2023.08.01.14
Files > Volume 8 > Vol 8 No 1 2023
Antibacterial effect of Cannabidiol oil against Propionibacterium acnes, Staphylococcus aureus, Staphylococcus epidermidis and level of toxicity against Artemia salina
Grace Pila 1* , Danny Segarra 1 and Marco Cerna 1
, Danny Segarra 1 and Marco Cerna 1
1 Universidad Politécnica Salesiana, Isabel La Católica Av. N 22-52 and Madrid.
* Correspondence: Grace Pila Rosero
[email protected]; Tel.: (+593) 0987747054;
http://dx.doi.org/10.21931/RB/2023.08.01.14
.
ABSTRACT
Acne is one of the most common skin pathologies; one of the causes is Propionibacterium acnes, an anaerobic and gram-positive microorganism that lives in the hair follicles of the skin and currently presents resistance to antibiotic-based treatments; this research topic has the purpose of evaluating the antibiotic activity of Cannabidiol oil against Propionibacterium acnes, Staphylococcus aureus and Staphylococcus epidermidis and the level of toxicity against Artemia salina.
For the methodology, antibiograms were used by the Kirby-Bauer method, where the concentrations were evaluated: 0,8 %; 0,6 %; 0,4 %; 0,3 % and 0,1 %; Amoxicillin for positive control and Dimethyl sulfoxide (DMSO) for negative control; the percentage of inhibition against Propionibacterium acnes and two control bacteria were calculated: Staphylococcus aureus and Staphylococcus epidermidis. Once the percentage of inhibition was tested, a toxicity study was carried out against Artemia salina to determine its LD50.
The Cannabidiol oil obtained from the Ecuadorian company was used as the antibiotic agent to be evaluated, and it was found that at a concentration of 0,8%, it presented a percentage of inhibition of 91,2 %; 98,7 % and 93,6 % against Propionibacterium acnes, Staphylococcus aureus and Staphylococcus epidermidis, respectively, data that do not present a significant difference against Amoxicillin; for the Artemia salina test, an LD50 of 4,8 % was obtained; taking into account that the commercial oil has a presentation of 1,6 % (500 mg/30 mL), it results in a relatively innocuous product. Thus concluding that Cannabidiol oil is a very promising antibiotic due to the inhibition percentages presented and low toxicity.
Keywords: CBD, antibiograms, bioassay, LD50.
INTRODUCTION
The use and abuse of antibiotics, not only in Ecuador but worldwide, is a fashionable and controversial topic; due to the efforts made by professionals, this is a practice that continues to leave in its wake several severe and irreversible consequences. one of them is the bacterial resistance acquired by microorganisms to antibiotics. Several resistances of Propionibacterium acnes have been reported over the years, as is the case of Clindamycin and Erythromycin, which were reported in 1979, and later in 1983 the first resistance to tetracycline was reported 10.
It has been reported in patients with severe acne that 70% of them present high biofilm formation and multi-resistance of Propionibacterium acnes to various antibiotics. For this reason, it is interesting to use new alternatives. It has been reported in patients with severe acne that 70% of them present high biofilm formation and multi-resistance of Propionibacterium acnes to various antibiotics. For this reason, it is interesting to use new alternatives against Propionibacterium acnes; it has been reported in studies 5,11 that Cannabidiol works excellent with biofilms of gram-positive microorganisms; therefore, studying the effect of Cannabidiol against Propionibacterium acnes is promising.
MATERIALS AND METHODS
Cannabidiol oil was obtained from an Ecuadorian company in a 500 mg/30mL presentation. The bacterial strain of Propionibacterium acnes ATCC 11827, Staphylococcus aureus ATCC 29213, and Staphylococcus epidermidis ATCC 14990 were obtained from the Cryobank of the Life Sciences Laboratories of the Salesian Polytechnic University. The manual described by 20 was used as a reference, and the tubes containing the bacterial beads were thawed with a punch to perform the striation in triplicate throughout the petri dish.
The recommendations of 7 were followed to prepare dilutions with oils and dimethyl sulfoxide. Five oil-based dilutions were prepared at concentrations of 0.1 %; 0.3 %; 0.4 %; 0.6 %, and 0,8 %, whose solvent was DMSO; the final volume for each dilution was 5 mL in every amber bottle.
A commercial antibiotic (Amoxicillin) was taken as a positive control, a beta-lactam antibiotic used for both gram-positive and gram-negative bacteria, due to its broad spectrum of bacterial activity 1. This antibiotic is used for antibiotic testing in the Staphylococcus and Propionibacterium families because of their sensitivity to its compounds 8,10.
P. acnes was incubated in TSB medium under anaerobic conditions at 35 °C for 16 hours, for S. aureus and S. epidermidis were incubated in TSB medium at 37 °C in an incubator.
After the established time passed, it was centrifuged at 350 rpm for 20 minutes, the supernatant was discarded in a beaker with alcohol, the bottom of the bacterial biomass was conserved, sterile saline was added to each tube, and vortexed for 2 minutes until reaching the 0,5 McFarland scale and read in the JASCO V-730 spectrophotometer with the spectra manager TM software until reaching an absorbance of 0,200 at 655 nm, obtaining an inoculum of 106 CFU/mL.
500 µL of bacterial inoculum was taken and dropped in the center of the Petri dish with Muller Hinton 15. One disc of antibiotic Amoxicillin was placed as the positive control, one blank disk with DMSO as the negative control and 5 blank discs with the respective dilutions from Cannabidiol oil, which would be at concentrations of 0,1 %; 0,3 %, 0,4 % 0,6 % and 0,8 % in a volume of 20 µL. Petro dishes with S. aureus and S. epidermidis were placed in an incubator at 37°C for 24 hours; P. Acnes was incubated in anaerobiosis at 35 °C.
When the time of 24 hours in the incubator for S. aureus, S. epidermidis and P. acnes had passed, each Petri dish was checked with a caliper ruler.
The percentage of inhibition of each bacterium concerning each concentration was calculated using the following reference formula (1) from 9.
7 grams of A. salina eggs were obtained from a commercial house, 2 g of egg were weighed, hydrated for 30 min with distilled water, then 25 mL of sodium hypochlorite were added (4 replicates); the eggs were recovered and rinsed with distilled water. For the incubation, a 3-liter bottle was used, 1500 mL of 2 % saline water was added, pH 8, temperature 24 °C and constant aeration for 48 hours 22.
To make the emulsions with Cannabidiol (CBD) oil, a 1:1 ratio of oil and tween 80 was used as a co-emulsifier used in the cosmetic and food area due to its low toxicity level and according to work carried out by 15 it is considered innocuous with Artemia. Cannabidiol oil was used to obtain emulsion at 3.2 %; 1.6 %; 0.8 %; 0.4 %, and 0.2 %, with which we worked in test tubes with A. salina to determine the LD50.
After 24 hours of incubation, dead nauplii were counted using a NIKON SMZ745 stereoscope, where those that did not show any seconds were considered dead
RESULTS

Table 1. Determination of the antibiotic activity of Cannabidiol oil against P. acnes.
The percentage of inhibition for P. acnes is 91.2% at a concentration of 0.8% of Cannabidiol oil, which gives a high percentage of inhibition compared to a commercial antibiotic, Amoxicillin, supporting inhibition compared with a commercial antibiotic, Amoxicillin, supporting the alternative hypothesis showing that Cannabidiol oil inhibits P. acnes.
A Tukey study showed an essential group of data, in which their averages are not significantly different; the group is formed by the positive control (commercial antibiotic), CBD5 (0.8% Cannabidiol oil).
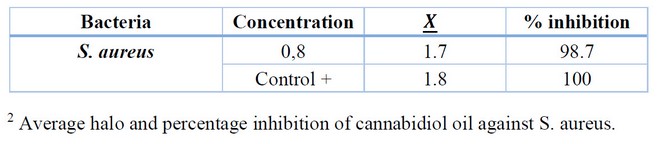
Table 2. Determination of the antibiotic activity of cannabidiol oil against S. aureus
The concentration of Cannabidiol oil at 0.8 % has an inhibition percentage of 98.7 %, a value very close to the positive control, which was the commercial antibiotic Amoxicillin.
A Tukey test proved that there is a group of interest where their averages are not significantly different; the group is formed by the control + (antibiotic Amoxicillin) and CBD5 (0.8% Cannabidiol oil).
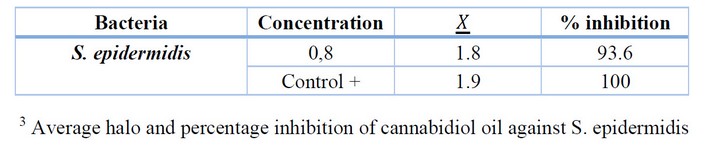
Table 3. Determination of the antibiotic activity of cannabidiol oil against S. epidermidis
The results show that the percentage of inhibition for S. epidermidis with 0.8 % oil was 93.6 %, affirming the alternative hypothesis on the inhibition of Cannabidiol oil against S. epidermidis.
The Tukey test shows a critical group where the positive control (Amoxicillin) and CBD5 (0.8 % Cannabidiol oil) are grouped. As a result, we would obtain that the 0.8 % Cannabidiol oil is similar in inhibition to the positive control (Amoxicillin), supporting the alternative hypothesis that there is at least one concentration that inhibits S. epidermidis
Toxicity test
Dilutions of cannabidiol oil were made at intervals of: 3.2 %; 2.8 %; 2.4 % 2.0 %. In order to determine the lethal dose, a linear regression was performed, and an LD50 of 4.86 % (48 mg/mL) was obtained.
Formatting of Mathematical Components

DISCUSSION
Following the studies of 18 where he mentions that Cannabidiol has a potential antimicrobial activity against gram-positive bacteria, such as P. acnes, with which using it could be beneficial for the treatment of acne vulgaris. Cannabidiol has a potential role as an antimicrobial agent 22; it was demonstrated through clinical studies that Cannabidiol oil acts on sebocytes, thus having an anti-acne function, controlling sebum production, mitigating the inflammatory process and functioning as a bactericidal agent by reducing bacterial proliferation 4,21.
Cannabidiol oil inhibits S. aureus; the results obtained by 2 can be compared with those of this work since Cannabidiol, one of the main cannabinoids of the plant, showed potent activity against the strain S. aureus.
The results obtained from the test with S. epidermidis can be compared with the study conducted by 19, where the mechanism of action of Cannabidiol in causing the death of gram-positive bacteria was evaluated due to the ability of this compound to inhibit the release of vesicles from the bacterial membrane; these vesicles are extremely important for cell communication and pathogen-host interaction.
In the negative control of the toxicity test with Artemia salina, saline water was used, and there was no dead individual, so the test is validated as there are no natural factors that can kill the study individuals, as indicated by 24, 13; the percentage of mortality in the negative controls did not exceed 10 %. In the positive control, where 96% alcohol was used, it was confirmed as an adequate positive control since the death of the individuals in the study was approved, as indicated by the study of 23.
The use of Cannabidiol oil at concentrations from 2 % onwards gradually increases the number of dead A. salina 14. A plant oil, when exceeding an LC50 of 1000 ppm in bioassays with A. salina does not have a high degree of toxicity due to the ability of the nauplii to present a very thin cuticle, which makes them sensitive to toxicants in the medium, which penetrate through the physiological barriers and are rapidly absorbed 6, 25
CONCLUSIONS
The valued Cannabidiol oil was obtained from an Ecuadorian company, which presents a concentration of 500 mg/30mL. Evaluation by means of the HPLC technique.
Cannabidiol oil showed antibacterial activity with halo averages of 1.8 cm, 1.7 cm and 1.8 cm for Propionibacterium acnes, Staphylococcus aureus and Staphylococcus epidermidis, respectively, at a concentration of 0.8 %, compared to the control antibiotic (Amoxicillin) with 2 cm of halo, using the statistical analysis it was possible to reject the null hypothesis and accept the alternative since Cannabidiol oil inhibits Propionibacterium acnes, Staphylococcus aureus and Staphylococcus epidermidis with the proposed concentrations. Likewise, the alternative ideas for the analysis of variance and Tukey are accepted; at least one degree of concentration of Cannabidiol oil inhibits the 3 bacteria with a similar effect to Amoxicillin. At the end of the experimental work, it was concluded that the results obtained under the laboratory test show that the use of Cannabidiol oil is effective for the control of the mentioned bacteria, and it is a promising field for the possible elaboration of phytoproducts for human use in order to improve and provide all the benefits offered by Cannabidiol oil.
For the toxicity bioassay where Artemia salina was used, an LD50 value of 4.8 % was obtained, which showed that the commercial Cannabidiol oil in a 500 mg/30mL presentation, equivalent to 1.6 %, is a relatively innocuous product at the highest concentration and non-toxic at deficient concentrations. Although at higher concentrations, survival may be negatively affected by swimming problems, the results confirmed the alternative hypothesis that the concentration of Cannabidiol oil is directly proportional to the percentage of mortality of Artemia salina.
Author Contributions: “Conceptualization, Grace Pila and Danny Segarra.; methodology, Grace Pila, and Danny Segarra.; software, Danny Segarra..; investigation, Grace Pila and Danny Segarra.; writing—original draft preparation, Grace Pila and Danny Segarra.; supervision, Marco Cerna; funding acquisition, Grace Pila and Danny Segarra. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding
Informed Consent Statement: Not applicable.
Data Availability Statement: https://docs.google.com/spreadsheets/d/1mGvmAHt5Q6ZsTLvIysinZDh_uoFFovcHAcjse0yb7ug/edit#gid=816728436
Acknowledgments: Our most sincere thanks to the technical team of the life sciences laboratories of the Salesian Polytechnic University, to our friends, colleagues and family.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Adm R, María J, Ramos F, Guadalupe M, Zaragoza O, Lorena López Rodríguez L, et al. www.medigraphic.org.mx [Internet]. Medigraphic.com. Disponible en: https://www.medigraphic.com/pdfs/adm/od-2016/od165c.pdf
2. Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, et al. Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod [Internet]. 2008;71(8):1427–30. Disponible en: http://dx.doi.org/10.1021/np8002673
3. Arima H, Danno G-I. Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation. Biosci Biotechnol Biochem [Internet]. 2002;66(8):1727–30. Disponible en: http://dx.doi.org/10.1271/bbb.66.1727
4. Baswan SM, Klosner AE, Glynn K, Rajgopal A, Malik K, Yim S, et al. Therapeutic potential of Cannabidiol (CBD) for skin health and disorders. Clin Cosmet Investig Dermatol [Internet]. 2020;13:927–42. Disponible en: http://dx.doi.org/10.2147/CCID.S286411
5. Blaskovich MAT, Kavanagh AM, Elliott AG, Zhang B, Ramu S, Amado M, et al. The antimicrobial potential of Cannabidiol. Commun Biol [Internet]. 2021;4(1):7. Disponible en: http://dx.doi.org/10.1038/s42003-020-01530-y
6. Berame JS, Cuenca SME, Cabilin DRP, Manaban ML. Preliminary phytochemical screening and toxicity test of leaf and root parts of the snake plant (Sansevieria trifasciata). J Phylogenetics Evol Biol [Internet]. 2017;05(03). Disponible en: http://dx.doi.org/10.4172/2329-9002.1000187
7. Bermúdez-Vásquez MJ, Granados-Chinchilla F, Molina A. Composición química y actividad antimicrobiana del aceite esencial de Psidium guajava y Cymbopogon citratus. Agron Mesoam [Internet]. 2019 30(1):147–63. Disponible en: https://www.scielo.sa.cr/scielo.php?pid=S1659-13212019000100010&script=sci_abstract&tlng=es
8. Castro Orozco R, Villafañe Ferrer L, Rocha Jiménez J, Alvis Guzmán N. Resistencia antimicrobiana en staphylococcus aureus y staphylococcus epidermidis : tendencia temporal (2010-2016) y fenotipos de multirresistencia, Cartagena (Colombia). Biosalud [Internet]. 2018;17(2):25–36. Disponible en: http://dx.doi.org/10.17151/biosa.2018.17.2.2
9. Corzo Barragán DC, Botánico J, Celestino J, Resumen M. Evaluación de la actividad antimicrobiana del extracto etanólico Evaluation of antimicrobial activity of ethanol extract of Cestrum buxifolium Kunth [Internet]. Org.mx. Disponible en: https://www.scielo.org.mx/pdf/rmcf/v43n3/v43n3a9.pdf
10. Dréno B, Pécastaings S, Corvec S, Veraldi S, Khammari A, Roques C. Cutibacterium acnes(Propionibacterium acnes) and acne vulgaris: a brief look at the latest updates. J Eur Acad Dermatol Venereol [Internet]. 2018;32:5–14. Disponible en: http://dx.doi.org/10.1111/jdv.15043
11. Dessinioti C, Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol [Internet]. 2017;35(2):163–7. Disponible en: http://dx.doi.org/10.1016/j.clindermatol.2016.10.008
12. Feldman M, Sionov RV, Mechoulam R, Steinberg D. Anti-Biofilm Activity of Cannabidiol against Candida albicans. Microorganisms [Internet]. 2021;9(2):441. Disponible en: http://dx.doi.org/10.3390/microorganisms9020441
13. Fernández A, Mendiola J, Monzote L, García M, Sariego I, Acuña D, et al. Evaluación de la toxicidad de extractos de plantas cubanas con posible acción antiparasitaria utilizando larvas de Artemia salina L. rEVISTA cUBANA MEDICINA TROPICAL [Internet]. 2009 [citado el 2 de febrero de 2023]; Disponible en: http://scielo.sld.cu/pdf/mtr/v61n3/mtr09309.pdf
14. Ferrante C, Chiavaroli A, Angelini P, Venanzoni R, Angeles Flores G, Brunetti L, et al. Phenolic Content and Antimicrobial and Anti-Inflammatory Effects of Solidago virga-aurea, Phyllanthus niruri, Epilobium angustifolium, Peumus boldus, and Ononis spinosa Extracts. Antibiotics (Basel) [Internet]. 2020;9(11):783. Disponible en: http://dx.doi.org/10.3390/antibiotics9110783
15. Hernández A, Cruz M, Guerra D, Guerra P, Rivera F, Ramírez-Marroquín O, et al. Estudio de la actividad antioxidante, antimicrobiana y toxicidad de tres extractos de heliocarpus appendiculatus turcz (malvaceae) [Internet]. [Cuernavaca, Morelos, México]: Universidad Autónoma del Estado de Morelos; 2021. Disponible en: http://aap.uaem.mx/index.php/aap/article/view/78/71
16. Ibarra M, Paredes E. Eficacia antibacteriana in vitro de marco (Ambrosia arborescens Mill.) y paico (Chenopodium ambrosioides L.) en una formulación cosmética [Internet]. [Quito, Ecuador]: Universidad Politécnica Salesiana; 2013. Disponible en: https://dspace.ups.edu.ec/bitstream/123456789/6007/1/UPS-QT03776.pdf
17. Juana MAC, Aguilera R, Yolanda ME, Romero M. MANUAL DE LABORATORIO DE MICROBIOLOGIA [Internet]. Www.uv.mx. Disponible en: https://www.uv.mx/qfb/files/2020/09/Guia-de-Microbiologia.pdf
18. Kircik LH. What’s new in the management of acne vulgaris. Cutis [Internet]. 2019;104(1):48–52. Disponible en: https://cdn.mdedge.com/files/s3fs-public/KircikCT104001048.PDF
19. Kosgodage US, Matewele P, Awamaria B, Kraev I, Warde P, Mastroianni G, et al. Cannabidiol is a novel modulator of bacterial membrane vesicles. Front Cell Infect Microbiol [Internet]. 2019;9:324. Disponible en: http://dx.doi.org/10.3389/fcimb.2019.00324
20. Moon SH, Roh HS, Kim YH, Kim JE, Ko JY, Ro YS. Antibiotic resistance of microbial strains isolated from Korean acne patients: Antibiotic resistance of microbial strains. J Dermatol [Internet]. 2012;39(10):833–7. Disponible en: http://dx.doi.org/10.1111/j.1346-8138.2012.01626.x
21. Oláh A, Markovics A, Szabó-Papp J, Szabó PT, Stott C, Zouboulis CC, et al. Differential effectiveness of selected non-psychotropic phytocannabinoids on human sebocyte functions implicates their introduction in dry/seborrhoeic skin and acne treatment. Exp Dermatol [Internet]. 2016;25(9):701–7. Disponible en: http://dx.doi.org/10.1111/exd.13042
22. Palmieri S, Maggio F, Pellegrini M, Ricci A, Serio A, Paparella A, et al. Effect of the distillation time on the chemical composition, antioxidant potential and antimicrobial activity of essential oils from different Cannabis sativa L. cultivars. Molecules [Internet]. 2021;26(16):4770. Disponible en: http://dx.doi.org/10.3390/molecules26164770
23. Quinchuela Barahona CD, Vaca Estrella IA. Estudio de las propiedades antibacterianas, antioxidantes y toxicidad de cuatro especies del género Huntleya (Orchidaceae) del Ecuador. 2020.
24. Saetama V, L. V, Vanegas ME, Cruzat C, D. B. Evaluación toxicológica de soluciones acuosas de ibuprofeno mediante bioensayos con Artemia salina, Allium schoenoprasum L y Lactuca sativa. Revista toxicológica [Internet]. 2018 [citado el 2 de febrero de 2023]; Disponible en: http://rev.aetox.es/wp/wp-content/uploads/2018/12/Revista-de-Toxicologia-35.2-34-40.pdf
25. Sarah QS, Anny FC, Misbahuddin M. Brine shrimp lethality assay. Bangladesh J Pharmacol [Internet]. 2017;12(2):5. Disponible en: http://dx.doi.org/10.3329/bjp.v12i2.32796
26. Valdés AF-C, Martínez JM, Fidalgo LM, Parra MG, Ramos IS, Rodríguez DA, et al. Evaluación de la toxicidad de extractos de plantas cubanas con posible acción antiparasitaria utilizando larvas de Artemia salina L. Revista Cubana de Medicina Tropical [Internet]. c 2009; Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0375-07602009000300009
Received: September 26, 2022 / Accepted: October 15, 2022 / Published:15 February 2023
Citation: Pila, Segarra, Cerna. Antibacterial effect oil against P. acnes, S. aureus, S. epidermidis and level of toxicity against A. salina.Revis Bionatura 2023;8 (1) 14. http://dx.doi.org/10.21931/RB/2023.08.01.14
