2021.06.03.27
Files > Volume 6 > Vol 6 No 3 2021
REPORTE DE CASOS / CASE REPORTS
Strongyloides stercoralis infestation in a pediatric patient
Ricardo Rubio-Sánchez 1, Esperanza Lepe-Balsalobre 2.
http://dx.doi.org/10.21931/RB/2021.06.03.27
ABSTRACT
Strongyloidiasis is a parasitic disease, very rare in countries like Spain, caused by the Strongyloides stercoralis nematode. We present a case of a 5-year-old patient from Ecuador who came to the Emergency Department due to fever, colicky abdominal pain, watery diarrhea, and occasional vomiting of several days of evolution. In laboratory studies, a marked leukocytosis with eosinophilia stands out, for which reason a microscopic study of the stool was carried out where larval forms compatible with Strongyloides stercoralis were observed. The diagnostic strategy of parasitic infection in developed countries is highly influenced by the low prevalence and diversity of the parasitic species, causing the diagnosis, on many occasions, to be a challenge. In the presence of eosinophilia and abdominal symptoms, it is recommended to orient the diagnosis towards a possible infection of parasitic origin to make an early diagnosis of the infection and avoid possible serious complications.
Keywords: Diarrhea; eosinophilia; larva; microscopy; parasite; Strongyloides stercoralis.
BACKGROUND
Strongyloidiasis is a parasitic disease caused by the Strongyloides stercoralis nematode that usually occurs asymptomatically but can manifest as a severe disseminated infection 1. The necessary forms for the diagnosis of this parasitosis are the rhabditiform larvae present in the intestinal mucosa, characterized by a prominent esophageal bulb, and the filariform larvae present in the feces, which are the infecting forms, measuring about 600 μm in length and having elongated fusiform esophagus 1,2.
The biological cycle of Strongyloides stercoralis is unique and unusual since the females are parthenogenetic; that is, they can give rise to a progeny without being fertilized. Due to this, there can be two types of cycles, the free life cycle that occurs in the external environment without the need for a mammalian host and the parasitic cycle inside a mammalian host. The invasion of this parasite occurs by penetration through the skin of filariform larvae present in the soil or the water 3.
Clinical case
A 5-year-old male patient from Ecuador with no relevant medical history came to the Emergency Department due to fever, colicky abdominal pain, watery diarrhea, and occasional vomiting of several days of evolution that intensified in the last two days. On physical examination, the abdomen was tender and painful on palpation, without masses or megaly, and increased peristalsis. The following complimentary tests were urgently requested: complete blood count, basic biochemistry with inflammatory markers (C-reactive protein), and urinary study. Laboratory results showed marked leukocytosis (24,000 leukocytes/µL) at the expense of 27% eosinophilia. The biochemistry and the study of the urinary sediment did not show alterations. Given the patient´s symptoms and origin, a microscopic study of parasites in feces was indicated, where larval forms compatible with Strongyloides stercoralis were observed (Figure 1).
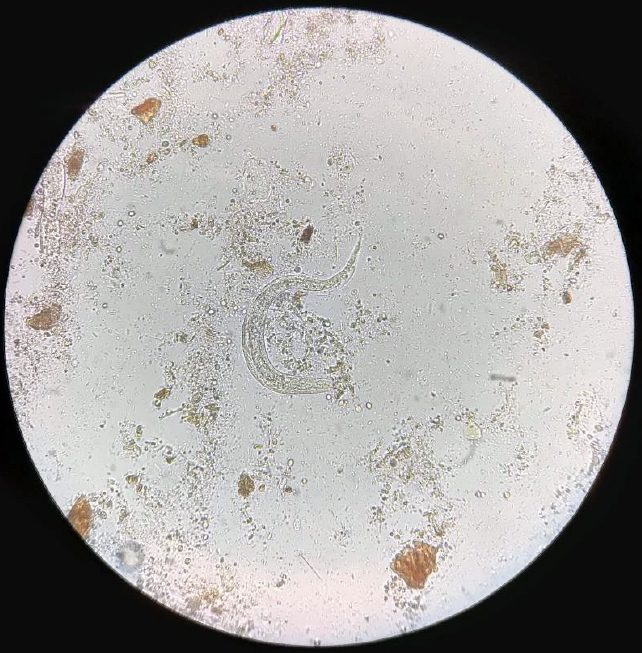
Figure 1. Strongyloides stercoralis larva in the stool.
Given this finding, treatment with oral ivermectin (200 mg/kg of body weight) was prescribed for two days, progressively decreasing diarrheal episodes and abdominal pain.
DISCUSSION
The diagnosis of this disease is epidemiological, clinical, and laboratory, and it is based on the microscopic visualization of the larvae of Strongyloides stercoralis in feces but, except in cases of hyperinfestation, the elimination is usually scarce and sporadic; therefore, the sensitivity of standard concentration procedures is very low 4. This, together with the fact that the diagnostic strategy of parasitic infection in countries such as Spain is highly influenced by the low prevalence and diversity of parasitic species, makes the diagnosis, on many occasions, a challenge 5,6.
Based on the above, many laboratories are replacing diagnostic methods based on conventional microscopy with enzyme immunoassay or molecular methods, which are techniques with higher sensitivity and specificity and less dependent on the observer. Thus, with more sensitive methods, work time is significantly reduced, and it is unnecessary to obtain the three classic fecal samples for parasitological examination. This approach would be similar to that already established for detecting microorganisms that cause bacterial and viral diarrhea (molecular stool culture), giving an entirely new dimension to the differential laboratory diagnosis of diarrheal diseases 6,7.
CONCLUSION
Currently, there are initiatives to organize quality assessment schemes for the molecular diagnosis of intestinal parasites. However, these systems have not yet been widely adopted due to the high cost of traditional microscopy and the uncertainty regarding the clinical importance of the molecular detection of each of the parasites included in the panels. Therefore, its impact on clinical practice has yet to be assessed.
In short, in the presence of eosinophilia and abdominal symptoms, it is recommended to orient the diagnosis towards a possible infection of parasitic origin to make an early diagnosis of the infection and avoid possible serious complications.
1. Greaves D, Coggle S, Pollard C, Aliyu SH, Moore EM. Strongyloides stercoralis infection. BMJ 2013;347:f4610.
2. Buonfrate D, Gobbi F, Angheben A, Bisoffi Z. Strongyloides stercoralis: the need for accurate information. Lancet 2018;391:2322-23.
3. Morales ML, López M, Ly P, Anjum S, Fernández-Baca MV, Valdivia-Rodríguez AM, et at. Strongyloides stercoralis infection at different altitudes of the Cusco region in Peru. Am J Trop Med Hyg 2019;101:422-27.
4. Barroso M, Salvador F, Sánchez-Montalvá A, Bosch-Nicolau P, Molina I. Strongyloides stercoralis infection: A systematic review of endemic cases in Spain. PLoS Negl Trop Dis 2019;13:e0007230.
5. Meningher T, Boleslavsky D, Barshack I, Tabibian-Keissar H, Kohen R, Gur-Wahnon D, et al. Giardia lamblia miRNAs as a new diagnostic tool for human giardiasis. PLoS Negl Trop Dis. 2019;13(6): e0007398.
6. De Boer RF, Ott A, Kesztyus B, Kooistra-Smid AM. Improved detection of five major gastrointestinal pathogens by use of a molecular screening approach. Clin Microbiol. 2010; 48: 4140-6.
7. Verweij JJ, Stensvold CR. Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin Microbiol. 2014; 27: 371-418.
Received: 1 April 2021
Accepted: 10 June 2021
Ricardo Rubio-Sánchez 1, Esperanza Lepe-Balsalobre 2.
1 UGC de Análisis Clínicos. Hospital Universitario Virgen de Valme. Sevilla. España.
2 Laboratorio de Análisis Clínicos. Hospital Universitario Virgen del Rocío. Sevilla. España.
*Autor para correspondencia:
Ricardo Rubio-Sánchez
Hospital Universitario de Valme
Dirección: Ctra. de Cádiz s/n. 1º planta. 41014. Sevilla.
Email: [email protected] Tfno.: 696867416
