2023.08.02.49
Files > Volume 8 > Vol 8 No 2 2023
Effects of In-Ovo Injection of sialic acid on Chick's embryonic development and physiological traits
1Department of Biology, College of Education for Pure Science, University of Anbar, Ramadi, Iraq
2Department of Animal Production, College of Agriculture, University of Anbar, Ramadi, Iraq
*Correspondence author: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.49
ABSTRACT
This study was conducted at the College of Agriculture – University of Anbar, Iraq. From 16 January to 5 February 2022, this study aimed to investigate the effect of injected egg hatching at different sialic acid times in growth and embryonic development. Four hundred eggs of hatching types (Ross 308) were injected with different sialic acid concentrations at 0 days (before placing in the incubator), 7 and 14 days of incubation. Eggs were divided into four groups (100 eggs each) as follows: T1: The control group was placed in the incubator without injection. T2: Injected with a dose of 100 μg sialic acid at the age of zero. T3: Injected with 100 μg sialic acid dose at 7 days. T4: Injected with 100 μg sialic acid dose at 14 days of incubation. Statistical analysis was performed (CRD) (P=0.05); results show: Increase in the e length of the embryo, the diameter of the vascular region and the number of pairs of somites at 3 days of incubation for T1. Increase in the percentage of embryonic weight, decrease in the percentage of Albumin and the percentage of shell at 7 days of incubation for T2 and T3. Increase in percentage of embryonic weight and amniotic sac and liquid, decrease in the percentage of Albumin and yolk, at 14 days of include sialic acid for T2. Increase the percentage of embryonic weight, and decrease the percentage of yolk at 14 days incubation for T2. They have concluded that In-Ovo injection of the hatching eggs with sialic acid contributed to increased physiological traits and embryonic development.
Keywords: In-Ovo, Sialic Acid, Erythropoietin, Oxygen, Anemia, and Anoxia.
INTRODUCTION
Utilization of supplements starts on the first day after incubation when both egg albumin and yolks assist in the development of the embryo. Bird embryos develop and grow from the energy and nutrients the hen stores in the egg. Nutrients are deposited in follicles over a long period but become essential during the week before ovulation. The amount and type of nutrients the egg delivers affect how successfully an embryo develops and how healthily a chick hatches 1. Sialic acids are an assortment of nine-carbon alpha-keto acid sugars. N-acetylneuraminic acid (Neu5Ac or NANA), found in animals and some prokaryotes, is the most prevalent member of this group. N-acetylglucosamine-6-P is produced by glucosamine 6 phosphate and acetyl-CoA using a transferase to produce sialic acid. This undergoes epimerization to become N-acetylmannosamine-6-P, which then interacts with phosphoenolpyruvate to form N-acetylneuraminic-9-P. (sialic acid). A monophosphate nucleoside is added to make sialic acid become cytidine monophosphate-sialic acid, which is then active to participate in the cell's oligosaccharide manufacturing process (CMP-sialic acid). The animal cell's nucleus is where this substance is made2. Sialic acid is known to serve as a red blood cell (RBC)-bound receptor for P. falciparum in humans. Also, it affords protection against oxidative stress, which may be highly important for RBCs, whose primary function is to transport oxygen3.
The development of chickens and the processes of breeding and improvement that occurred in domestic chickens during the previous years led to a change in some physiological and immunological characteristics4, the most important of which was a weakness in the membranes of Chorion, Amnion and Allantois, and thus leads to a lack of oxygen supply as a result of a decrease in the formation of cells red blood and the occurrence of a suffocation process as a result of a lack of oxygen in the body5, which is expressed by the term Anoxia in the last stages of hatching and thus the death of the embryos6. It is possible to use natural nutrients that contribute to the development of the breathing process in embryos, such as the use of sialic acid, which is the primary catalyst for the formation of the hormone erythropoietin, which works to produce red cells through its action on the bone marrow and increases their numbers in the lining of blood vessels and increases the production of nitric oxide, which facilitates it controls blood flow, which improves oxygen supply to the heart, brain, and other tissues. 7.
This study aims to investigate the use of natural nutrients that contributes to solving the problem of suffocation of bird embryos and the lack of oxygen supply by increasing the production of Erythropoietin, which is one of the factors used in treating anemia in animals and humans.
MATERIALS AND METHODS
Animal Study
The study was conducted according to the protocol approved by the University of Anbar Ethics Committee, Iraq. Fertile eggs from Ross (308) strain broiler breeder hens were gotten from a commercial farm.
Experimental study
In this study, 400 eggs were Collected from Ross 308 Broiler breeders (53 weeks old). The egg was divided into 4 groups, each distributed to 100 eggs, and every one of this group was sub-divided into 4 replicates, every replicate consisting of 25 eggs. Eggs were injected using an automatic syringe that was used for oil vaccination by using a needle of 25 millimeters.8
In Ovo
The eggs were injected from the broad side by making more (13mlm) in the air sac than every egg injected with 100 μg sialic acid (Latex CO. Ltd, Germany) as follows: T1: The control group was placed in the incubator without injection. T2: Injected with a dose of 100 μg sialic acid at the age of zero. T3: Injected with 100 μg sialic acid dose at 7 days. T4: Injected with 100 μg sialic acid dose at 14 days of incubation. Egg candling was conducted to determine the Amnion sac for making the second injection (18 days of egg incubation), and the injection surface was sterilized with antiseptic (Dettol) before injection. The pores were closed by using Dye pedicures9. Eggs were incubated in (AFLO) mark setter by distributing the groups randomly. Prepare 400 mL of sterile water divided into four glass containers. Weighed quantities of 100 mg of sialic acid and the amount of each dissolved in 100 ml of sterile water. Injection method of eggs at aged 0, 7, and 14 days of incubation10, by a needle of size 25 mm, The needle is inserted from the petition of the eggshell after piercing through the air gap depth of 13 mm and the injection dose of 100 μg of the sialic acid prepared in both dates and then underwent four tests embryonic.
Embryonic test
The first embryonic test is conducted 3 days from incubation, where we put the eggs horizontally. The shell is opened, and the following traits are measured: Embryo length, vascular region, and pairs of somites. The second embryonic test was conducted 7 days from incubation, where we broke the eggshell and removed the egg's contents. The following traits are measured: Embryo weight, Albumin, and shell. The third embryonic test was conducted 14 days after incubation, where we broke the eggshell and took out the egg's contents; the following traits were measured: Embryo weight, yolk, amniotic sac, and liquid and Albumin. The fourth embryonic test was conducted 17 days after incubation, where we broke the eggshell and removed the egg's contents. The following traits were measured: embryo weight and yolk11.
Statistical Analysis
Complete randomization was used for this experiment (CRD). Then the SAS tool for statistical analysis was used to evaluate the data12. To find significant changes between the averages, Duncan's polynomial was used to compare the means for each treatment at significance levels of 0.05 and 0.01.13.
RESULTS AND DISCUSSION
The results shown in Table (1) refers to that treated the eggs with sialic acid led to a significant increase (P<0.05) in Embryo Length, Vascular length, and Pairs of somites for treatment (T2) at 3 days from incubation in the Table (2) the results refer to significant increasing (P<0.05) in Embryo weight, and significant decreasing (P<0.05) in Albumin and Shell weight for (T2) treatment compared with other treatments in 7 days of incubation. In Table (3), the results refer to a significant increase (P<0.05) in Embryo weight for (T2) treatment compared with (T0), a significant increase (P<0.05) in Amniotic Sac and Liquid compared with another treatment, significant decreasing in yolk and Albumin weight for (T2) treatment compared with another treatment.
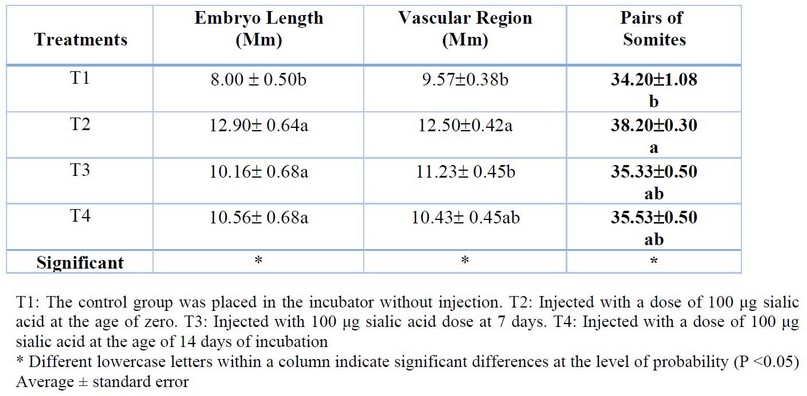
Table 1. Effect of injection of eggs hatching in different concentrations of sialic acid in embryonic growth at the age of 3 days from incubation ( %As a percentage relative to egg weight at examination )
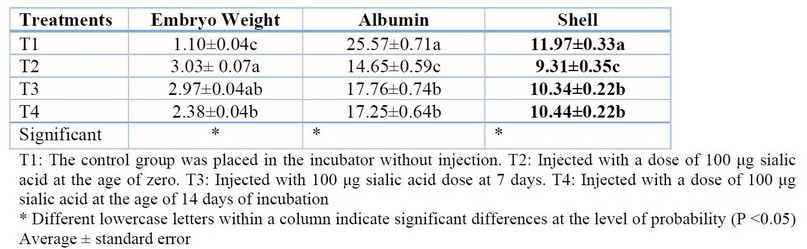
Table 2. Effect of injection of eggs hatching in different concentrations of Sialic acid in embryonic growth at the age of 7 days from incubation ( %As a percentage relative to egg weight at the examination
The crucial erythropoietin response to ischemia stress is provided by the erythropoietin (EPO) regulation of red blood cell formation and its activation at low oxygen tension. Since its cloning and manufacturing, recombinant human Erythropoietin has been used clinically in anemia patients for two and a half decades, making it easier to study how it works14. Reports of ischemia stress in vitro and injury in animal and cell models point to possible erythropoietin benefits beyond red blood cell formation. Including vascular endothelial response to increasing nitric oxide production15 facilitates oxygen delivery to the brain16, heart and non-hematopoietic tissues. This review addresses these and other findings of erythropoietin function outside of the generation of red blood cells, including how it affects metabolism and animal models of obesity. Observations of erythropoietin activity in cell and animal model systems17, including mice with tissue-specific deletion of erythropoietin receptor (EpoR), suggest the potential for erythropoietin response in metabolism and disease7, 18.
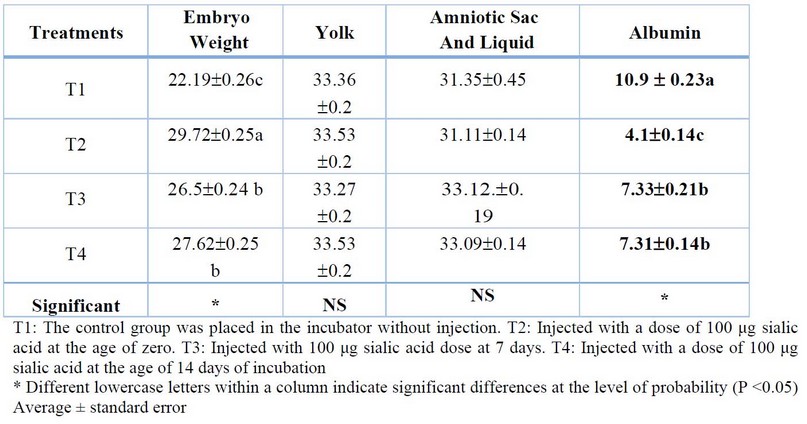
Table 3. Effect of injection of eggs hatching in different concentrations of sialic acid in embryonic growth at the age of 7 days from incubation (%As a percentage relative to egg weight at the examination
As a function of animals, Kumar and Rizvi (2013)19 note a considerable drop in RBC sialic acid content and an increase in plasma sialic acid. A reliable indicator of aging is the sialic acid content of the erythrocyte membrane, which decreases as rat age increases3. Greater expression of acute phase proteins and increased organ damage are two possibilities for the cause of elevated plasma sialic acid. Hemoglobin6, a protein that makes up 95% of red blood cells, cooperatively binds oxygen for transportation to the tissues from the lungs20. As a result, Erythropoietin's main job is to control how much oxygen is delivered through the synthesis of red blood cells21. This job is made more accessible by the hypoxia-induced elevation of erythropoietin gene transcription, which makes erythropoietin production sensitive to the local oxygen environment22. Erythropoietin stimulates cell survival, proliferation, and differentiation by attaching to a particular receptor on the surface of erythroid progenitor cells23. Mice lacking Erythropoietin or the erythropoietin receptor have severe anemia, which causes embryonic mortality24.
Injecting the eggs with sialic acid resulted in the embryos benefiting from the acid by manufacturing erythropoietin21; since sialic acid is a significant factor in the production of the hormone erythropoietin and a crucial component of erythropoiesis, Erythropoietin has been commercialized in several forms to treat anemia in animals25. This study observed the development of the embryos of the treated injected with sialic acid. So, as mentioned above, the production of red blood cells as a result of the increase in the hormone erythropoietin led to an increase in the acceleration of biological processes and, thus, an increase in oxygen transfer and the ability of hemoglobin to transport it inside living cells, and thus helps to complete the process Embryo growth and development26.
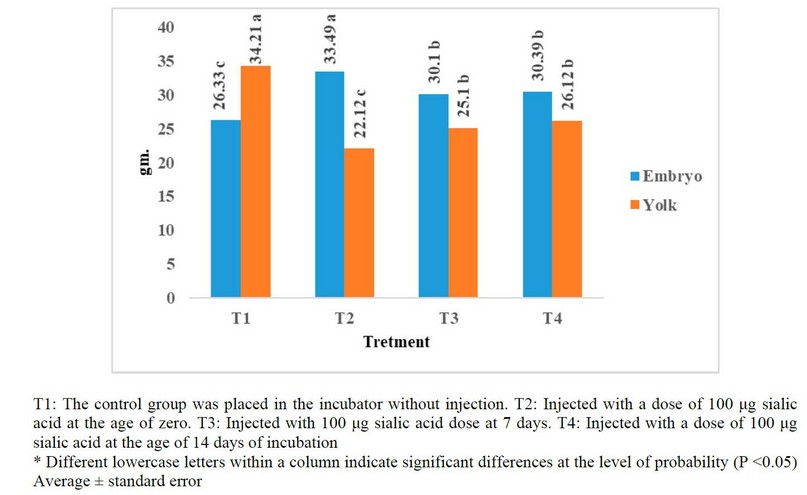
Figure 1. Effect of injection of eggs hatching in different concentrations of sialic acid in embryo weight and yolk weight at the age of 17 days from incubation (%As a percentage relative to egg weight at the examination
In Figure 1. the results refer to a significant increase (P<0.05) in Embryo Weight at the age of 17 days from incubation for (T2) compared with (T1). In comparison, there is a significant increase (P<0.05) in Embryo Weight at the age of 17 days from incubation for (T3 and T4) compared with T1. However, the results show a significant improvement (P<0.05) in Yolk Weight at the age of 17 days from incubation for (T2) compared with (T1) and a significant improvement (P<0.05) at the period of 17 days from incubation for (T2) compared with another treatment. At the same time, there is a significant improvement (P<0.05) in Yolk Weight at the age of 17 days from incubation for (T3 and T4) compared with T1.
As we mentioned earlier, EPO has an essential role in the availability and improvement of the manufacture of red blood cells, increasing oxygen availability and thus improving the growth process27. The increase in oxygen in the egg means an increase in the gas exchange process between the embryo and the external environment. Thus this leads to an increase in biological processes such as metabolism and protein production in the body and obtains an increase in the number and size of muscle cells and, thus, an increase in weight for embryos28. At the same time, this above hypothesis can be confirmed by observing the decrease in the weight of the yolk concerning the treatments injected with sialic acid. The Chick's embryo begins to switch its nutrition from albumen to yolk within 14 days of incubation; therefore, the indicator of measuring the yolk weight is significant. If the yolk weight decreases, this indicates the consumption of a more significant amount of it29, and it means that there is an increase in the feeding process for the embryo, which works to increase the weight10, as we indicated previously, and this is what was done in the injected experimental treatments. So from Figure 1, we notice a significant decrease in yolk weight for all treatments except T1.
CONCLUSION
A problem occurs in the embryos that prevent the completion of the hatching; a shortage of oxygen occurs at the end of the incubation period. Thus, in this study, we solved this problem by injecting eggs with sialic acid and measuring some physiological and biological indicators that biologics contribute to generating an amount of oxygen that helps the embryo overcome the stressful hatching process.
REFERENCES
1. Vieira, S. L. Chicken embryo utilization of egg micronutrients. Revista Brasileira de Ciencia Avicola vol. 9 (2007).
2. Tanner, M. E. The enzymes of sialic acid biosynthesis. Bioorganic Chemistry vol. 33 (2005).
3. Mehdi, M. M., Singh, P. & Rizvi, S. I. Erythrocyte sialic acid content during aging in humans: Correlation with markers of oxidative stress. Dis. Markers 32, (2012).
4. Givisiez, P. E. N. et al. Chicken embryo development: metabolic and morphological basis for in ovo feeding technology. Poultry Science vol. 99 (2020).
5. Nechaeva, M., Alekseeva, T., Dobretsov, M. & Kubasov, I. Chicken embryos can maintain heart rate during hypoxia on day 4 of incubation. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 190, (2020).
6. Influence, S. Dynamics of Hematological Indicators of Chickens under Stress-Inducing Influence. Ukr. J. Ecol. 10, (2020).
7. Zhang, Y. et al. Erythropoietin action in stress response, tissue maintenance and metabolism. International Journal of Molecular Sciences vol. 15 (2014).
8. Ohta, Y. & Kidd, M. T. Optimum site for in ovo amino acid injection in broiler breeder eggs. Poult. Sci. 80, (2001).
9. Alkubaisy, S. A. et al. Effects of In-Ovo injection of Biotin on Chick's embryonic development and physiological traits. in IOP Conference Series: Earth and Environmental Science vol. 761 (2021).
10. Abdul-Lateif, K. M. & Abdulateef, S. M. The effect of injecting hatching eggs with different concentrations of biotin on the quality and physiological characteristics of the hatched chicks. Iraqi J. Vet. Sci. 26, (2012).
11. Al-Bayar, M.A., Abdulateef, S.M. ,Farhan, S.M., Shawkat, S.S. , Mohammed, T. . Role of nitroglycerine injection in Japanese Quail (Coturnix japonica) testes tissues parameters. Indian J. Ecol. 47, 251–255 (2020).
12. System., S. a S. S. A. SAS user's guide. Statistical (2004).
13. Duncan, D. B. Multiple Range and Multiple F Tests. Biometrics 11, (1955).
14. Baseman, J. B., Banai, M. & Kahane, I. Sialic acid residues mediate Mycoplasma pneumoniae attachment to human and sheep erythrocytes. Infect. Immun. 38, (1982).
15. Jankowski, M. D., Glaberman, S. R., Kimball, D. B., Taylor-McCabe, K. J. & Fair, J. M. Sialic acid on avian erythrocytes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 238, 110336 (2019).
16. Mahmood, N. A. & Abdulateef, S. M. Determining Some Undesirable Behavioral Traits and Their Impact on the Behavioral Performance of Broiler Chicks. in IOP Conference Series: Earth and Environmental Science vol. 904 (IOP Publishing Ltd, 2021).
17. Yap, K. N. & Zhang, Y. Revisiting the question of nucleated versus enucleated erythrocytes in birds and mammals. American Journal of Physiology - Regulatory Integrative and Comparative Physiology vol. 321 (2021).
18. A. AL-Bayar, M. Effect of testosterone injection to native layer breeder Mezo on primary and secondary sex ratio, fertility and hatchability. ANBAR J. Agric. Sci. 14, (2016).
19. Kumar, D. & Rizvi, S. I. Erythrocyte membrane bound and plasma sialic acid during aging. Biol. 68, (2013).
20. Al-Obaidi, O. H. S. et al. Preparation of some oxygenic compounds and comparing their antibacterial activity with pomegranate puniea granatum peels extracts. Asian J. Chem. 24, 5602–5604 (2012).
21. Y. S. Najim, Th. T. Mohammed & F. M. Hussain. The effect of the use of different levels of azolla to male broilers diets in the productive and physiological performance. J. Life Sci. Appl. Res. 3, 37–41 (2022).
22. Kwon, M. S. et al. Generation of transgenic chickens expressing the human Erythropoietin (hEPO) gene in an oviduct-specific manner: Production of transgenic chicken eggs containing human Erythropoietin in egg whites. PLoS One 13, (2018).
23. Wu, H., Liu, X., Jaenisch, R. & Lodish, H. F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require Erythropoietin or the erythropoietin receptor. Cell 83, (1995).
24. Lin, C. S., Lim, S. K., D'Agati, V. & Costantini, F. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erytnropoiesis. Genes Dev. 10, (1996).
25. Thomann, M. et al. Effects of sialic acid linkage on antibody-fragment crystallizable receptor binding and antibody dependent cytotoxicity depend on levels of fucosylation/bisecting. Bioanalysis 11, (2019).
26. Beleslin-Cokic, B. B. et al. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 104, (2004).
27. Straat, M., Van Bruggen, R., De Korte, D. & Juffermans, N. P. Red blood cell clearance in inflammation. Transfusion Medicine and Hemotherapy vol. 39 (2012).
28. McMullin, M. F. Genetic background of congenital erythrocytosis. Genes vol. 12 (2021).
29. Abdulateef, S. M. et al. Effect of exposure to different light colors on embryonic development and neurophysiological traits in the chick embryo. Vet. World 14, 1284–1289 (2021).
Received: May 15 2023/ Accepted: June 10 2023 / Published:15 June 2023
Citation: Thabit S S, Awad M M, Abdulateef S M. Effects of In-Ovo Injection of sialic acid on Chick's embryonic development and physiological traits. Revis Bionatura 2023;8 (2) 49. http://dx.doi.org/10.21931/RB/2023.08.02.49
