2022.07.02.50
Files > Volume 7 > Vol 7 No 2 2022
1Department of Biology, Faculty of Science, Cankiri Karatekin University, Cankiri, Turkey; [email protected]
2 Department of Biology, Faculty of Science, Cankiri Karatekin University, Cankiri, Turkey; [email protected]
3*Department of biology, College of Science, University of Misan, Iraq; [email protected].
*Correspondence:[email protected]
Available from: http://dx.doi.org/10.21931/RB/2022.07.02.50
ABSTRACT
Tuberculosis is considered one globally public health problem, particularly in unindustrialized countries. The disease has an increasing threat to public health, causing high morbidity and mortality rates. So, rapid diagnosis is critical to diminish the death and interfere with transmission. In this study, GeneXpert MTB/RIF and Loop-Mediated Isothermal Amplification (TB-LAMP) assays were utilized for the rapid molecular diagnosis of Mycobacterium tuberculosis bacteria and compared sensitivity and specificity of Mycobacterium tuberculosis bacteria; these tests with acid-fast bacilli staining in pulmonary and extra-pulmonary clinical specimens from Iraqi patients in Baghdad capital. 141 pulmonary and extra-pulmonary samples were collected and tested for tuberculosis detection. Acid-fast bacilli, TB-LAMP and GeneXpert MTB/RIF methods were used to diagnose tuberculosis. Of 141 tuberculosis specimens, 58 were acid-fast bacilli smear-positive, while 90 samples were positive for Mycobacterium tuberculosis by GeneXpert MTB/RIF assay and 78 by TB-LAMP assay. The results reported a high sensitivity rate of GeneXpert MTB/RIF assay compared to the TB-LAMP and bacilli staining method. The sensitivity of GeneXpert was 63.83%, specificity 50%; positive and negative predictive values were 58.36% to 68.96% and 42.73% to 57.27%, respectively. TB-LAMP sensitivity was 55.32%, specificity 50%; positive and negative predictive values were 49.61% to 60.89% and 43.70% to 56.30%, respectively, while the sensitivity of bacilli staining was 41.13%, specificity 50%; positive and negative predictive values were 35.26% to 47.27% and 44.88% to 55.12%, respectively, these values are dependent on disease prevalence. We concluded that the GeneXpert MTB/RIF test is more sensitive and specific than the TB-LAMP and both techniques are more sensitive and specific than the conventional acid-fast staining method for both pulmonary and extra-pulmonary specimens. Despite culture remaining the gold standard for laboratory confirmation of TB, GeneXpert MTB/RIF and TB-LAMP should become standard practice for patients with suspected TB, and all clinicians and public health programs for TB control should have access to molecular testing for TB to shorten diagnosis time.
Keywords. Mycobacterium tuberculosis; Pulmonary TB; Extrapulmonary TB; GeneXpert MTB/RIF; TB-LAMP; A.F.B
INTRODUCTION
Tuberculosis (TB) remains a primary infectious disease, particularly in developing countries, and it is a global public health concern₁.In 2019, there were approximately 10.4 million cases of TB worldwide. 90% of them were adults₂. Tuberculosis is a significant cause of death among infectious diseases, with about 25% of the world's population being infected₃. According to the estimations of the Iraqi ministry of planning for 2020, the population of Iraq is approximately 40 million. In Iraq, an incidence of 42 TB per 100,000 and the case detection rate in Iraq is 54% for all forms of TB cases₂, mainly due to the poor security situation in some governorates, which report fewer cases as many cases are displaced or lost to follow up. TB clinics in these governorates cannot function normally and staffs are unable to report to their clinics₄. It is estimated that Iraq is one of the seven countries with the highest TB burden, accounting for 3% of TB patients in the WHO Eastern Mediterranean region₅. In Iraq, the total TB incidence in 2019 was 41.000 (36.000 - 46.000)₂.
The pathogen that causes TB disease is Mycobacterium tuberculosis which continuously develops strains containing resistance genes, increasing the challenge of TB treatment and prevention₆. Initially, TB targets the lungs; however, the pathogen can invade other organs, including the skin, lymphatic, circulatory, central nervous, and digestive system₇. Although speedy and inexpensive, both the radiographic microscopy and the smear had poor sensitivity and positive predictive values; however, they remained a valuable prognosis in most resource-limited settings, from smear microscopy, which has low sensitivity and specificity compared to culture₈. The culture method is still the golden tool for final determination; nevertheless, it is slow and might take up to eight weeks due to the long incubation time of the pathogen₉. Thus, rapid and accurate methods of diagnosing Mycobacterium tuberculosis are essential for early initiation of treatment, prevention and improvement of patient outcomes. With the demand and urgency for rapid and simple diagnostic tests, especially in developing countries, polymerase chain reaction-based techniques have been developed for tuberculosis detection and antibiotic resistance testing₁₀.GeneXpert MTB / RIF and TB-LAMP assays are two modern molecular technologies that are widely used worldwide₁₁.
GeneXpert MTB / RIF is a nucleic acid test established by Xpert, Cepheid, Sunnyvale, CA, USA, developed to simultaneously diagnose TB and detect RIF resistance by detecting mutations in the RIF resistance determining region RRDR of the rpoB gene₁₂,₂. The Xpert MTB/RIF assay employs a molecular beacon to detect DNA sequences amplified in a hemi-nested rt-PCR assay with the ability to utilize 5 different nucleic acid hybridization probes performed in the same multiplex reaction. Every probe matches a different target sequence in the rpoB gene of rifampicin-susceptible M. tuberculosis, labeled with a different colored fluorophore, and these overlapping probes extend along the entire 81 bp core region of the rpoB gene₁₃.
Xpert results can be obtained in 2 hours, compared to the 8 weeks required to complete the traditional TB diagnosis by culture₁₄.
Loop-mediated isothermal amplification (LAMP) is the technique developed in Japan (Eiken Chemical Co., Ltd.)₁₅, which amplifies DNA under isothermal conditions₁₆.
The LAMP technique can increase small amounts of DNA to 109 copies in less than an hour using four to six primers that recognize six to eight regions of the target DNA. It initiates DNA amplification using different oligonucleotides simultaneously. As a result, the technique is particular, precise and fast. Reaction intermediates include several inverted repetitive loop structures. The large amounts of DNA produced, turbidity or fluorescence are observed, allowing the result to be determined visually. It prevents detection by gel electrophoresis or labeled probes. The LAMP technique is considered superior to other nucleic acid amplification methods because it is much faster, more efficient and economical than other NAATs₁₇, ₁₈
MATERIALS AND METHODS
Study design and samples collection
This study was conducted from September 3 to December 23 in 2020 on patients suspected of having pulmonary and extra-pulmonary tuberculosis at the specialized center for chest and respiratory diseases in Baghdad's capital. A total of 141 samples (107 pulmonary and 34 extra-pulmonary) were collected from patients with symptoms suggestive of tuberculosis. In this study, suspected tuberculosis patients were assigned to sample collection based on the presentation of symptoms and chest radiographic findings.
The pulmonary samples were taken from sputum, bronchial lavage, Broncho-alveolar lavage (BAL), and gastric lavage. Patients with clinical fitness criteria were requested to provide three sputum specimens within two days, two spot specimens furthermore one gotten in the morning.
Extra-pulmonary samples of pleural fluid, lymph node biopsy, cerebrospinal fluid, skin, ascitic fluid, sinus swab, and pus were taken.
The following steps are taken according to the type and location of the sample taken:
1-Sputum samples: Instruct each patient to cough deeply from the chest and spit sputum into the sterile container, avoiding nasal secretions or saliva, and three samples were collected. The first one was taken from the patient after arriving at the center; the second sample was collected early in the morning before breakfast; the third was collected at any time of the day.
2- Body fluids and other samples
Each body fluid was concentrated by centrifuging the samples at 3000 rpm for 15 min. The sediment has been processed. All of the above samples were digested and purified with 4% sodium hydroxide (NaOH); Samples were mixed with equal volumes of NaOH in a sterile falcon tube and mixed with a vortex mixer for a few seconds. The tube was then left at room temperature for 15 minutes. The mixture was then centrifuged at 3000 rpm for 15 min. Samples were neutralized by drops of HCL solution containing phenol red as an indicator and shaken until the color changed from red to yellow. The third sputum sample was directly tested by Ziehl-Neelsen staining and the TB-LAMP and GeneXpert MTB/RIF assays. Non-respiratory specimens from closed, sterile sites were not naturally cleared before smear preparation but were centrifuged at 3000 g for 20 min₁₉.
Tuberculosis detection
Ziehl-Neelsen’s stain (ZNS)
According to the guidelines of manual laboratory work, clinical decontaminated specimens were examined by Ziehl-Neelsen's stain₂₀ as follows
Using a sterile bacteriological loop, a smear was prepared on a new and clean slide and allowed to dry by air. Heat-fixed smears were flooded with carbon fuchsin stain, and the slides were heated slowly without boiling the stain. The stain was left for 5min., then rinsed the slides with tap water. Decolorization was performed by adding 25% sulfuric acid solution (H2SO4) to each slide and left for 2 min. Then rinsed the slides with tap water. Methylene blue was added to each slide for 1 min. As a counterstain, then rinsed the slides with tap water. Smears were allowed to dry in the air and then examined under a light microscope (Model CH 30, Olympus, Tokyo, Japan); the results of direct examination appear within fewer than 1 an hour. Smear results were reported for the presence or absence of AFB using the World Health Organization/International Union against Tuberculosis and Lung Disease scale, with a positive result corresponding to ≥ 1 AFB per 100 high-power fields.
GeneXpert MTB / RIF assay
The GeneXpert MTB/RIF (Cepheid Inc. /USA) assay consists of a single-use multi-chamber plastic cartridge with liquid buffers and lyophilized reagent beads required for sample processing and DNA extraction, and real-time polymerase chain reaction interference. Clinical samples or disinfected sputum pellets are treated with sodium hydroxide and a sample reagent containing isopropanol (SR). SR is added in a 2:1 ratio to the sample or sputum pellets and incubated for 15 minutes at room temperature. The treated sample is transferred to the cartridge, the cartridge is loaded into the GeneXpert instrument, and the automatic process completes the remaining assay steps. The assay cartridge also contains lyophilized Bacillus globigii, internal sample treatment and PCR control. Spores are automatically resuspended and processed during the sample processing step, and B. globigii DNA is amplified during the polymerase chain reaction step. The standard user interface indicates the presence or absence of Mycobacterium tuberculosis, the presence or absence of resistance to RIF, and a semi-quantitative estimation of the concentration of Mycobacterium tuberculosis (high, medium, low, very low). Negative tests for Mycobacterium tuberculosis and negative internal control with B. globigii were reported as invalid₂₁, GeneXpert MTB/RIF kit containing; cartridge (disposable), reagent 1, reagent 2, sample reagent and disposable transfer pipettes, the same company makes the kits.
The TB-LAMP technique
The TB-LAMP was performed on clinical decontaminated specimens following the manufacturer's recommendations (Eiken Chemical Co., Ltd., Tokyo, Japan). The Loopamp™ PURE DNA Extraction Kit extracted DNA from the sputum specimens. In a heating tube, a sixty microliter aliquot of purulent sputum was mixed with the DNA extraction solution and incubated at 90°C in an LF-160 incubator for 5 minutes to let bacterial cells lysis and DNA release into the solution. The process of DNA solution purification was conducted using porous absorbent powder in an adsorbent tube. A thirty microliter aliquot of purified DNA was distributed in the LAMP reaction tube comprising the lyophilized LAMP components (Bst DNA polymerase, dNTPs, calcein, and MTBC-specific primers targeting DNA gyrase subunit B and insertion sequence 6110). Reconstitution of the dried LAMP reagents (stored in the cap of the reaction tube) was performed by mixing it with the DNA solution. Then the mixture tube was incubated at 67.0°C for 40 minutes to permit DNA amplification by the LAMP reaction. The LAMP amplified products were visually detected based on their fluorescence under ultraviolet light.
RESULTS
The study included 141 patients (102 male, 39 female) with symptoms suggestive of TB based on clinical, pathological, or radiological evidence of tuberculosis. The age groups included in this study are between 6 and 65 years old. Male cases were 70 (78.77%), and female cases were 20 (22.3%). Most of the patients were in the 25-35 age group, while the lowest were under 15 years.
As the results appeared in table 1 and figure 1 the distribution of positive results for 54 lung samples tested by microscope (sputum 47, bronchial lavage 5, alveolar Broncho-alveolar lavage BAL 1, gastric lavage 1) and distribution of 4 positive results for extra-pulmonary samples diagnosed with Microscope (pleural fluid 3, Lymph node 1).
At the same time as the distribution of positive results for 76 lung samples tested with GeneXpert MTB / RIF (sputum 63, bronchial lavage 8, alveolar bronchial lavage BAL 2, gastric lavage 3) and distribution of 14 positive results for extra-pulmonary specimens diagnosed with GeneXpert MTB / RIF (pleural fluid 8, skin swab 4, lymph node swab 2). Distribution of positive results for 70 lung samples tested with TB-LAMP (sputum 57, bronchial lavage 8, alveolar broncho-alveolar lavage BAL 2, gastric lavage 3) and distribution of 8 positive results for extra-pulmonary samples diagnosed with TB-LAMP (pleural fluid 6, Lymph node 2).
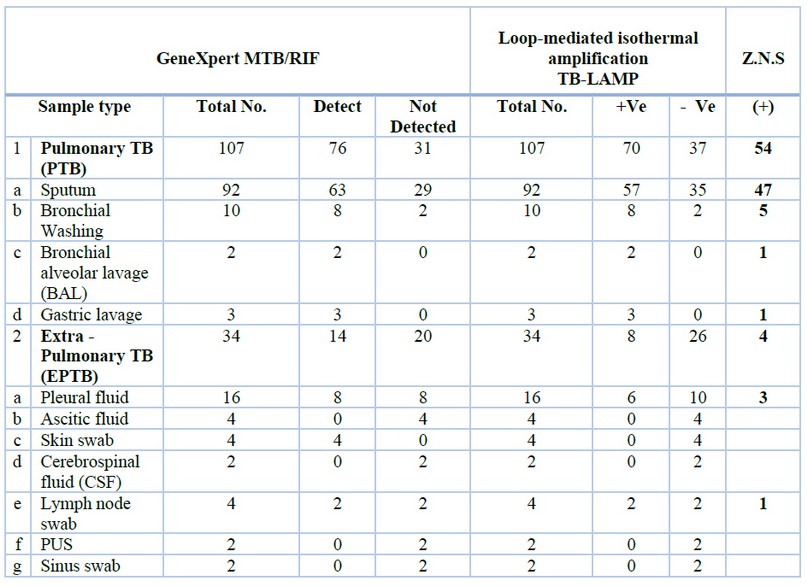
Table 1. Results by GeneXpert MTB / RIF, TB-LAMP and Z.N.S
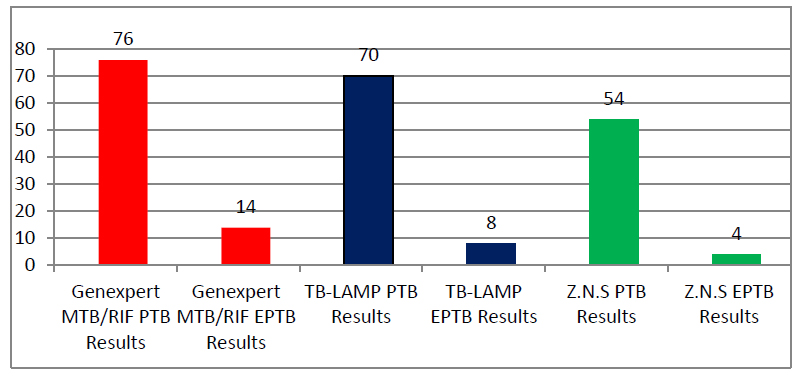
Figure 1. Distribution of PTB and EPTB samples diagnosed by the three methods
As shown in table 1 and figure 2, the results of the Ziehl-Neelsen stain showed that the sum of the positive results was 58 (54 pulmonary and 4 extra-pulmonary).
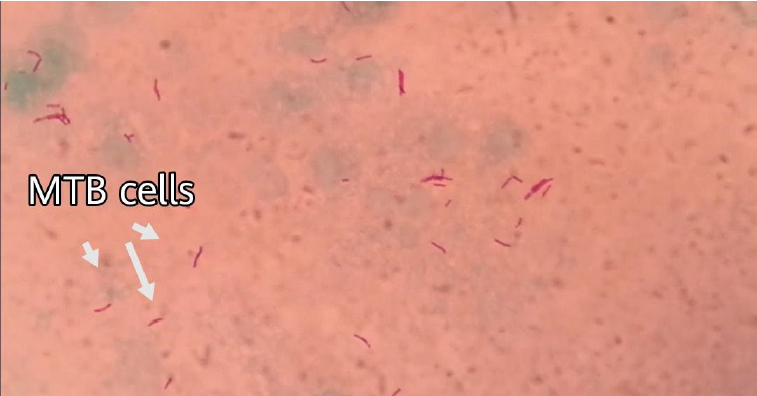
Figure 2. Mycobacterium tuberculosis bacteria stained by Ziehl-Neelsen stain, under light microscope (magnification power is 1000X).
Molecular diagnosis of Mycobacterium tuberculosis bacteria by GeneXpert MTB / RIF test revealed that 90 samples were positive (76 pulmonary and 14 extra-pulmonary). The results appeared on the computer screen according to the number of TB bacilli isolated by the GeneXpert MTB / RIF system, and they were (very low, low, medium or high, very high)(table 1 figure 3).
Only two cases of rifampicin resistance were diagnosed due to therapeutic relapse and referred to a respiratory consultant for evaluation.
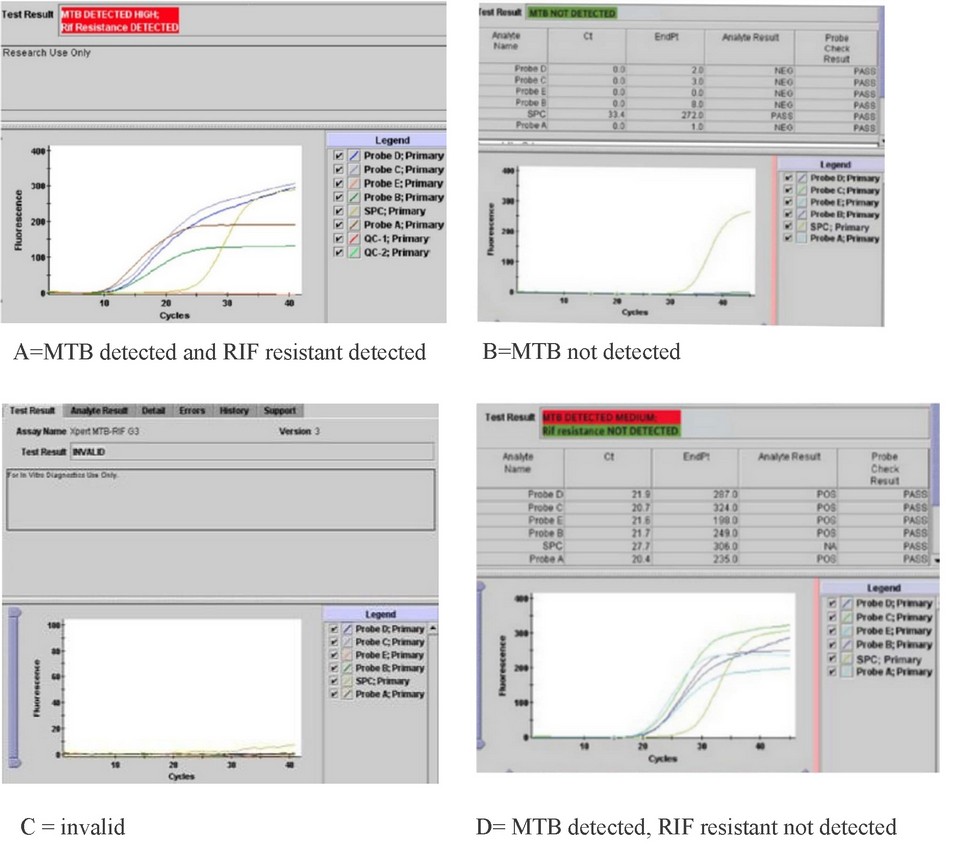
Figure 3. GeneXpert MTB / RIF test results
Table 1 and figure 4 showed that molecular diagnosis of Mycobacterium tuberculosis using the TB-LAMP test showed 78 samples were positive (70 pulmonary and 8 extra-pulmonary). The study results appeared in the fluorescence detection unit of the TB-LAMP device, and when the UV light was turned on, the color of the pattern was green in case of a positive result, while negative results remained unchanged in color.
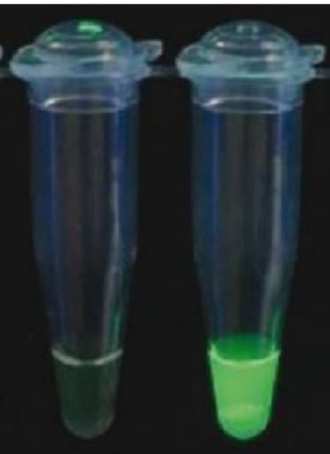
Figure 4. TB-LAMP test results, - means negative result while + means a positive result
As shown in table 2, GeneXpert MTB / RIF sensitivity was 63.83% while 50% was specificity; the positive and negative predictive values were 58.36% to 68.96% and 42.73% to 57.27%, respectively whereas the sensitivity and specificity of TB-LAMP was 55.32% for sensitivity and 50% was the specificity ; the positive and negative predictive values were 49.61% - 60.89% and 43.70% - 56.30 respectively, while the acid-fast bacilli (AFB) sensitivity was 41.13%, and the specificity 50%; The positive and negative predictive values are 35.26% to 47.27% and 44.88% to 55.12%, respectively. These values depend on disease prevalence; statistical analysis data were analyzed using SPSS version 24. Percentages were calculated when the results of the AFB test, GeneXpert test and TB-LAMP test of the same patients as shown in table 2.
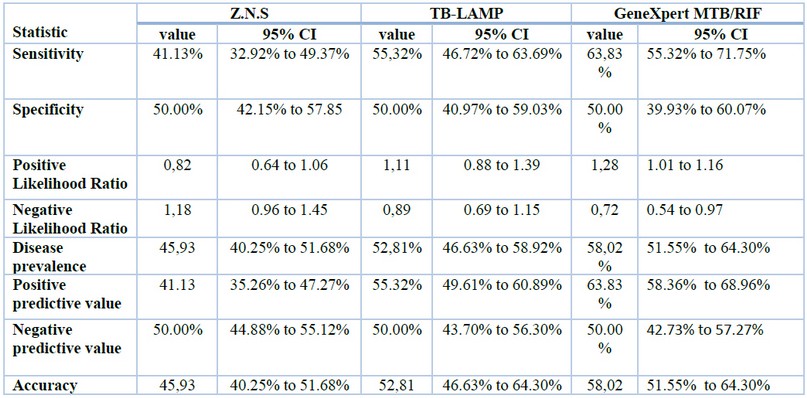
Table 2. Statistical analysis Z.N.S, GeneXpert MTB/RIF and TB-LAMP.
DISCUSSION
The availability of affordable, fast and reliable diagnostic tests that can be used in resource-limited settings is a critical gap in the battle against TB. The production of nucleic acid-based tests has created new avenues for producing susceptible point-of-care tests. The GeneXpert MTB/RIF, which has turned the TB diagnostic landscape into well-fitted environments, is the latest model that has been rolled out worldwide, but it has major resource-poor setting limitations₂₂, while the TB-LAMP test needs fewer infrastructures as an alternative and may therefore be applicable for use in second-tier diagnostic laboratories₂₃.
In this study, the age groups included are between 6 and 65. Male TB positive cases were 70 (78.77%), and female TB positive cases were 20 (22.2%) from 141 pulmonary and extra-pulmonary samples. The higher percent of the patients were recorded in the 25-35 age groups, whereas the lowest was below 15 years. A similar study described different percentages in different places around the world. In this respect, our data were in the same line with a study conducted in Turkey in 2015 on pulmonary and extra-pulmonary TB Established that positive results for 372 patients were 227 (61%) men, 145 (39%) women, 168 (45.2%) EPTB and 204 (54.8%) PTB₂₄, however, our study conflicts with the study conducted by Al-Otaibi and Al-Hazmi (2010) in Saudi Arabia, which reported that the rate of EPTB was 57.5% versus 42.5% according to PTB. The most dominant age group impacted was 20-29 years (n=38; 33%) followed by age 60 and more (n=25; 21.7%)₂₅; these findings were different from data obtained by (Sharma et al., 2017), who reported that 41-60 age group was the most affected one in their study₂₆.
A study by Rakotosamimanana in Madagascar found the high sensitivity and specificity was confirmed for both GeneXpert MTB/RIF® (86.6% (95% CI 81.1-90.7%) and 97.4% (95% CI 94.9-98.8%)) and TB-LAMP (84.6% (95% CI 78.9-89.0%) and 98.4% (95% CI 96.2-99.4%)). Implementation of GeneXpert MTB/RIF and TB-LAMP increased tuberculosis diagnostic algorithms sensitivity from 73.6% (95% CI 67.1-79.3%) up to 88.1% (95% CI 82.8-91.9%). This increase was highest when molecular assays were used as add-on testing following negative smear microscopy, as add-on testing, GeneXpert MTB/RIF and TB-LAMP respectively improved case detection by 23.8 and 21.2% (p < 0.05)₂₇.
The results of a study in Gambia (2016) demonstrate the diagnostic utility of both GeneXpert and TB-LAMP. While the TB-LAMP program requires fewer infrastructures, it cannot detect drug resistance patterns and is thus more suitable as a screening test for new TB cases in peripheral health clinics. When using culture as the reference standard, it was found to have an overall sensitivity of 99% (95% CI: 94.5-99.8) and a specificity of 94% (95% CI: 89.3-96.7) for TB-LAMP. When performing a latent class analysis, TB-LAMP has a sensitivity of 98.6% (95% CI: 95.9-100) and a sensitivity of 99% (95% CI: 98 compared to 91.1% (95% CI: 86.1-96). 2-100) and 100% (95% CI: 98.2-100) specificity to MGIT culture. GeneXpert had the highest sensitivity of 99.1% (95% CI: 97.1-100), but the lowest specificity of 96% (95% CI: 92.6-98.3). TB-LAMP and GeneXpert showed high sensitivity and specificity regardless of age or type of infection₂₈.
GeneXpert test with smear microscopy and the gold standard of Mycobacterial culture and TB-LAMP were compared to a diagnosis of TB. Sputum Specimens were collected from patients with pulmonary TB symptoms who attended a primary health care clinic. The results showed that the TB-LAMP test had significantly higher sensitivity than smear microscopy (72.6% versus 45.4%, P, 0.001) but was significantly higher than GeneXpert (92.9%, P¼0.004). There was no significant difference in the sensitivity of smear-positive, culture-positive and smear-negative, culture-positive sputum samples using GeneXpert versus TB-LAMP (95.9% / 55.9% versus 97.6% / 66.1%, respectively; P ¼0.65, P ¼ 0.27). The positive predictive value of TB LAMP was significantly higher than that of GeneXpert (87.5% versus 77.0%; P 0.02), but similar to that of the smear microscope (94.2%; P 0.18). The negative predictive value was 91.9%, 92.5% (P ¼ 0.73) and 83.1% (P ¼ 0.0001 respectively₂₉.
A study conducted in North India (2017) stated that 530 patients with PTB symptoms with one sputum sample was collected from each patient. The TB-LAMP assay was performed. The rest of the sample was used for microscopy and culture. Xpert® MTB/RIF assay was also enrolled in this study; the findings of this study show the sensitivity and specificity of the TB-LAMP assay in culture-positive samples obtained from 453 patients presenting with PTB symptoms were respectively 100% (95%CI 94.7-100) and 99.2% (95%CI 97.8-99.8). The sensitivity and specificity of Xpert in culture-positive samples were respectively 82.6% (95%CI 71.5-90.6) and 94.9% (95%CI 92.2-96.8). The study concludes that the TB-LAMP assay showed high sensitivity and specificity with the limited requirement of testing infrastructure and is thus a promising diagnostic tool for TB diagnosis in resource-poor settings₃₀.
GeneXpert MTB/RIF and TB-LAMP provide a definitive diagnostic test for identifying MTB with the advantage of GeneXpert MTB/RIF over TB-LAMP contrasting conventional methods. The main disadvantage of AFB smear is low sensitivity, and essential benefits of GeneXpert MTB/RIF and TB-LAMP are the early diagnoses of TB and early initiation of treatment; Thus. They were improving patient consequences. Although culture remains the necessary standard test for laboratory confirmation of TB, GeneXpert MTB/RIF should become the standard first and then TB-LAMP. This study is for patients suspected of having TB, and all physicians and public health programs must obtain molecular TB tests to shorten the diagnostic time. Through this study, we found some advantages and disadvantages in both techniques, which are as follows, advantages of GeneXpert MTB / RIF; fast completion time (two hours), the ability to detect very few living tubercles, detection of rifampicin-resistant and full automation is possible to eliminate personal errors and cross-contamination of samples, while the disadvantages were; Incorrect results due to the presence of amplification inhibitors in samples, high cost and technical skill requirement, Nucleic acid contamination - false results, amplification from dead bacillus and both Cartridges and the machine must be stored appropriately (at optimum temperature), connection with a computer and a continuous optimum power supply are the absolute fundamental. On the other hand, the advantages of TB-LAMP were; fast technique (one hour), low cost, simple skills, and cheaper than GeneXpert kits, while the disadvantages were; It is not automatic; all processes (DNA extraction, amplification and result interpretation) are manual, it is challenging to run multiplex LAMP reactions in one tube, and Unlike the GeneXpert device, the resistance of Mycobacterium tuberculosis bacteria to rifampicin is not detected.
CONCLUSION
This study firmly concluded the high sensitivity and specificity of molecular tests as compared with conventional methods, which need more time and heavy labor. Also, this study confirmed that the GeneXpert MTB/RIF test is more sensitive and specific than the TB-LAMP, and both are more sensitive and specific than the conventional acid-fast staining method for both pulmonary and extra-pulmonary specimens and recommended to use of these two techniques especially suspected cases of TB.
Funding: "This research received no external funding."
Conflicts of Interest: "The authors declare no conflict of interest."
REFERENCES
1. Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop Dis Travel Med Vaccines. 2018 June 14;4:5. doi: 10.1186/s40794-018-0065-5. PMID: 29942536; PMCID: PMC6000958.
2. World Health Organization. Global TB 2020 report. World Health Organization; 2020
3. da Silva Escada RO, Velasque L, Ribeiro SR, Cardoso SW, Marins LMS, Grinsztejn E, da Silva Lourenço MC, Grinsztejn B, Veloso VG. Mortality in patients with HIV-1 and tuberculosis co-infection in Rio de Janeiro, Brazil - associated factors and causes of death. BMC Infect Dis. 2017 May 30;17(1):373. doi: 10.1186/s12879-017-2473-y. PMID: 28558689; PMCID: PMC5450415.
4. WHO/ Eastern Mediterranean Regional Office (EMRO). (2015).Tuberculosis/ programmes/ Iraq. Eastern Mediterranean Health Journal (EMHJ). http://www.emro.who.int /iraq/programmes/tuberculosis.htm
5. WHO (2019). Global Tuberculosis report. https://www.who.int/teams/global tuberculosisprogramme/tb-reports/global-report-2019
6. Singh, R., Dwivedi, S. P., Gaharwar, U. S., Meena, R., Rajamani, P., & Prasad, T. (2020). Recent updates on drug resistance in Mycobacterium tuberculosis. Journal of applied microbiology, 128(6), 1547-1567.
7. Moule MG, Cirillo JD. Mycobacterium tuberculosis Dissemination Plays a Critical Role in Pathogenesis. Front Cell Infect Microbiol. 2020;10:65. Published 2020 February 25. doi:10.3389/fcimb.2020.00065
8. Ahmed, S., Shukla, I., Fatima, N., Varshney, S. K., Shameem, M., & Tayyaba, U. (2019). Light-emitting diode-fluorescent microscopy: Determining its sensitivity and specificity in diagnosis of pulmonary tuberculosis in a high-burden tuberculosis region and resource-limited country like India. CHRISMED Journal of Health and Research, 6(1), 44. doi: 10.4103/cjhr.cjhr_46_18
9. Afsar, I.; Gunes, M.; Er, H. and Gamze Sener, A. (2018).Comparison of culture, microscopic smear and molecular methods in diagnosis of tuberculosis. Rev Esp Quimioter. 2018 Oct;31(5):435-438. Epub 2018 September 19. PMID: 30229645; PMCID: PMC6194869.
10. Maldonado-Bernal, C., Ramos-Garibay, A., Rios-Sarabia, N., Serrano, H., Carrera, M., Navarrete-Franco, G., ... & Isibasi, A. (2019). Nested polymerase chain reaction and cutaneous tuberculosis. The American Journal of dermatopathology, 41(6), 428-435. doi:10.1097/DAD.0000000000001315
11. Garg, R. K. (2019). Microbiological diagnosis of tuberculous meningitis: Phenotype to genotype. The Indian journal of medical research, 150(5), 448. doi: 10.4103/ijmr.IJMR_1145_19
12. Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-López EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010 Jan;48(1):229-37. doi: 10.1128/JCM.01463-09. Epub 2009 October 28. PMID: 19864480; PMCID: PMC2812290.
13. Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011 Sep;6(9):1067-82. doi: 10.2217/fmb.11.84. Erratum in: Future Microbiol. 2012 Aug;7(8):1024. PMID: 21958145; PMCID: PMC3252681.
14. Boehme CC, Nabeta P, Hillemann D. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-15. doi: 10.1056/NEJMoa0907847
15. Mori Y, Kanda H, Notomi T. Loop-mediated isothermal amplification (LAMP): recent progress in research and development. J Infect Chemother. 2013 Jun;19(3):404-11. doi: 10.1007/s10156-013-0590-0. Epub 2013 March 29. PMID: 23539453; PMCID: PMC7088141.
16. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000 June 15;28(12):E63. doi: 10.1093/nar/28.12.e63. PMID: 10871386; PMCID: PMC102748.
17. Aryan E, Markvandi M, Farajzadeh A, Huygen K, Bifani P, Mousavi S-L, et al. A novel and more sensitive loop mediated isothermal amplification assay target IS6110 for detection of Mycobacterium tuberculosis complex. Microbiol Res. 2010; 165:211-22. doi: 10.1016/j.micres.2009.05.001
18. Neonakis IK, Spandidos DA, Petinaki E. Use of loop-mediated isothermal amplification of DNA for the rapid detection of Mycobacterium tuberculosis in clinical specimens. Eur J Clin Microbiol Infect Dis. 2011 Aug;30(8):937-42. doi: 10.1007/s10096-011-1195-0. Epub 2011 February 18. PMID: 21331481.
19. Forbes, B. A., Hall, G. S., Miller, M. B., Novak, S. M., Rowlinson, M. C., Salfinger, M., Somoskövi, A., Warshauer, D. M., & Wilson, M. L. (2018). Practical Guidance for Clinical Microbiology Laboratories: Mycobacteria. Clinical microbiology reviews, 31(2), e00038-17. https://doi.org/10.1128/CMR.00038-17
20. Tessema B, Beer J, Emmrich F, Sack U, Rodloff AC. Rate of recovery of Mycobacterium tuberculosis from frozen acid-fast-bacillus smear-positive sputum samples subjected to long-term storage in Northwest Ethiopia. J Clin Microbiol. 2011;49(7):2557-2561. doi:10.1128/JCM.00059-11
21. Ajbani, Kanchan; Naik, Swapna; Kazi, Mubin; Shetty, Anjali; Rodrigues, Camilla Interpreting very low Mycobacterium tuberculosis detected on Xpert Mycobacterium tuberculosis/rifampicin, Lung India: Nov–Dec 2019 - Volume 36 - Issue 6 - p 555-557 doi: 10.4103/lungindia.lungindia_463_18
22. Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014 January 21;2014(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. Update in: Cochrane Database Syst Rev. 2019 June 7;6:CD009593. PMID: 24448973; PMCID: PMC4470349.
23. Shete, P.B., Farr, K., Strnad, L. et al. Diagnostic accuracy of TB-LAMP for pulmonary tuberculosis: a systematic review and meta-analysis. BMC Infect Dis 19, 268 (2019). https://doi.org/10.1186/s12879-019-3881-y
24. Guler SA, Bozkus F, Inci MF, Kokoglu OF, Ucmak H, Ozden S, Yuksel M. Evaluation of pulmonary and extra-pulmonary tuberculosis in immunocompetent adults: a retrospective case series analysis. Med Princ Pract. 2015;24(1):75-9. doi: 10.1159/000365511. Epub 2014 October 17. PMID: 25341702; PMCID: PMC5588178.
25. Al- Otaibi, F. and El Hazmi, M. M. (2010). Extra-pulmonary tuberculosis in Saudi Arabia. Indian J. Pathol. Microbiol. 53(2): 227 31. doi: 10.4103/0377-4929.64327
26. Sharma P, Katoch K, Chandra S, Chauhan DS, Sharma VD, Couvin D, Rastogi N, Katoch VM. Comparative study of genotypes of Mycobacterium tuberculosis from a Northern Indian setting with strains reported from other parts of India and neighboring countries. Tuberculosis (Edinb). 2017 Jul;105:60-72. doi: 10.1016/j.tube.2017.04.003. Epub 2017 April 10. PMID: 28610789.
27. Rakotosamimanana, N., Lapierre, S.G., Raharimanga, V. et al. Performance and impact of GeneXpert MTB/RIF® and Loopamp MTBC Detection Kit® assays on tuberculosis case detection in Madagascar. BMC Infect Dis 19, 542 (2019). https://doi.org/10.1186/s12879-019-4198-6
28. Bojang AL, Mendy FS, Tientcheu LD, Otu J, Antonio M, Kampmann B, Agbla S, Sutherland JS. Comparison of TB-LAMP, GeneXpert MTB/RIF and culture for diagnosis of pulmonary tuberculosis in The Gambia. J Infect. 2016 Mar;72(3):332-7. doi: 10.1016/j.jinf.2015.11.011. Epub 2015 December 24. PMID: 26724771.
29. Reddy S, Ntoyanto S, Sakadavan Y, Reddy T, Mahomed S, Dlamini M, Spooner B, Ramjee G, Coutsoudis A, Ngomane N, Naidoo K, Mlisana K, Kiepiela P. Detecting Mycobacterium tuberculosis using the loop-mediated isothermal amplification test in South Africa. Int J Tuberc Lung Dis. 2017 October 1;21(10):1154-1160. doi: 10.5588/ijtld.16.0863. PMID: 28911361.
30. Yadav R, Sharma N, Khaneja R, et al. Evaluation of the TB-LAMP assay for the rapid diagnosis of pulmonary tuberculosis in Northern India. The International Journal of Tuberculosis and Lung Disease : the Official Journal of the International Union Against Tuberculosis and Lung Disease. 2017 Oct;21(10):1150-1153. DOI: 10.5588/ijtld.17.0035.
Received: 31 January 2022 / Accepted: 15 March 2022 / Published:15 May 2022
Citation. Jabber Benellam Y K, Ozkan O, Saadoon Aziz Z . Diagnosis of Mycobacterium tuberculosis using GeneXpert MTB / RIF and TB-LAMP techniques from pulmonary and extra-pulmonary TB patients in Iraq. Revis Bionatura 2022;7(2) 50. http://dx.doi.org/10.21931/RB/2022.07.02.50
