shouthwest Iran. Bulgar J Vet Med. 2012;15(2): 123-130.
36. Dehkordi FS, Haghighi Borujeni MR, Rahimi E, Abdizadeh R. Detection of Toxoplasma gondii in raw caprine, ovine, buffalo, bovine, and camel milk using cell cultivation, cat bioassay, capture ELISA, and PCR methods in Iran. Foodborne Pathog Dis. 2013;10(2):120-5.
37. Safarpordehkordi F, Yahaghi E, Khodaverdi Darian E. Prevalence of antibiotic resistance in Escherichia coli isolated from poultry meat supply in Isfahan. Iran J Med Microbiol. 2014;8(2):41-7.
38. Safarpour Dehkordi F, Hosseini S, Rahimi E, Momeni M, Yahaghi E, Khodaverdi Darian E. Investigate the frequency of virulence genes Vibrio parahaemolyticus isolated from fish, lobsters and crabs caught from Persian Gulf. Iran J Med Microbiol. 2014;8(2):1-7.
39. Safarpour Dehkourdi F, Momtaz H, Esmailzade S, Khayyat Khameneie M, Yahaghi E. Detection of virulence factors of Uropathoigenic Escherichia coli isolates from infertile women high vaginal swabs. Iran J Med Microbiol. 2014;7(4):1-8.
40. Nejat S, Momtaz H, Yadegari M, Nejat S, Safarpour Dehkordi F, Khamesipour F. Seasonal, geographical, age and breed distributions of equine viral arteritis in Iran. Kafkas Univ Vet Fak Derg. 2015;21(1):111-6.
41. Ziebarth, D., Spiegelhalder, B., & Bartsch, H. (1997). N-nitrosation of medicinal drugs catalyzed by bacteria from human saliva and gastrointestinal tract, including Helicobacter pylori. Carcinogenesis, 18(2), 383–389. https://doi.org/10.1093/carcin/18.2.383
42. Michaud DS, Mysliwiec PA, Aldoori W, Willett WC, Giovannucci E. Peptic ulcer disease and the risk of bladder cancer in a prospective study of male health professionals. Cancer Epidemiol Biomarkers Prev. 2004;13(2):250-254. doi:10.1158/1055-9965.epi-03-0174
43. Siomek A, Rytarowska A, Szaflarska-Poplawska A, et al. Helicobacter pylori infection is associated with oxidatively damaged DNA in human leukocytes and decreased level of urinary 8-oxo-7,8-dihydroguanine. Carcinogenesis. 2006;27(3):405-408. doi:10.1093/carcin/bgi238
44. Matsumoto, T., & Iida, M. (1997). Regression of mucosa-associated lymphoid-tissue lymphoma of rectum after eradication of Helicobacter. The Lancet, 350(9071), 115-116.
45. Brown L. M. (2000). Helicobacter pylori: epidemiology and routes of transmission. Epidemiologic reviews, 22(2), 283–297. https://doi.org/10.1093/oxfordjournals.epirev.a018040
46. Karatas, O. F., Turkay, C., Bayrak, O., Cimentepe, E., & Unal, D. (2010). Helicobacter pylori seroprevalence in patients with chronic prostatitis: a pilot study. Scandinavian journal of urology and nephrology, 44(2), 91-94.
47. Vaira, D., Holton, J., Menegatti, M., Gatta, L., Ricci, C., Alì, A., Landi, F., Moretti, C., & Miglioli, M. (1998). Routes of transmission of Helicobacter pylori infection. Italian journal of gastroenterology and hepatology, 30 Suppl 3, S279–S285.
48. Li, L. J., Shen, Z. J., Lu, Y. L., & Fu, S. Z. (2001). The value of endotoxin concentrations in expressed prostatic secretions for the diagnosis and classification of chronic prostatitis. BJU international, 88(6), 536–539. https://doi.org/10.1046/j.1464-410x.2001.02354.x
49. Colovic, M., Hadzi-Djokic, J., Cemerikic, V., Colovic, R., Jankovic, G., & Dacic, M. (1999). Primary MALT lymphoma of the kidney. Hematology and cell therapy, 41(5), 229-232.
50. Kumar, S., Metz, D. C., Ellenberg, S., Kaplan, D. E., & Goldberg, D. S. (2020). Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology, 158(3), 527–536.e7. https://doi.org/10.1053/j.gastro.2019.10.019
51. Heidari, M., Irani, D., & Khezri, A. (2005). THE efficacy of intravesical bacillus calmette-guerin in the treatment of female patient with interstitial cystitis. A double-blind, prospective, placebo controlled trial. J Urol. 1998;159(5):1483-7.
52. Godolo, G., Munari, F., Fassan, M. and de Bernard, M., 2015. Evaluation of the efficacy of the H. pylori protein HP-NAP as a therapeutic tool for treatment of bladder cancer in an orthotopic murine model. . J Vis Exp. 2015;(99):e52743. doi:10.3791/52743.
Received: 26 June 2021 / Accepted: 7 August 2021 / Published: date. 15 february 2022
Citation: Heidari M, Eshagh Hosaini S K, Fatemi Manesh H. Study the relationship between Helicobacter pylori and bladder cancer. Revis Bionatura 2022 ;7(1). 5. http://dx.doi.org/10.21931/RB/2022.07.01.5
Academic Editors: Prof. Zohreh Mashak , Dr. Mohammadhossein Sakhaei Shahreza
