2022.07.02.45
Files > Volume 7 > Vol 7 No 2 2022
1, Department of Horticulture and Landscape design, College
of Agriculture, Tikrit University, Salahaddin, Iraq.
*Corresponding Author: [email protected],
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.45
ABSTRACT
This study was conducted in order to test some agricultural wastes from fruits and sterilization method in the production of oyster mushroom Pleurotus sapidus. This study included two factors, the first is the type of substrate which consisting of seven combinations: A0 (wheat straw 80% + flour bran 20%), A1 (pomegranate peel 25% + wheat straw 75%), A2 (pomegranate peel 50% + wheat straw 50%), A3 (pomegranate peel 75% + wheat straw 25%), A4 (date fruit residues 25% + wheat straw 75%), A5 (date fruit residues 50% + wheat straw 50%), A6 (date fruit residues 75% + straw wheat 25%). The second factor is the sterilization method, represented by two methods of sterilization, the first is sterilization by Autoclave (P0) and the second is sterilization using hydrogen peroxide H2O2 at two concentrations of 3% (P1) and 5% (P2). A2 achieved the shortest period of growth and spread of mycelium which was 51.67 days and the highest amount of total wet yield was 199.2 g.kg-1. A0 and A6 had the shortest period for primordia formation which was 61.0 and 62.8 days, respectively. The mixture of A6 substrate gave the longest production cycle duration of 40.8 days and the highest biological efficiency rate was 44.78%. A1 and A2 gave the shortest fruiting period of 6.22 days. The P0 sterilization method recorded the shortest period for the growth and spread of mycelium, shortest period for the formation of primordia and the longest production cycle resulting in 48.88, 47.4 and 43.6 days, respectively, while the P1 sterilization method recorded highest wet yield of 175.6 g.kg-1 and the highest biological efficiency rate of 39.85%.
Keywords. Pleurotus sapidus, hydrogen peroxide, sterilization, substrate
INTRODUCTION
Mushrooms are large, heterotrophic, spore-producing fungi belonging to the kingdom of fungi1. Most of the cultivated edible mushrooms belong to the phylum Basidiomycota, although some genera of the phylum Ascomycota such as species of the genus Morchella and Tuber have been successfully cultivated and commercially exploited2. Mushroom usually grows either above the ground on the soil or on a substrate prepared industrially and used as a substrate3. The species belonging to the genus Pleurotus are economically important and its importance comes after the white button mushroom. Its importance is due to the fact that it is rich in proteins, essential amino and fatty acids, sugars, vitamins and important minerals such as calcium, iron, manganese and copper, as well as this mushroom had several medicinal properties in resisting cancer diseases, hypersensitivity and atherosclerosis4 5. Oyster mushroom contains protein in abundant quantities, it is also rich in vitamin B, oyster mushrooms do not contain cholesterol that causes hypertention, in addition to the fact that it contains high percentage of lovastatin, which lowers cholesterol levels, this led to encourage the study of oyster mushrooms for their benefits in adjusting cholesterol levels in the blood6. Oyster mushroom was grow on dead wood and other substrates rich in cellulose and lignin, it can be grown on agricultural and industrial wastes containing lignocellulosic biomass, such as wheat straw, soybean straw, cotton stalks, corn cobs, etc.7. Wheat straw was used as an ideal substrate for the production of oyster mushroom, but it is not available at all times of the year, in addition to its use as animal feed at high prices, which prompted researchers to find other substrates as alternatives to the substrate of wheat straw, such as wild plants, agricultural residues and date palm waste8. The productivity and biological efficiency of oyster mushroom were increased in some mixtures when compared to wheat straw alone, due to the difference in the ability of these substrates to provide nutritional and environmental requirements, in addition to the difference in the contents of cellulose, hemicellulose and lignin9. Several methods have been used to sterilize oyster mushroom substrate such as pasteurization, steam sterilization, hot water sterilization, chemical sterilization and sterilization with plant extracts in order to get rid of unwanted microorganisms present in the substrate10 11.
MATERIALS AND METHODS
Treatments of experiment
This experiment was carried out in Department of Horticulture and Landscape design/College of Agriculture/ Tikrit University in Iraq for production of oyster mushrooms Pleurotus sapidus, was conducted to test seven different mixtures of substrates (wheat straw 80% + 20% wheat bran, pomegranate peel 25% + wheat straw 75%, pomegranate peel 50% + wheat straw 50%, pomegranate peel 75% + wheat straw 25%, molasses factory wastes (date husk only) 25% + wheat straw 75%, molasses factory waste (date husk only) 50% + wheat straw 50%, molasses factory waste (date husk only) 75% + wheat straw 25%), with two types of substrates sterilization, the first is thermal by Autoclave and the second is chemical using hydrogen peroxide (H2O2) at concentrations of 3% and 5% .
Substrates sterilization
In the first sterilization method, the substrates were prepared and placed in mesh bags, they were soaked in tap water for 4-5 hours, then lifted and suspended to get rid of excess water for 24 hours, then they were mixed well with 2% calcium carbonate (CaCO3) on the basis of wet weight12 in order to ensure an even distribution of calcium carbonate in the substrate and adjust the acidity of the substrate to approximately pH 6.5, then the substrates (each substrate weight 1 kg) was placed in polypropylene plastic bags resistant to heat and pressure and sealed with heat-resistant thread, then placed in Autoclave device in the Plant Tissue Culture Laboratory/ Department of Horticulture and Landscape design / College of Agriculture/ Tikrit University, the sterilization was carried out under pressure of 1.5 bar and temperature of 121°C for 20-30 minutes13. Polypropylene plastic bags were taken out and left until its temperature decreased, then 2% of Pleurotus sapidus spawn was added to each bags by making a small hole in the bag and then closed well to prevent contamination of the substrate. In the second sterilization method, commercial hydrogen peroxide (50%) was used after diluting it to two concentrations, the first 3% and the other 5% 14, after the substrates was prepared and placed in perforated nylon bags, the bags were immersed in a plastic container with a diameter of 40 cm and a height of 80 cm containing dilute hydrogen peroxide for 5-6 hours, then lifted and placed on an iron shelf for 24 hours to get rid of excess water and hydrogen peroxide, then mixed well with calcium carbonate (2% on a wet weight basis) as it was found to be more suitable12 to ensure equal distribution of calcium carbonate in the substrate and adjusting the acidity of the substrate to pH 6.5, then the sterile substrates were filled with both concentrations in transparent plastic bags of the polyethylene 35 × 50 cm in dimensions and weighed so that each bag contains 1 kg of the wet substrate, then 2% (approximately 20 g.bag-1) of the fungal spawn was added to the substrate15.
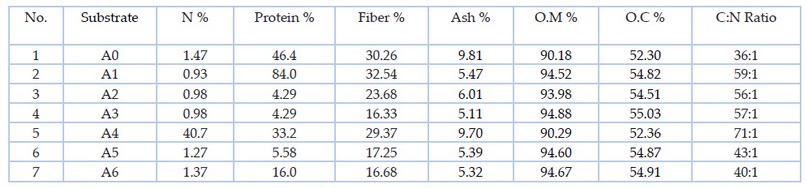
Table 1. some laboratory test to substrates combination according to Al-Amiri16
Studied traits
A different set of traits were studied, including:
Time period for the mycelium run: which represented the number of days from the beginning of the spawning substrate until the completion of the mycelium full growth in the substrates.
Primordia formation period: The number of days from the beginning of the spawning until the beginning of the emergence of the first primordia outside the bag in the form of pinheads.
Formation of the fruiting bodies: which is the number of days from the emergence of the primordia until the fruiting bodies are ready to be harvested16.
Number of fruit bodies: represented the number of fruit bodies resulting from one bag containing 1 kg of wet substrate for all flashes.
Total yield: total yield was estimated on the basis of substrate wet weight where the sum of all the yields produced from one bag containing 1 kg of substrate, and expressed on the basis of g. kg-1 substrate16.
Biological efficiency: the ability of a substrate to produce the largest amount of fruit bodies and is expressed in terms of the percentage of substrate production.
Production cycle: the number of days from the beginning of the harvesting stage to the last harvest.
Statistical analysis
All experiments units were performed at least in triplicate according to the Randomized Complete Block Design (RCBD). The obtained data were evaluated by two-factor analysis of variance, and significant differences were determined by Fisher’s test (L.S.D) at significance level of 0.05.
RESULTS
Effect of the substrates combination and the sterilization method and the interaction between them on the mycelium run of the of the oyster mushroom Pleurotus sapidus
The results of Table 2. showed that there were significant differences between the averages of the substrates combination, where the A2 substrate combination (50% pomegranate peel and 50% wheat straw) was superior on the most other mixtures with the shortest period of mycelium spread of 51.67 days. For the sterilization method, P0 sterilization method (autoclave sterilization) gave the shortest period for the spread of mycelium, reaching 76.45 days, superior on the sterilization method using hydrogen peroxide. In the interaction between combinations and sterilization methods, A0P0 treatment (80% wheat straw and 20% flour bran with autoclave sterilization) gave the shortest mycelium completion time of 35.67 days, superior on the most of the other treatments.
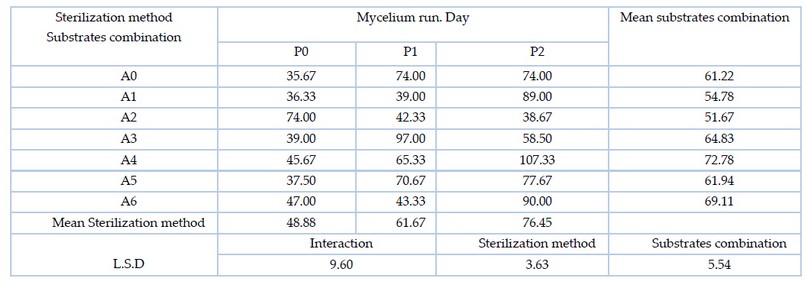
Table 2. Effect of the substrates combination and the sterilization method and the interaction between them on the mycelium run of the of the oyster mushroom Pleurotus sapidus (day)
Effect of the substrates combination and the sterilization method and the interaction between them on the time period for the primordia formation of the oyster mushroom Pleurotus sapidus
Statistical analysis of the data in Table 3 showed there are significant differences between the combination of the substrates, the two factors of substrates combinations A0 (wheat straw 80% and flour bran 20%) and A6 (date fruit residues 75% and wheat straw 25%) gave the shortest period for primordial formation reached 61.00 and 62.80 days, respectively, significantly superior on the factors of substrates combination A3 (75% pomegranate peel and 25% wheat straw) and A4 (25% date residues and 75% wheat straw). For the sterilization method, P0 (autoclave sterilization) outperformed the sterilization method using hydrogen peroxide with both concentrations, which gave the shortest period for primordial formation reached 47.40 days. In interaction between substrates combination and sterilization methods, the treatment A0P0 (wheat straw 80% and flour bran 20% with autoclave sterilization) gave the shortest duration of primordial formation reaching 34.00 days, superior on the most other treatments.
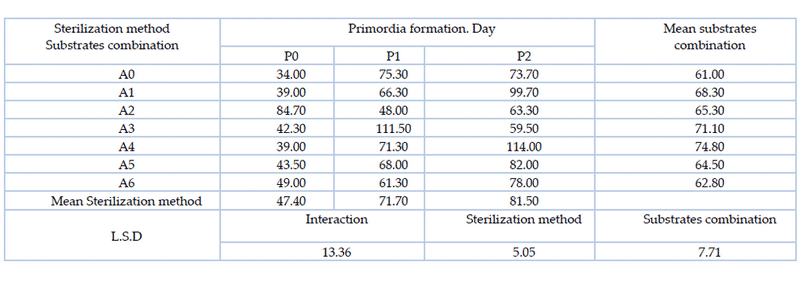
Table 3. Effect of substrates combination and sterilization method and the interaction between them on the time for the primordial formation of the oyster mushroom Pleurotus sapidus (day)
Effect of substrates combination and the sterilization method and the interaction between them on the formation of the Pleurotus sapidus fruit bodies .
It was shown through the statistical indications in Table 4. there were significant differences between the substrates combination, as the substrates combination A0 (wheat straw 80% and flour bran 20%) and A4 (date fruit residues 25% and wheat straw 75%) recorded the longest period for the fruit bodies formation reached 7.89 days, superior on the substrate combination A1 (pomegranate peel 25%, wheat straw 75%) and A2 mixture (pomegranate peel 50% and wheat straw 50%), which recorded the shortest period for the formation of the fruit bodies reached 6.22 days, while there were no significant differences between the other substrates. For the sterilization method, there were no significant differences between the means of sterilization methods. The interaction between the substrates combination and the sterilization method had significant differences, as the two treatments A0P0 (wheat straw 80% and flour bran 20% with autoclave sterilization) and A1P2 (pomegranate peel 25% and flour bran 75% with sterilization using hydrogen peroxide at 5%) showed superiority over most of the other treatments, where these treatments recorded the longest period for the formation of fruit bodies, which was 8.33 days.
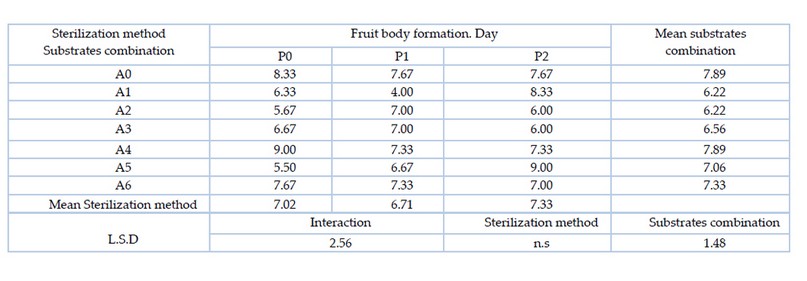
Table 4. Effect of substrates combination and the sterilization method and the interaction between them on the formation of the Pleurotus sapidus fruit bodies (day)
Effect of substrates combination and sterilization method and their interaction on the total fresh weight of oyster mushroom Pleurotus sapidus
The results of Table 5. Showed there are significant differences between the substrates combination, where the mixture of substrate A2 (pomegranate peel 50% and wheat straw 50%) significantly outperformed most of the treatments with the highest wet yield of 199.2 g.kg-1. As for the sterilization methods, the sterilization method P1 (hydrogen peroxide at 3%) outperformed the sterilization method P2 (hydrogen peroxide at 5%) with the highest wet yield of 175.6 g. The results of the interaction between the two factors (substrates combination and sterilization method) showed that there were significant differences between the treatments, whereby A5P1 treatment (date fruit residues 75% and wheat straw 25% sterilized with 3% hydrogen peroxide) significantly outperformed most of the other treatments with the highest wet yield of 233.1 g. kg-1.
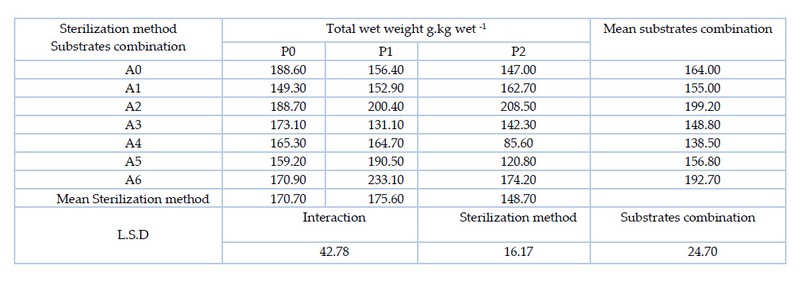
Table 5. Effect of substrates combination and sterilization method and their interaction on the total fresh weight of oyster mushroom Pleurotus sapidus (g.kg-1)
Effect of substrates combination and sterilization method and their interaction on the number of Pleurotus sapidus fruit bodies
The results of the statistical analysis in Table 6. indicate that there are no significant differences between the substrates combination factor in the characteristic of the number of fruit bodies, and the sterilization method used did not affect the number of fruit bodies, and thus no significant differences appeared between the sterilization methods. The results of the interaction between the two factors (substrates combinations and sterilization method) indicate that there are significant differences between the treatments, as the A4P0 treatment (date fruit residues 25%, wheat straw 75% with autoclave sterilization) outperformed most of the other treatments with the highest number of fruit bodies reaching to 6.33 fruit bodies.
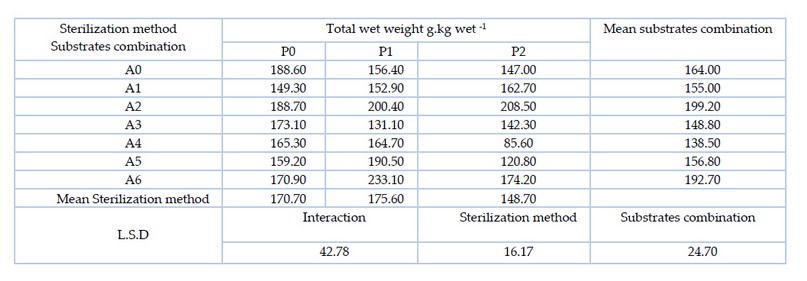
Table 6. Effect of substrates combination and sterilization method and their interaction on the number of Pleurotus sapidus fruit bodies.
Effect of substrates combination and the sterilization method and the interaction between them on the production cycle (days) for the oyster mushroom Pleurotus sapidus
It is clear from table 7. presence of significant differences between the substrates combination, where the mixture of A6 substrate (date fruit residues 75% and wheat straw 25%) outperformed the mixture of substrate A4 (date fruit residues 25% and wheat straw 75%) with the highest production cycle duration of 40.80 days. In case of the sterilization method, P0 (Autoclave sterilization) outperformed all other sterilization methods, as it recorded the highest period of 43.60 days. The interaction, A6P0 (date fruit residues 75% and wheat straw 25% sterilized by Autoclave) outperformed most of the other treatments with a production period of 68.70 days.
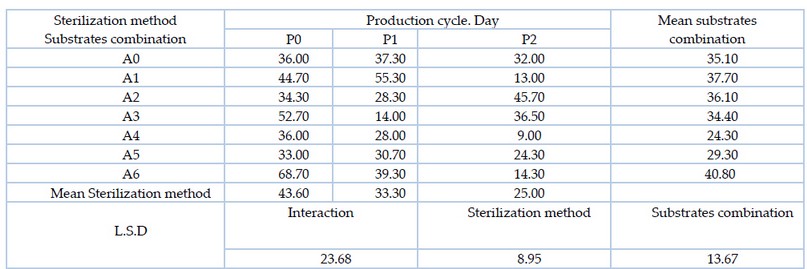
Table 7. Effect of substrates combination and the sterilization method and the interaction between them on the production cycle (days) for the oyster mushroom Pleurotus sapidus
Effect of substrates combination and the sterilization method and the interaction between them on the biological efficiency (%) of the oyster mushroom Pleurotus sapidus
In table 8, it is clear that there are significant differences between the factor of substrates combination, where the mixture of substrate A6 (date fruit residues 75% and wheat straw 25%) significantly outperformed most of the other substrates combination with the highest biological efficiency rate of 44.78%. However, a significant difference was found between the sterilization methods, where the sterilization method P1 (3% hydrogen peroxide) recorded the highest biological efficiency rate of 39.85%, significantly superior on the sterilization method P2 (5% hydrogen peroxide). In the interaction between the substrates combination and sterilization method, A6P1 (date fruit residues 75% and wheat straw 25% sterilized with 3% hydrogen peroxide) significantly outperformed all other treatments, recording the highest biological efficiency rate of 61.34%.
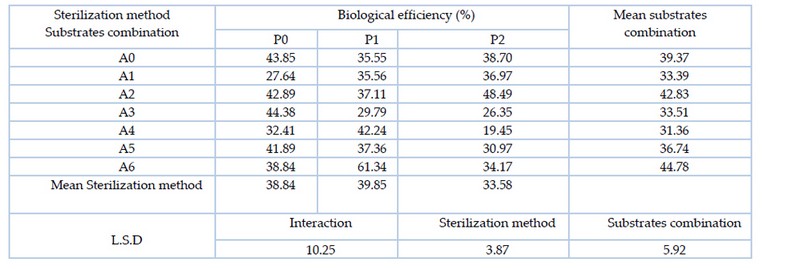
Table 8. Effect of substrates combination and sterilization method and the interaction between them on the biological efficiency (%) of the oyster mushroom Pleurotus sapidus.
DISCUSSION
The difference in the period of completion of mycelium growth may be due to several reasons such as the method of sterilization, type of material used for sterilization, moisture of the culture substrate, amount and method of adding the spawn to the mushroom substrate and requirements for the growth of the fungal strain used and its characteristics17. The reason for the variation in the growth period of the mycelium and the primordial formation as well as the completion of the fruit bodies growth may be due to the different substrates and their components, this is consistent with18 19. The growth stages of oyster mushrooms are affected by the type of substrate and its components. The variation in the amount of the total mushroom yield (on the basis of wet weight) may be due to the different substrates in their ability to provide the mycelium with the nutrients, with different substrates content of cellulose and hemicellulose compounds, hemicellulose degrades faster than cellulose compounds due to their low polymerization and amorphous nature20. The biological efficiency and production cycle differ according to the different components of the substrate itself from cellulose and hemicellulose in addition to the strength of the mushroom spawn and the generation and the strain used of the spawn21, the additives to the substrate and the amount of the mushroom spawn22. On the other hand, research did not agree on a specific efficacy and vitality for a particular species of mushroom or a substrate8 19.
CONCLUSION
The growth and production of oyster mushrooms was affected by different substrates and their combinations, as well as the sterilization method. Oyster mushrooms can be grown on substrates with pomegranate peels in their combinations, and they may be effective in producing mushrooms if some additives were added to them. The method of sterilization with hydrogen peroxide H2O2 can be used as a successful method for sterilization of cultivation substrates for oyster mushrooms, taking into account the soaking for a longer period and increasing the period of time before adding the mushroom spawn to the substrates to get rid of the largest possible amount of hydrogen peroxide.
Funding: This research received no external funding.
Acknowledgments: The authors are thankful to the Department of Horticulture and Landscape design, College of Agriculture, Tikrit University, for supporting this study's achievement.
Conflicts of Interest: There are no conflicts of interest reported by the authors.
REFERENCES
1. Kumar, V.; Subha Chandra, M. P.; Shancy, S. C.; Sabnam, V. S.; Lamya, T. V. Cultivation of edible mushroom in India: Precautions, opportunities and challenges. Journal of Plant Development Sciences 2015, 7(5), 409-413.
2. Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial cultivation of true morels: current state, issues and perspectives. Critical Reviews in Biotechnology 2018, 38(2), 259-271.
3. Thakur, M. P. Advances in mushroom production: Key to food, nutritional and employment security: A review. Indian Phytopathology 2020, 73, 377-395.
4. Sulistiany, H.; Sudirman, L. I.; Dharmaputra, O. S. Production of fruiting body and antioxidant activity of wild Pleurotus. HAYATI Journal of Biosciences 2016, 23(4), 191-195.
5. Oloke, J. K. Oyster mushroom (Pleurotus species); a natural functional food. The Journal of Microbiology, Biotechnology and Food Sciences, 2017. 7(3), 254.
6. Caz, V., Gil-Ramírez, A., Largo, C., Tabernero, M., Santamaría, M., Martin-Hernandez, R., ... & Soler-Rivas, C. 2015. Modulation of cholesterol-related gene expression by dietary fiber fractions from edible mushrooms. Journal of agricultural and food chemistry, 63(33), 7371-7380.
7. Sánchez, C. Cultivation of Pleurotus ostreatus and other edible mushrooms. Applied microbiology and biotechnology 2010, 85(5), 1321-1337.
8. Al-Saadawi, A. K. A. Evaluation of the efficiency of medium type and coverage in the productive and qualitative traits of Flammulina velutipes and Pleurotus erygii and their effect on resistance to some plant pathogens. PhD thesis. College of Agriculture - University of Baghdad. Iraq. 2015.
9. Owaid, M.N.; Abed, A.M.; Nassar, B.M. Recycling cardboard wastes to produce blue oyster mushroom Pleurotus ostreatus in Iraq. Emir. J. Food Agric 2015, 27, 537–541.
10. Al-Rubaie, W. L. T. Effectiveness of some plant extracts in controlling green mold disease caused by the fungus Trichoderma longibrachiatum associated with Pleurotus eryngii culture media. Master Thesis. College of Agriculture - University of Baghdad. Iraq. 2020.
11. Saritha, B.; Pandey, M. Evaluation of alternate substrate pasteurization techniques for culinary-medicinal white oyster mushroom, Pleurotus ostreatus var. florida (Agaricomycetideae) cultivation. International Journal of Medicinal Mushrooms 2010, 12(3).
12. Grace, A.; Ayandele, A. Comparative study of yield performance and nutrient composition of the edible mushroom pleurotus pulmonariu, cultivated on different substrates. African Journal of plant science 2018, 12(8), pp. 148-154.
13. Mubarak, M. T.; Ozsahin, I.; Ozsahin, D. U.Evaluation of sterilization methods for medical devices. In 2019 Advances in Science and Engineering Technology International Conferences (ASET) 2019, (pp. 1-4). IEEE.
14. Gowda, N. A.; Manvi, D. Agro-residues Disinfection Methods for Mushroom Cultivation. Agricultural Reviews 2019, 40(2), 93-103.
15. Vieira, F. R.; de Andrade, M. C. N. Optimization of substrate preparation for oyster mushroom (Pleurotus ostreatus) cultivation by studying different raw materials and substrate preparation conditions (composting: phases I and II). World Journal of Microbiology and Biotechnology 2016, 32(11), 1-9.
16. Al-Amiri, S. R. Effect of Substrate type, Supplement material and Spawn level on growth and yield of oyster mushroom (Pleurotus sapidus). PhD thesis, College of Technical- University of Al-furat Al-ausat Technical, Iraq. 2017
17. Imtiaj, A.; Rahman, S.A. Economic viability of mushrooms cultivation to poverty production in Bangladesh .Tropical and subtropical Agroecosystems 2008, 8(1): 93 - 99.
18. Iqbal, S. M.; Rauf, C. A.; Sheikh, M. I. Yield Performance of oyster mushroom on different substrates. Int. J. Agri. bio. 2005, 7(6): 900–903.
19. Al-Badrany, K. I. The effect of some local agricultural circles on the productivity of oyster mushrooms and their storage potential. Master's thesis, College of Agriculture - University of Baghdad, Iraq. 2010.
20. Kuhad, R. C.; Singh, A.; Eriksson, K. E. L. Microorganisms and enzymes involved in the degradation of plant fiber cell walls. Biotechnology in the pulp and paper industry 1997, 45-125.
21. Al-Douri, A. A. H. Mushroom production, Pleurutus spp. For human consumption on agricultural residues and the use of its products for animal consumption. Master Thesis. College of Science - University of Baghdad. Iraq. 2010.
22. Bhatti, M. I.; Jiskani, M. M.; Wagan, K. H.; Pathan, M. A.; Magsi, M. R. Growth, development and yield of oyster mushroom, Pleurotus ostreatus (Jacq. Ex. Fr.) Kummer as affected by different spawn rates. Pak. J. Bot 2007, 39(7), 2685-2692.
Received: 16 January 2022 / Accepted: 16 March 2022 / Published:15 May 2022
Citation. Hasan Najm S, Rasheed Majeed Alqaisi M. Investigated of the Hydrogen peroxide's efficacy in sterilizing various substrates for the formation of the oyster mushroom Pleurotus sapidus. Revis Bionatura 2022;7(2) 45. http://dx.doi.org/10.21931/RB/2022.07.02.45
