2023.08.03.13
Files > Volume 8 > Vol 8 No 3 2023
Beyond reproduction: Exploring the Non-Canonical roles of the Kisspeptin System in Diverse Biological Systems
1 Medicine Program, Faculty of Health Sciences, Universidad Autonoma de Bucaramanga;
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.03.13
ABSTRACT
G protein-coupled receptors are integral membrane proteins in cell signaling processes. Activation of G protein-coupled receptors by specific agonists promotes the activation of different G-proteins, activating different intracellular signaling pathways, including adenylate cyclase activation and intracellular calcium release. One of the G protein-coupled receptors studied is the kisspeptin receptor, which regulates reproduction and gonadotropin secretion. However, recent studies have shown that kisspeptin and its receptor have non-canonical roles in cell signaling and several biological systems. In the present review, we will present these different functions exerted by the kisspeptin system in different biological systems, such as the central nervous system, the cardiovascular system, and the immune system, as well as the role of this system in pathologies such as preeclampsia, diabetes, and cancer. Understanding their non-canonical roles in cell signaling may have important implications in developing new therapies for various diseases.
Keywords: Kisspeptin-1 Receptor, Kisspeptins, G-protein coupled receptor, Signal Transduction, Cancer, Diabetes Mellitus, Preeclampsia.
INTRODUCTION
Kisspeptins are a family of related peptides identified as the natural ligands of the G protein-coupled receptor (GPCR) GPR541-3, also known as the kisspeptin receptor (KISS1R). The physiological ligand for the KISS1R receptor was identified by several groups in 20012-4 and is encoded by the KISS1 gene, which produces a 145 amino acid protein, Kisspeptin54 (KP-54), also known as metastin (Figure 1). The C-terminal region of KP-54 is responsible for binding to the receptor, and this region is the most conserved among different species. The peptides in this 10-, 13- and 14-amino acid portion (KP-10, KP-13, and KP-14) exhibit similar activities at the Kiss1R receptor in vitro assays2-4.
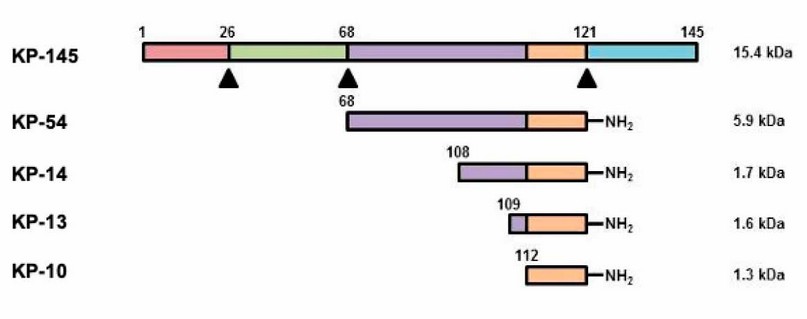
Figure 1. Production of kisspetins. The primary protein product of the KISS1 gene is cleaved (triangles) to produce small amidated peptides (kisspeptins, Kp) capable of binding to the GPR54 receptor. All peptides containing the same C-terminal portion are biologically active. Source: Author.
The kisspeptin-KISS1R system plays an essential role in the neuroendocrine control of the reproductive axis5. However, in recent years, the kisspeptin system has also been found to be involved in various biological processes non-related to reproduction. These non-canonical roles of kisspeptin have been identified in multiple methods, including the cardiovascular system 6, central nervous system 7, immune system 8, preeclampsia9, diabetes10, and cancer11.
Non-canonical signaling refers to biological effects that occur through pathways other than classical GPCR signaling. In the case of the kisspeptin system, these effects may be mediated by the activation of sex hormone receptors12, translation factors13, and ionic channels14. It has also been shown that kisspeptin can interact with other signaling systems, such as the insulin signaling system and the insulin-like growth factor (IGF) system15. The precise mechanisms of kisspeptin non-canonical signaling and its physiological effects have yet to be fully elucidate and are still under study.
In this review, recent findings on the non-canonical roles of kisspeptin in various biological systems, including the cardiovascular system, central nervous system, preeclampsia, immune system, diabetes, and cancer, will be presented. In addition, possible underlying mechanisms of non-canonical kisspeptin signaling and their potential clinical significance will be discussed.
Non-Canonical Signaling of Kisspeptins
Studies have revealed that Kisspeptins, primarily acting via the KISS1R receptor, activate the Gq/11 protein, increasing intracellular calcium through PLC activation2. Also, kisspeptins can activate critical non-canonical pathways, allowing us to understand the complexity of the signaling processes. One of the most studied examples of non-canonical kisspeptin signaling is the activation of the ERK1/2 signaling pathway through the direct binding of kisspeptin to the MET tyrosine kinase receptor16, and this signaling pathway has been implicated in promoting progesterone secretion. Also, according to a study conducted by Kim and Cho13, the activation of EIF2AK2 induced by kisspeptin may require the presence of RhoA. This suggests that the signaling pathway responsible for the effects of kisspeptin may be illustrated by the sequence KISS1R/Gq/11/p63RhoGEF/RhoA/EIF2AK2. This signaling pathway leads to inhibit cancer growth and metastasis.
In addition, it has been shown that kisspeptin can activate the Ca2+ mobilization and cAMP reduction levels through interaction with receptors for other ligands, such as Neuropeptide FF receptors (NPFFR1 and NPFFR2)17, with highly potent activity leading to the possible localization of a secondary kisspeptin receptors such as NPFFRs (Figure 2.).
In summary, non-canonical cell signaling of the kisspeptin system is an emerging area of research that may have essential implications in regulating physiological and pathological function. Although several non-canonical signaling pathways activated by kisspeptin have been identified, further studies are needed to understand their role in regulating physiology and pathology.
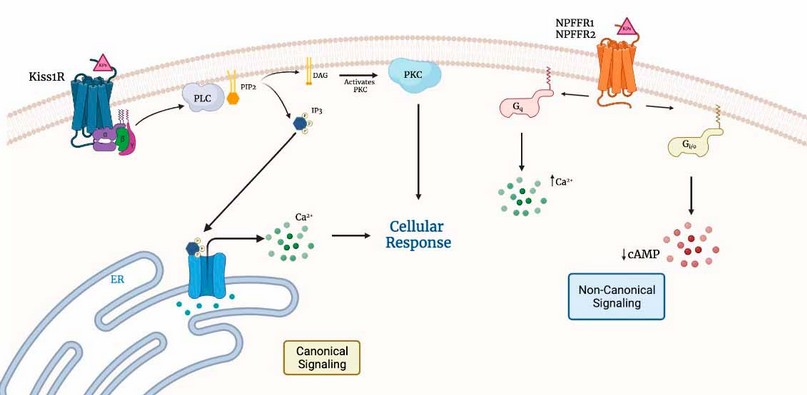
Figure 2. Distinct signaling pathways are triggered following the activation of the Kiss1 receptor by the endogenous agonist's KPs. The canonical KISS1 receptor signal transduction pathways triggered by the endogenous agonist KPs include coupling to the Gq-protein with the activation of PLC, which processes the membrane PIP2 to produce IP3 and DAG. IP3 interacts with IP3 receptors (IP3R) at the endoplasmic reticulum and releases Ca2+, which, together with DAG, leads to PKC activation. In the non-canonical signaling pathway, KPs bind to NPFFR receptors, activating two Gq- and Gi/o-dependent signaling that promote an increase in intracellular calcium and a decrease in cAMP concentration.
Non-Canonical Roles of Kisspeptins
Kisspeptins and the Cardiovascular System
The kisspeptin system is a peptide signaling important in regulating reproduction, appetite, metabolism and energy homeostasis.2-4. In addition, the kisspeptin system has also been shown to be involved in regulating cardiovascular function.
Kisspeptin and its receptor KISS1R can be found in endothelial cells, vascular smooth muscle, and other cardiovascular tissues, including the aortic artery 18. In the isolated human coronary artery and umbilical vein, it was observed that KP-10, KP-13, and KP-54 act as powerful vasoconstrictors. The response of these tissues to KP was similar to that of angiotensin (AngII) in the coronary artery, as reported in that study18.
Also, in 2011, Maguire et al. showed that kisspeptins are a potent positive inotrope in the atria of humans, rats, and mice19. KP-10 vascular effects are considered significant due to hypertension and edema as symptoms of preeclampsia in late-term pregnancies, which subside after delivery.
In summary, understanding the potential "off-target" cardiovascular effects can guide the development and usage of kisspeptin-derived treatments, such as improved analogs and antagonists with increased efficacy21.
Kisspeptins and Preeclampsia
Preeclampsia is a pregnancy complication that involves high blood pressure and dysfunction of the body's endothelial cells. Studies suggest that changes in the levels of KISS1 and KISS1R, both at the mRNA and protein levels, may be responsible for causing preeclampsia. These changes lead to a decrease in KISS1 expression and an increase in KISS1R expression compared to a normal, healthy pregnancy22. Kisspeptin is expressed in the placenta and has been shown to play an essential role in regulating fetal growth and development23, as well as in angiogenesis and placental endothelial function23 24. In addition, lower levels of plasma kisspeptin-10 have been associated with more severe forms of the disease25.
Kisspeptin may play a role in the development of preeclampsia by inhibiting the growth of new vessels from placental vessels24. A clinical study demonstrated that women with preeclampsia had lower levels of plasma kisspeptin-10, which was inversely correlated with the severity of their condition. Maternal plasma KP-10 levels were also associated with estimated fetal weight during the second and third trimesters26. Another study found that kisspeptin levels at 16 weeks in maternal plasma were positively correlated with the birthweight of fetuses in uncomplicated pregnancies27. The results indicate that plasma levels of kisspeptin may function as a biomarker of insufficient blood flow to the uterus and placenta, along with limited fetal development within the uterus, lead to restricted growth.
Kisspeptins and the Central Nervous System
Several lines of evidence indicate kisspeptin directly affects Gonadotropin hormone-releasing Hormone (GnRH) neurons. Firstly, most GnRH neurons express KISS1R28. Furthermore, the fibers of KISS1R are close to GnRH neurons and stimulate the release of gonadotropin-releasing hormone by activating multiple ion channels through a pathway dependent on phospholipase C and calcium29. Thirdly, kisspeptin can directly depolarize and increase the firing rates of GnRH neurons in vitro28. It is important to note that kisspeptin may not only act through traditional synaptic mechanisms to stimulate GnRH secretion but also directly in a non-synaptic manner, especially in the median eminence30. Increasing evidence suggests that kisspeptin also affects intermediary neurons, such as GABAergic cells, to regulate GnRH secretion 31,32.
Additionally, the study conducted by Khonacha et al. demonstrated that KP-13 can enhance spatial memory consolidation and retrieval. Also, when administered in the presence of Amiloid β (Aβ), KP-13 significantly improved reference memory impairment caused by Aβ. These findings suggest that KP-13, as a neuropeptide, may possess neuroprotective properties against amyloid-beta-induced pathology and has the potential to enhance spatial memory33.
Kisspeptins and the Immune System
Due to the expression of kisspeptin receptors on various immune system cells 2-4, it has been proposed that kisspeptin, in conjunction with other pregnancy hormones and proteins, has the potential to regulate immune responses. Also, kisspeptin may play a role in regulating cytokines, which are proteins that control the immune response and can be regulated through modulation of their production. In particular, it has been shown that kisspeptin can increase the production of anti-inflammatory cytokines such as interleukin-10 (IL-10) and decrease the production of proinflammatory cytokines such as interleukin-17A (IL-17A)8.
Studies on the immune system response to kisspeptin have covered how the hormone regulates CD4+T lymphocytes through molecular mechanisms35. By binding to KISS1R, kisspeptin increases intracellular cAMP concentrations, boosting iTreg production and the quantity of these cells in the culture. The heightened cAMP also activates cAMP response element binding protein (CREB) and MAPK/ERK (MEK1/2)36, which helps CD4+ lymphocytes differentiate into iTreg and reduces RORC expression. The capacity of [Ca2+] governs the control of the cAMP-dependent activity of kisspeptin in lymphoid cells I to trigger protein kinase A (PKA) through Ca2+/CaM, Ca2+/CaM dependent protein kinase 2 (CaMKK2), and AMP-activated protein kinase (AMPK)37. PKA, in turn, inhibits mTOR38, which activates RORC transcription39 and phosphorylates CREB 40. The role of kisspeptin in coordinating reproductive and immune functions may help to discover new mechanisms of this control system.
Kisspeptins and Diabetes
Diabetes is a long-term condition marked by high glucose levels in the blood. This occurs either because the pancreas does not produce enough insulin or because it is resistant to the effects of insulin, a hormone responsible for regulating blood glucose levels. The kisspeptin system has been interested in diabetes because it regulates energy metabolism and pancreatic function10.
It has been shown that kisspeptin and its receptor KISS1R are expressed in the pancreas, the organ responsible for the production of insulin4. In particular, it has been demonstrated that kisspeptin administration can increase insulin production in animal models of type 2 diabetes (DM2)41.
The role of KP in DM2 indicates that KP-10 may benefit diabetic patients, as it has been found to increase testosterone secretion in men with DM2 and central hypogonadism 42. Animal studies have also shown that KP may regulate insulin secretion43, but in vivo, experiments are necessary to explore its effects on peripheral organs that control metabolism.
In summary, pancreatic function, insulin production, energy metabolism, and insulin sensitivity are significantly influenced by the regulation of the kisspeptin system. However, the effects of kisspeptin on pancreatic function may be contradictory, and additional research is required to investigate the relationship between KP and insulin in DM2 patients.
Kisspetins and Cancer
The regulation of cancer cell proliferation and invasion in various types of cancer has been linked to the kisspeptin system and its receptor, KISS1R. Studies have demonstrated that kisspeptin inhibits cell proliferation and invasion in breast44, prostate45, ovarian46, lung47, and gastric48 cancers.
In breast cancer, kisspeptin and KISS1R expression have been shown to correlate inversely with the degree of tumor malignancy, suggesting that the kisspeptin system may have a role in suppressing invasion and metastasis in this type of cancer49. In addition, it has been shown that kisspeptin administration can inhibit cell proliferation and tumor formation in animal models of breast cancer50. In prostate cancer, it has been reported that kisspeptin and KISS1R expression is reduced in advanced tumors45, suggesting that the kisspeptin system may also have a role in suppressing invasion and metastasis in this type of cancer. In ovarian cancer, high concentrations of KISSR lead to increased inhibition of cell migration51 and hypersensitization of cells to chemotherapy52. It has also been shown to act as a suppressor of metastasis and suppresses NF-kB and MMP9 expression53.
In gastric cancer, the kisspeptin system also has metastasis suppressor activity, as it inhibits cell growth, proliferation, and invasion48.
To summarize, the kisspeptin system seems to significantly inhibit the spread and metastasis of various cancer types, indicating its potential as a therapeutic target for cancer treatment. However, additional research is necessary to understand better the kisspeptin system's role in cancer progression and its efficacy in treating diverse cancer types.
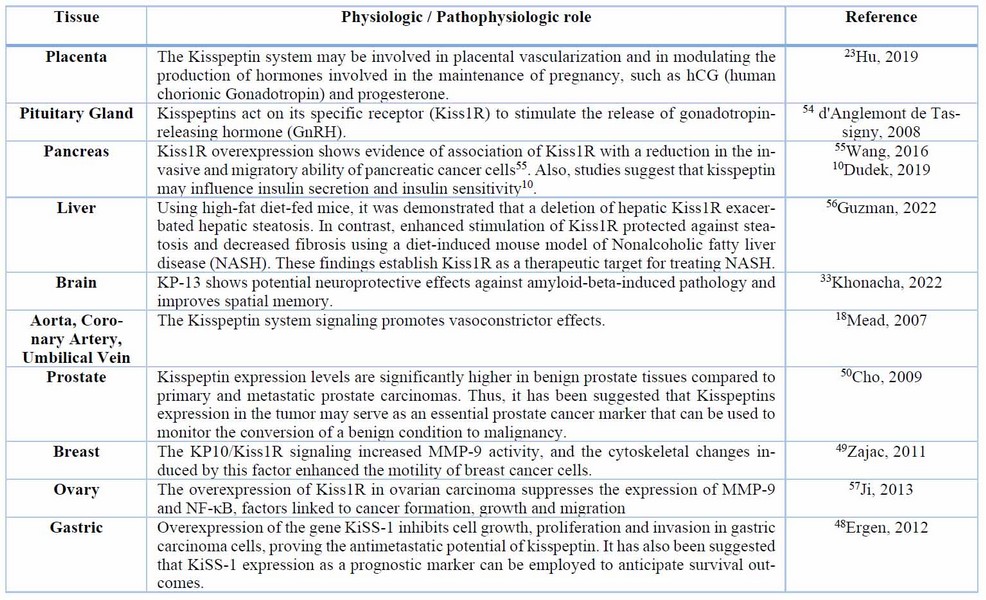
Table 1. Physiological and pathophysiological roles of the Kisspeptin system in different tissues.
CONCLUSION
Investigating the kisspeptin system is crucial for both its reproductive and non-reproductive functions. Regarding reproduction, kisspeptin is essential in regulating the hypothalamic-pituitary-gonadal axis, which controls puberty, menstrual cycles, fertility, and overall reproductive health. Understanding the mechanisms behind this regulation can provide valuable insights into reproductive disorders and offer new avenues for treatment.
Moreover, the kisspeptin system has been found to play non-reproductive roles in various physiological systems (Table 1.), including diabetes, metabolism, memory, cardiovascular function, preeclampsia, and cancer. These findings suggest that kisspeptin has a broader scope and is vital in maintaining organismal homeostasis.
Therefore, studying the non-canonical roles of kisspeptin can provide a more comprehensive understanding of its function and lead to the development of therapies and treatments for various health issues, such as obesity, diabetes, cardiovascular disorders, and stress.
Funding: This research was funded by MINCIENCIAS, Government of Colombia, grant number 808-2017.
Conflicts of Interest: The author declares no conflict of interest.
REFERENCES
1 Lee, D. K. et al. Discovery of a receptor related to the galanin receptors. FEBS Lett 446, 103-107, doi:10.1016/s0014-5793(99)00009-5 (1999).
2 Muir, A. I. et al. AXOR12, a novel human G protein-coupled receptor, is activated by the peptide KiSS-1. J Biol Chem 276, 28969-28975, doi:10.1074/jbc.M102743200 (2001).
3 Ohtaki, T. et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 411, 613-617, doi:10.1038/35079135 (2001).
4 Kotani, M. et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem 276, 34631-34636, doi:10.1074/jbc.M104847200 (2001).
5 Popa, S. M., Clifton, D. K. & Steiner, R. A. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol 70, 213-238, doi:10.1146/annurev.physiol.70.113006.100540 (2008).
6 Sawyer, I. et al. The vasoactive potential of kisspeptin-10 in the peripheral vasculature. PLoS One 6, e14671, doi:10.1371/journal.pone.0014671 (2011).
7 Mills, E. G. A., O'Byrne, K. T. & Comninos, A. N. Kisspeptin as a Behavioral Hormone. Semin Reprod Med 37, 56-63, doi:10.1055/s-0039-3400239 (2019).
8 Gorbunova, O. L. & Shirshev, S. V. Role of Kisspeptin in Regulation of Reproductive and Immune Reactions. Biochemistry (Mosc) 85, 839-853, doi:10.1134/S0006297920080015 (2020).
9 Perez-Lopez, F. R. et al. Preeclampsia and gestational hypertension are associated with low maternal circulating kisspeptin levels: a systematic review and meta-analysis. Gynecol Endocrinol 37, 1055-1062, doi:10.1080/09513590.2021.2004396 (2021).
10 Dudek, M., Ziarniak, K., Cateau, M. L., Dufourny, L. & Sliwowska, J. H. Diabetes Type 2 and Kisspeptin: Central and Peripheral Sex-Specific Actions. Trends Endocrinol Metab 30, 833-843, doi:10.1016/j.tem.2019.07.002 (2019).
11 Stathaki, M. et al. The role of the kisspeptin system in cancer biology. Crit Rev Oncol Hematol 142, 130-140, doi:10.1016/j.critrevonc.2019.07.015 (2019).
12 Munoz de la Torre, L. P., Trujillo Hernandez, A., Eguibar, J. R., Cortes, C. & Morales-Ledesma, L. Sex-specific hypothalamic expression of kisspeptin, gonadotropin-releasing hormone, and kisspeptin receptor in progressive demyelination model. J Chem Neuroanat 123, 102120, doi:10.1016/j.jchemneu.2022.102120 (2022).
13 Kim, T. H. & Cho, S. G. Kisspeptin inhibits cancer growth and metastasis via activation of EIF2AK2—Mol Med Rep 16, 7585-7590, doi:10.3892/mmr.2017.7578 (2017).
14 Zhang, C., Bosch, M. A., Ronnekleiv, O. K. & Kelly, M. J. Kisspeptin activating TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology 154, 2772-2783, doi:10.1210/en.2013-1180 (2013).
15 Izzi-Engbeaya, C. & Dhillo, W. S. Emerging roles for kisspeptin in metabolism. J Physiol 600, 1079-1088, doi:10.1113/JP281712 (2022).
16 Peng, J. et al. Kisspeptin stimulates progesterone secretion via the Erk1/2 mitogen-activated protein kinase signaling pathway in rat luteal cells. Fertil Steril 99, 1436-1443 e1431, doi:10.1016/j.fertnstert.2012.12.008 (2013).
17 Oishi, S. et al. Activation of Neuropeptide FF Receptors by Kisspeptin Receptor Ligands. ACS Med Chem Lett 2, 53-57, doi:10.1021/ml1002053 (2011).
18 Mead, E. J., Maguire, J. J., Kuc, R. E. & Davenport, A. P. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor, G protein-coupled receptor 54, to atherosclerosis-prone vessels. Endocrinology 148, 140-147, doi:10.1210/en.2006-0818 (2007).
19 Maguire, J. J. et al. Inotropic action of the puberty hormone kisspeptin in rat, mouse and human: cardiovascular distribution and characteristics of the kisspeptin receptor. PLoS One 6, e27601, doi:10.1371/journal.pone.0027601 (2011).
20 Zhang, J., Meikle, S. & Trumble, A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy 22, 203-212, doi:10.1081/PRG-120021066 (2003).
21 Curtis, A. E. et al. A kisspeptin-10 analog with greater in vivo bioactivity than kisspeptin-10. Am J Physiol Endocrinol Metab 298, E296-303, doi:10.1152/ajpendo.00426.2009 (2010).
22 Cartwright, J. E. & Williams, P. J. Altered placental expression of kisspeptin and its receptor in preeclampsia. J Endocrinol 214, 79-85, doi:10.1530/JOE-12-0091 (2012).
23 Hu, K. L. et al. Potential roles for the kisspeptin/kisspeptin receptor system in implantation and placentation. Hum Reprod Update 25, 326-343, doi:10.1093/humupd/dmy046 (2019).
24 Ramaesh, T. et al. Kisspeptin-10 inhibits angiogenesis in human placental vessels ex vivo and endothelial cells in vitro. Endocrinology 151, 5927-5934, doi:10.1210/en.2010-0565 (2010).
25 Adali, E. et al. Metastin levels in pregnancies complicated by preeclampsia and their relation with disease severity. J Matern Fetal Neonatal Med 25, 2671-2675, doi:10.3109/14767058.2012.708369 (2012).
26 Ziyaraa, M. A., Hamdan, F. B. & Mousa, L. R. Correlation of Kisspeptin-10 level and fetal well-being in preeclamptic patients. Taiwan J Obstet Gynecol 55, 840-846, doi:10.1016/j.tjog.2015.10.028 (2016).
27 Smets, E. M. et al. Decreased plasma levels of metastin in early pregnancy are associated with small for gestational age neonates. Prenat Diagn 28, 299-303, doi:10.1002/pd.1969 (2008).
28 Han, S. K. et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25, 11349-11356, doi:10.1523/JNEUROSCI.3328-05.2005 (2005).
29 Liu, X., Lee, K. & Herbison, A. E. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 149, 4605-4614, doi:10.1210/en.2008-0321 (2008).
30 Ramaswamy, S., Guerriero, K. A., Gibbs, R. B. & Plant, T. M. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 149, 4387-4395, doi:10.1210/en.2008-0438 (2008).
31 Pielecka-Fortuna, J., Chu, Z. & Moenter, S. M. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149, 1979-1986, doi:10.1210/en.2007-1365 (2008).
32 Zhang, C., Bosch, M. A., Ronnekleiv, O. K. & Kelly, M. J. Gamma-aminobutyric acid B receptor-mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology 150, 2388-2394, doi:10.1210/en.2008-1313 (2009).
33 Khonacha, S. E. et al. Kisspeptin-13 prevented the electrophysiological alterations induced by amyloid-beta pathology in rat: Possible involvement of stromal interaction molecules and pCREB. Brain Res Bull 184, 13-23, doi:10.1016/j.brainresbull.2022.03.003 (2022).
34 Gorbunova, O. L. & Shirshev, S. V. The role of kisspeptin in immune tolerance formation during pregnancy. Dokl Biol Sci 457, 258-260, doi:10.1134/S0012496614040085 (2014).
35 Gorbunova, O. L., and Shirshev, S. V. . Molecular mechanisms of the regulation by kisspeptin of formation and functional activity of TREG and TH17. Biol. Membr. 33, 47-55, doi:10.7868/S0233475516020067 (2016).
36 Zhang, H. T., Zhao, Y., Huang, Y., Dorairaj, N. R., Chandler, L. J., and O'Donnell, J. M. . Inhibition of the phosphodiesterase 4 (PDE4) enzymes reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CAI subregion of the rat hippocampus. Neuropsychopharmacology 39, 1432-1439, doi:10.1038/ sj.npp.1300440 (2004).
37 Quan, J., He, M., Ko, W. K., and Wong, A. O. Kisspeptin induction of somatolactinαrelease in goldfish pituitary cells: functional role of cAMP/PKA, PLC/PKC, and Ca2+/calmodulindependent cascades. Am. J. Physiol. Endocrinol. Metab. 307, 872-884, doi:10.1152/ajpendo. 00321.2014 (2014).
38 Wen, A. Y., Sakamoto, K. M. & Miller, L. S. The role of the transcription factor CREB in immune function. J Immunol 185, 6413-6419, doi:10.4049/Immunol.1001829 (2010).
39 Ivanov, II et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121-1133, doi:10.1016/j.cell.2006.07.035 (2006).
40 Schwartz, J. H. The many dimensions of cAMP signaling. Proc Natl Acad Sci U S A 98, 13482-13484, doi:10.1073/pnas.251533998 (2001).
41 Dudek, M. et al. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides 56, 41-49, doi:10.1016/j.npep.2016.01.005 (2016).
42 George, J. T., Veldhuis, J. D., Tena-Sempere, M., Millar, R. P. & Anderson, R. A. Exploring the pathophysiology of hypogonadism in men with type 2 diabetes: kisspeptin-10 stimulates serum testosterone and LH secretion in men with type 2 diabetes and mild biochemical hypogonadism. Clin Endocrinol (Oxf) 79, 100-104, doi:10.1111/cen.12103 (2013).
43 Dufourny, L., Delmas, O., Teixeira-Gomes, A. P., Decourt, C. & Sliwowska, J. H. Neuroanatomical connections between kisspeptin neurons and somatostatin neurons in female and male rat hypothalamus: possible involvement of SSTR1 in kisspeptin release. J Neuroendocrinol, e12593, doi:10.1111/jne.12593 (2018).
44 Martin, T. A., Watkins, G. & Jiang, W. G. KiSS-1 expression in human breast cancer. Clin Exp Metastasis 22, 503-511, doi:10.1007/s10585-005-4180-0 (2005).
45 Wang, H. et al. Clinical and biological significance of KISS1 expression in prostate cancer. Am J Pathol 180, 1170-1178, doi:10.1016/j.ajpath.2011.11.020 (2012).
46 Jayasena, C. N. et al. Plasma kisspeptin: a potential biomarker of tumor metastasis in patients with ovarian carcinoma. Clin Chem 58, 1061-1063, doi:10.1373/clinchem.2011.177667 (2012).
47 Popper, H. H. Progression and metastasis of lung cancer. Cancer Metastasis Rev 35, 75-91, doi:10.1007/s10555-016-9618-0 (2016).
48 Ergen, A. et al. Plasma Kisspeptin-54 levels in gastric cancer patients. Int J Surg 10, 551-554, doi:10.1016/j.ijsu.2012.08.014 (2012).
49 Zajac, M. et al. GPR54 (KISS1R) transactivates EGFR to promote breast cancer cell invasiveness. PLoS One 6, e21599, doi:10.1371/journal.pone.0021599 (2011).
50 Cho, S. G. et al. Kisspeptin-10, a KISS1-derived decapeptide, inhibits tumor angiogenesis by suppressing Sp1-mediated VEGF expression and FAK/Rho GTPase activation. Cancer Res 69, 7062-7070, doi:10.1158/0008-5472.CAN-09-0476 (2009).
51 Wiiger, M. T., Bideli, H., Fodstad, O., Flatmark, K. & Andersson, Y. The MOC31PE immunotoxin reduces cell migration and induces gene expression and cell death in ovarian cancer cells. J Ovarian Res 7, 23, doi:10.1186/1757-2215-7-23 (2014).
52 Prentice, L. M. et al. Kisspeptin and GPR54 immunoreactivity in a cohort of 518 patients defines a favorable prognosis and clear cell subtype in ovarian carcinoma. BMC Med 5, 33, doi:10.1186/1741-7015-5-33 (2007).
53 Gao, G. L., Liu, L. D., Zou, X. S. & Chen, W. X. Expression of KiSS-1, matrix metalloproteinase-9, nuclear factor-kappaBp65 in ovarian tumor. Zhonghua fu chan ke za zhi 42, 34-38 (2007).
54 d'Anglemont de Tassigny, X., Fagg, L. A., Carlton, M. B. & Colledge, W. H. Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology 149, 3926-3932, doi:10.1210/en.2007-1487 (2008).
55 Wang, C. H., Qiao, C., Wang, R. C. & Zhou, W. P. KiSS‑1‑mediated suppression of the invasive ability of human pancreatic carcinoma cells is not dependent on the level of KiSS‑1 receptor GPR54. Mol Med Rep 13, 123-129, doi:10.3892/mmr.2015.4535 (2016).
56 Guzman, S. et al. Targeting hepatic kisspeptin receptor ameliorates nonalcoholic fatty liver disease in a mouse model. J Clin Invest 132, doi:10.1172/JCI145889 (2022).
57 Ji, K., Ye, L., Mason, M. D. & Jiang, W. G. The Kiss-1/Kiss-1R complex as a negative regulator of cell motility and cancer metastasis (Review). Int J Mol Med 32, 747-754, doi:10.3892/ijmm.2013.1472 (2013).
Received: 28 May 2023/ Accepted: 15 July 2023 / Published:15 September 2023
Citation: Rodríguez Sarmiento D Y. Beyond reproduction: Exploring the Non-Canonical roles of the Kisspeptin System in Diverse Biological Systems. Revis Bionatura 2023;8 (3) 13. http://dx.doi.org/10.21931/RB/2023.08.03.13
