2023.08.02.64
Files > Volume 8 > Vol 8 No 2 2023
Electropolymerization of some amino acids on platinum electrode
Taleb F. Hassen1,*, Tijani Gharbi2 , Helene Cattey2, Guillaume Herlem2
1 Collage of Medicine , University of Sumer, Rifai, Thi-Qar, Iraq.
2Nanomedicine Laboratory EA4662, Bat. E, University of Bourgogne Franche-Comte, UFR Sciences & Techniques, 16 route de ́Gray, 25030 Besancon Cedex, France ̧: [email protected]
3Nanomedicine Laboratory EA4662, Bat. E, University of Bourgogne Franche-Comte, UFR Sciences & Techniques, 16 route de ́Gray, 25030 Besancon Cedex, France ̧: [email protected]
4Nanomedicine Laboratory EA4662, Bat. E, University of Bourgogne Franche-Comte, UFR Sciences & Techniques, 16 route de ́Gray, 25030 Besancon Cedex, France ̧: [email protected]
* Correspondence: [email protected]; Tel.: 009647802738919
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.64
ABSTRACT
This paper studied the anodic oxidation of L-amino acids on smooth platinum and modified Pt electrodes (Pt/AAO). The oxidation of L-amino was carried out by cyclic voltammetry coupled with electrochemical quartz crystal microbalance (EQCM). The Amino acid concentration, pH of the electrolyte and the scan number effects on cyclic voltammetry were examined. Spectroscopic analysis, such as attenuated total reflectance FT infrared spectroscopy (ATR-FTIR) and X-ray photoelectron spectroscopy (XPS), characterize the resulting thin film coatings. Scanning electron microscopy (SEM) is used to study the morphology of thin film surfaces and their solubility. Spectroscopic measurements favor L-amino acids electropolymerization into poly-L-amino acids in an irreversible way. The electrosynthesis of poly-L-amino acids was used as a proton receptor for the solid-state pH solid sensor.
Keywords. Anodic oxidation, L-amino acids, electropolymerization, pH sensor.
INTRODUCTION
Amino acids are organic molecules used as basic building blocks in proteins. Twenty amino acids are essential in biomineralization and catalysis; they have carboxylic and amine groups and some other functional groups such as (–CHx, -OH, and -S). The amino acids are based on a structural monomer unit, which will compose linear or branched polymers by coupling at least two neighboring amino acid molecules 1.
They have various properties such as nutrition value, taste, medical work, chemical properties, food additives and drugs, nutrition supplements, cosmetics, polymer materials and agricultural chemicals 2. Electropolymerization involves electrochemical reactions such as initiator reactions depending on the polymerized monomer concentration and the electrical charge required 3. Electrochemical oxidation includes the transferred electrons between a molecule and the electrode. The polymer coatings on conducting or semiconducting substrates absorb and can be applied to surface decoration, optical activity alteration, heat and fractional resistance enhancement and corrosion protection 4.
Many authors in the literature studied the electrochemical oxidation of amino acids on platinum and other electrodes; 5 Synthesis polyglycine by anodic oxidation of glycine concentrated electrolyte on the smooth platinum electrode as working electrode. Cyclic voltammetry, AFM topography, XPS and ATR-FTIR techniques were used to characterize produced polyglycine thin films: Marx and Zhou 6 electropolymerized tyrosine and decyl ester of tyrosine on platinum electrodes. A three-electrode cell setup was used with a platinum disc, Ag/AgCl as a counter and reference electrodes, respectively. The resulting coating was used as a potentiometric pH sensor.
Adsorbed L-serine on Pt single crystal electrode in H2SO4 electrolyte 7 and used cyclic voltammetry and FTIR to identify species. 8 studied L-alanine and L-serine adsorption on Au electrodes in perchloric acid solution. Cyclic voltammetry and ATR-IR were used for comparative characterization with theoretical study and found an agreement between theoretical and experimental results 9 studied the oxidation of glycine, serine and threonine on platinum and gold electrodes in sodium hydroxide solution. Cyclic voltammetry and Raman spectroscopy showed amino acid deposition on working electrodes 10 showed the electrochemical properties of amino acids without sulfur atoms (L-tryptophan, L-tyrosine and L-histidine) by using smooth gold and glassy carbon electrodes in anodic oxidation.
Electropolymerized L-alanine on smooth platinum, gold and glassy carbon electrodes; results of electrochemical oxidation between diluted and concentrated amino acid were compared at zwitterion state and in basic solution by cyclic voltammetry. Spectroscopic analyses such as FTIR and XPS characterized polymeric thin films. The product's thin film was also as a pH sensor 11.
Studied the L-threonine electrooxidation on carbon paste electrodes. The electrochemical behavior of L-tyrosine on a poly(L-threonine) film was studied at pH = 7 by cyclic voltammetry technique12. Scan rate and pH effect were taken into account. 13 prepared a poly-L-serine thin film on a glassy carbon electrode (as a working electrode). They used a modified electrode (GCE/ poly-L-serine) as a biosensor towards estradiol to determine its concentration in blood serum with a detection limit of 2x10-8 M. 14 used the electrochemical oxidation method to prepare a poly(L-lysine) electrodeposition on the glassy carbon electrode. They used cyclic voltammetry, FTIR and electrochemical impedance spectroscopy techniques to characterize poly-L-lysine thin film.
In this study, the electropolymerization of aromatic amino acids was studied and characterized by spectroscopic methods. The product thin films were a pH sensor as application.
MATERIAL AND METHOD
Chemicals
L-amino acids: L-phenylalanine, L-tyrosine, and L-tryptophan were purchased from Sigma – Aldrich company (France).
NaOH, NaBF4, and H2SO4 were purchased from Sigma-Aldrich (France).
Ultrapure water (Milli-Q-Millipore) was used.
Anodic aluminum oxide templates (AAO) were purchased from Synkra Technologies, Inc. (USA) with a thickness of 50 nm, pore diameter of 150 nm, pore density of 2 × 109 pore/ cm2, and porosity of 35%.
pH buffer solutions used for solid potentiometric pH sensing were in the pH range 2-12 and obtained from Merck company (Germany).
Procedure
L-Amino acids were dissolved in ultrapure water to prepare diluted 0.05 M and concentrated 0.5 M solutions, charged with 0.01 M NaBF4 as supporting electrolytes. H2SO4 and NaOH were added sufficiently to give pH = 1 and 13, respectively.
Apparatus
Cyclic voltammetry measurements were carried out with a µAutolab potentiostat (ECO Chimie, The Netherlands) coupled to a quartz crystal microbalance (QCM) PM710 frequency meter (Maxtek, USA). The cyclic voltammetry measurements were performed either on smooth platinum or on polished platinum-coated quartz crystal (5 MHz resonance frequency, area: 1.37 cm2) mounted on a PTFE probe and plugged into the microbalance or on glassy carbon electrodes (1 mm diameter).
The smooth platinum was first polished with alumina powder (0.03µ) on a polishing cloth after being sonicated and washed with water for 4 min. The reference electrode was Ag/AgCl in all the experiments, and a platinum square (1 cm²) was the counter electrode.
Attenuated total reflection infrared spectroscopy (ATR-FTIR) was performed using a PerkinElmer Spectrum Version 10.01.00 model 2010.
X-ray photoelectron spectroscopy (XPS) experiments were carried out using a VG Esclab220iXL spectrometer comprising a rotating anode X-ray source (Al Kα).
Scanning electron microscope (SEM) measurements were performed using an FEI Helios Nanolab 600i apparatus (FEI Winning Dual Beam Company, USA).
pH measurements in voltmeter mode were performed with a voltmeter/pH meter ± 1 mV precision (Mettler M220) with high input impedance (100 MΩ) and connected with shielded cables. Notice that 1 mV causes about 0.02 pH variation in pH measurement.
RESULTS
The aromatic L-amino acids concern L- phenylalanine, L-tyrosine and L-tryptophan as shown in scheme 3-8 below 17
Figure 1. Structure of aromatic L-amino acids
Figures 2 a and b. show the cyclic voltammograms of diluted and concentrated L-phenylalanine zwitterion (NaBF4 0.01 M as support electrolyte). There is an anodic oxidation peak at about 1.50 V for diluted and concentrated L-phenylalanine. The first scan has a higher current value, decreasing with an increased scan number. The cyclic voltammograms of L-phenylalanine on Pt/AAO are gathered in figures (3 c and d). There is one irreversible anodic peak for every two concentrations at about 1.27 V, and the higher current value is related to the high concentration of L-phenylalanine. The current value for the first scan is higher than the fifth scan. The effect of pH is illustrated in figures (3e and f); there is one anodic peak at about 1.30 V for diluted and concentrated electrolytes at pH = 13 and none at acidic pH where gas is bubbling on the working electrode as Figure 3g.
Figure 4 Cyclic voltammetry of L-phenylalanine at zwitterion state on smooth platinum electrode at (a) 0.05 M and (b) 0.5 M. Cyclic voltammetry of L-phenylalanine on smooth platinum electrode masked by AAO, diluted (c) and concentrated (d). Cyclic voltammetry of L-phenylalanine on the smooth platinum electrode at pH = 13, diluted (e) and concentrated (f) electrolyte, and (g) at pH = 1 for concentrated electrolytes. Scan rate: 50 mV/s.
The cyclic voltammogram of diluted L-tyrosine is shown in Figure 5a, with two well-defined anodic oxidation peaks. The first is at about 1.06 V and the second is at 1.40 V with no cathodic peak on reverse scans. The first scan shows a high current value and a necessary deposition amount, as shown from the EQCM curve in Figure 5b. There is considerable adsorption of L-tyrosine on the crystal surface of the Pt electrode at the first scan, and the mass increases with the increasing scan number. During the electrodeposition process, the current value decreases with scan number in favor of polymeric film formation 18, 19. There is no peak for concentrated L-tyrosine-based electrolytes. Figure 2c, the cyclic voltammogram of L-tyrosine on Pt/AAO shows one anodic peak centered at 0.85 V. The same electrochemical behavior as on smooth Pt is found on Pt/AAO. The effect of pH is shown in Figure 2d. There are two anodic peaks at basic pH. One is at about ~ 0. 98V, while the second one is at 1.36 V. Figure 2e shows the cyclic voltammetry of L-tyrosine at acidic electrolyte pH = 1, and there is a single anodic oxidation peak at about 0.8V associated to a cathodic peak at about 0.12 V on the reverse scan. The same behavior is observed with the zwitterionic state: the current value decreases with the increasing scan numbers.
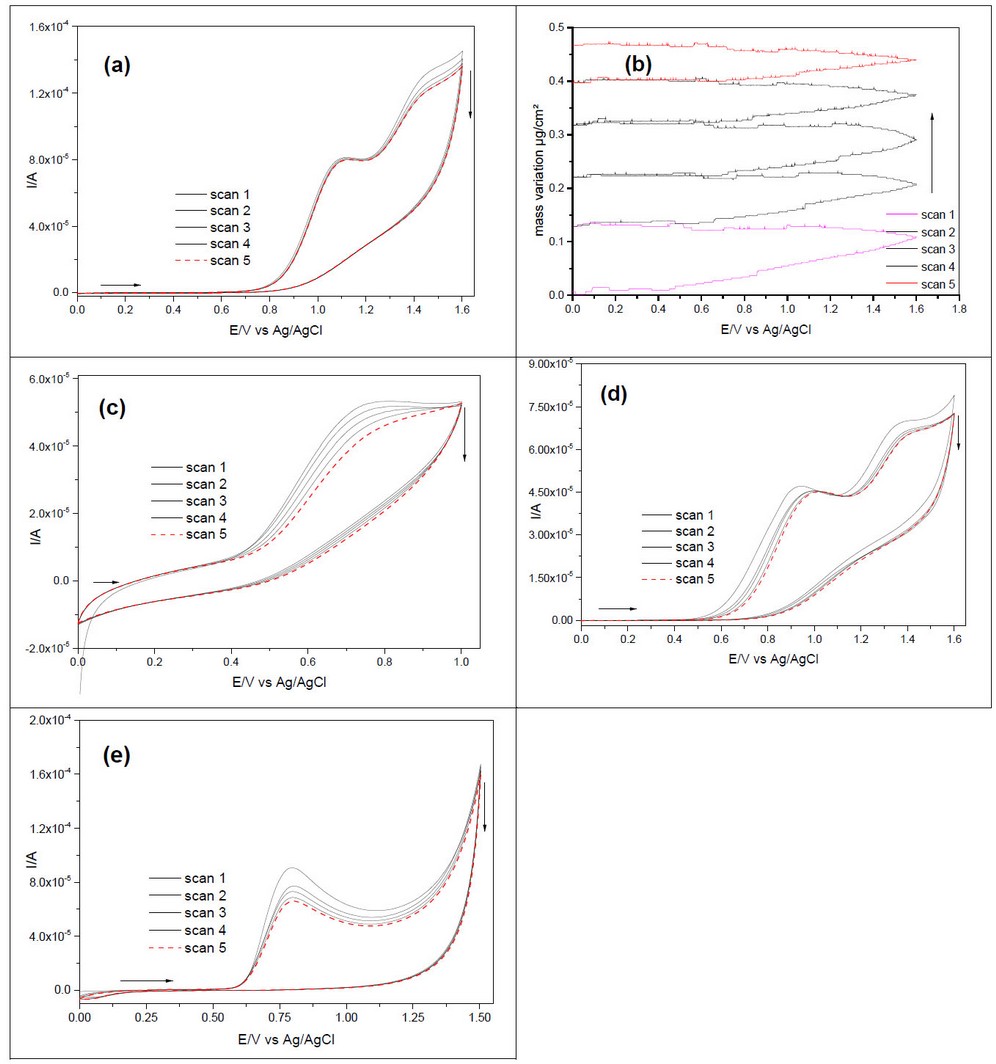
Figure 2. Cyclic voltammetry of 0.05 M L-tyrosine at (a) zwitterion state on smooth platinum electrode and (b) on Pt/AAO. (c) EQCM curve of diluted L-tyrosine and (d) cyclic voltammetry of L-tyrosine 0.05 M on the smooth platinum electrode at pH = 13 and (e) at pH = 1. Scan rate: 50 mV/s.
The cyclic voltammograms of diluted and concentrated L-tryptophan in a zwitterionic state are shown in Figures 3a and b. There are two irreversible anodic peaks for both L-tryptophan concentrations. For diluted electrolytes, the two peaks are at about 0.87 V and 1.30 V, while they are at about 0.9 V and 1.32 V for concentrated L-tryptophan. The first scan has a high current value and then decreases with scan numbers for each electrolyte. An EQCM measurement with concentrated L-tryptophan is given in Figure 3c, where the mass electrodeposition on Pt increases with scan numbers. The cyclic voltammetry of L-tryptophan on Pt/ AAO is shown in Figures (3d and e). There are two anodic peaks at about 0.83 and 1.30 V for diluted and concentrated L-tryptophan. The first scan has a higher current value in each electrolyte concentration and decreases with increasing scan numbers. Figure 3 f,g and h displays the L-tryptophan cyclic voltammograms at different pH. There is only one peak at 0.40 V in acidic media (pH = 1) and two in primary media at 0.80 and 1.25 V for diluted and concentrated L-tryptophan electrolytes, respectively.
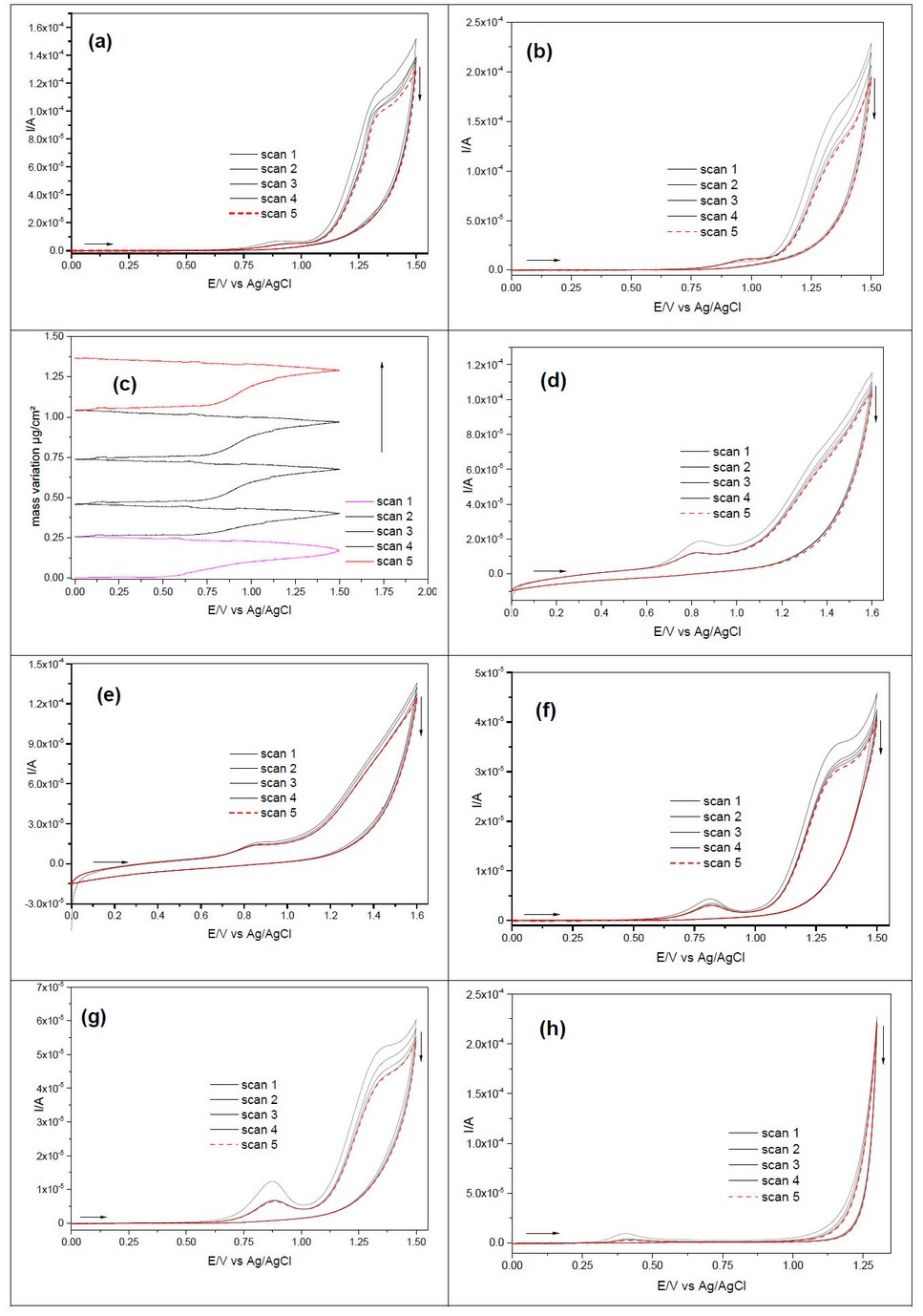
Figure 3. Cyclic voltammetry of L-tryptophan zwitterion on the smooth platinum electrode at (a) 0.05 M, (b) 0.5 M, and (c) EQCM response at 0.5 M. Cyclic voltammetry of L-tryptophan on Pt/AAO, diluted (d) and concentrated (e). Cyclic voltammetry of L-tryptophan on the smooth platinum electrode at pH = 13, diluted (f) and concentrated (g) electrolyte, and (h) at pH = 1 for concentrated L-tryptophan. Scan rate: 50 mV/s.
For ATR-FTIR spectra of the aromatic L-amino acids deposited on Pt electrode figures 4a-, b and c, amide A, I, II, and III bands for the polymers are indicated. For instance, the amide A is observed at 3200-3400 cm-1 for the N-H group in the amide band.
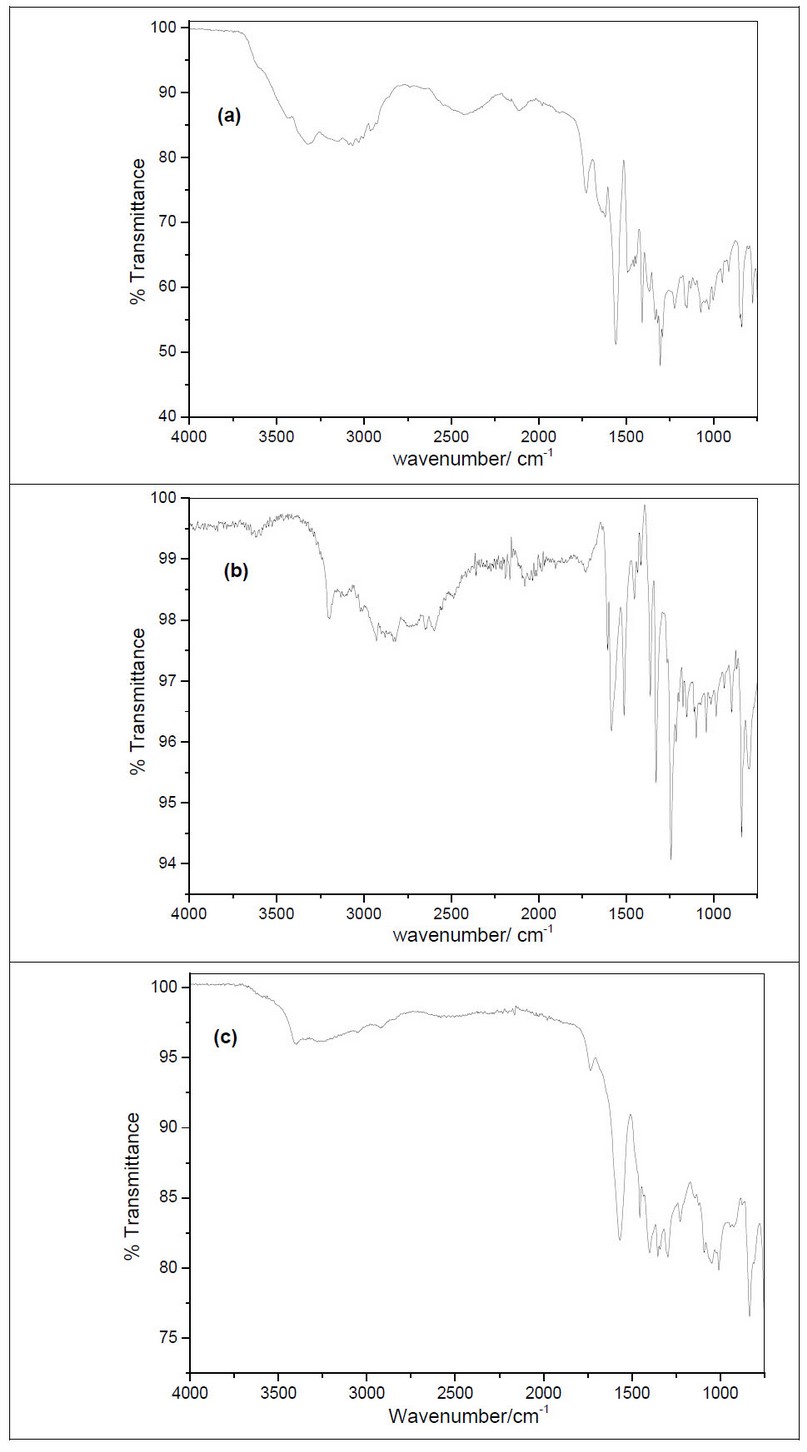
Figure 4. ATR-FTIR spectra from electrodeposition of (a) poly-L-phenylalanine, (b) poly-L-tyrosine, and (c) poly-L-tryptophan on smooth platinum electrode
XPS spectra of the three aromatic poly-L-amino acids (phenylalanine, tyrosine, and tryptophan) electrodeposited on the Pt electrode in the C1s, N1s, and O1s regions are shown in Figure 5.
The XPS analysis of electrodeposited L-tryptophan in C1s, N1s, and O1s regions is shown in Figures 5g-i. Three peaks at 288.2, 289 and 291.9 eV in the C1s region figure 5 g are related to C-C, C-N and C=O, respectively. Two peaks at 403.2 and 404 eV correspond to nitrogen atoms in peptide bonds and NH3+, respectively. Finally, two peaks in the O1s region centered at 535 and 539.5 eV Figure 5 I concern oxygen atoms in carbonyl and hydroxyl groups 11.
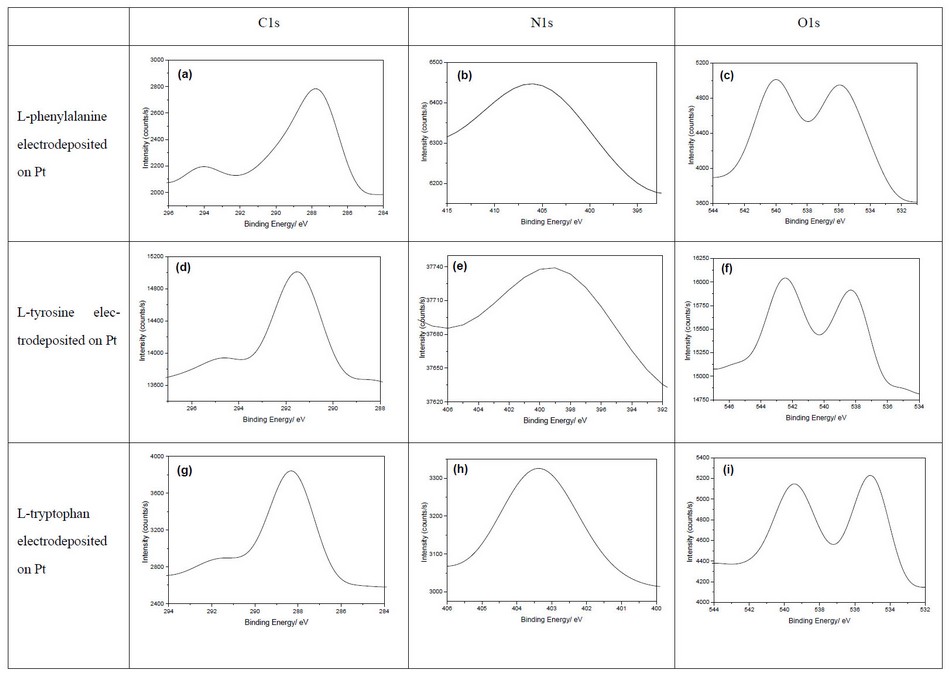
Figure 5. XPS spectra of aromatic L-amino acids (a-c) Poly-L-phenylalanine on Pt electrode, (d-f) Poly-L-tyrosine on Pt electrode, and (g-i) Poly-L-tryptophan on Pt electrode
The morphology of electrosynthesized products was characterized by the SEM technique in Figure 6. The products are composed of poly-L-amino acids electrodeposited on the Pt electrode surface; The poly-L-amino acids are localized in the aperture area of a PTFE mask between AAO/Pt and the electrolyte. The electrodeposition of poly-L-amino acids can be observed with a good contrast through the electrochemical growth across AAO nanopores. We conclude that the poly-L-amino acids mass increases on the AAO surface.
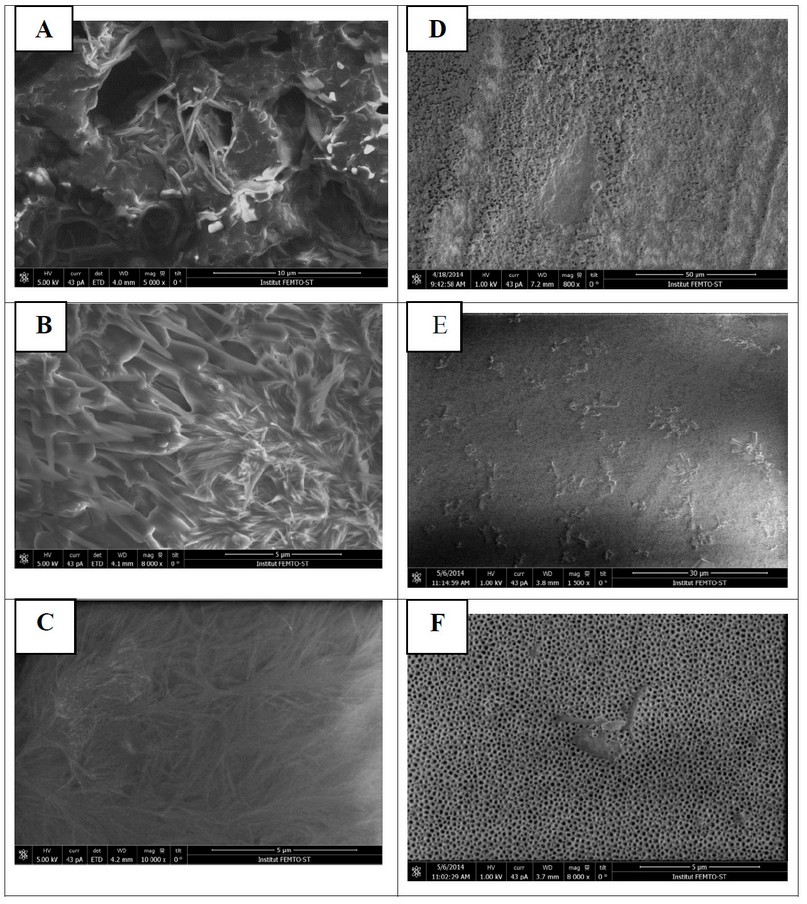
Figure 6. SEM images of (A) poly-L-phenylalanine, (B) poly-L-tyrosine, (C) poly-L-tryptophan, (D) AAO/ poly-L-phenylalanine, (E) AAO/ poly-L-tyrosine, (F) AAO/ poly-L-tryptophan
The poly-L-amino acids like coating electrodeposited on smooth Pt electrodes were tested as receptors for solid-state potentiometric pH sensors in the pH range 2-12 versus Ag/AgCl as the reference electrode. Indeed, the amine and amide groups on the electrode surface can be protonated and are well-suited for solid-state pH sensor application.
Poly-L-amino acids were electrodeposited on Pt electrode by anodic oxidation of concentrated L-amino acids electrolyte at zwitterion state by cyclic voltammetry during 10 scans versus Ag/AgCl. The modified Pt electrode was stored and kept in a KCl 0.1 M solution when unused. Figure 7 shows the potential changes versus pH. The potential decreases with pH increasing for the modified electrode (Pt/ poly-L-amino acids). We have a linear response between the modified Pt electrode and pH changes.
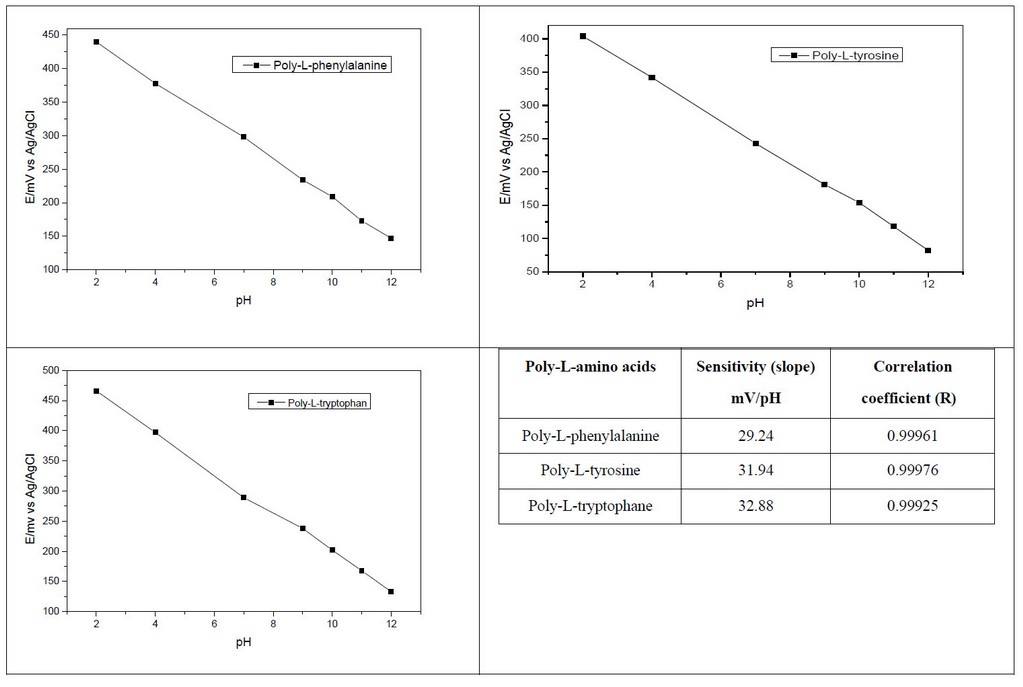
Figure 7. poly-L-amino acids sensitivity as pH sensor
DISCUSSION
Polymeric coatings prepared on conducting or semiconducting substrates have been desirable since electropolymerization offers a facile synthesis route and control of the electro-generation process in addition to characterization and the development of many applications 15. The electropolymerization method is a one-pot process to produce polymers and allows adding chemical agents inside polymer chains 16.
The band between 1690-1750 cm-1 is assigned to the amide I (-C=O group), the band in the range 1550-1570 cm-1 concerns –N-H group bending pointed to the amide II while the amide III is indicated at 1200-1350 cm-1. On the other hand, the bands around 1480-1520 cm-1 are assigned to the C=C stretching of the aromatic ring 20. Our results are in agreement with Literature 21.
For phenylalanine Figure 5 a, there are three peaks at 287.9, 288.9 and 294 eV in the C1s region related to carbon atoms of polymer chains backbone, functionalized with hydroxyl or amino groups and carbonyl group 22. For the N1s region Figure 5 b, two peaks are visible at 405 and a smaller one at 407 eV assigned to nitrogen atoms of the peptide bond and NH3+ groups. Two peaks in the O1s region Figure 5c correspond to the C=O and –OH groups centered at 536 and 540.3 eV, respectively 11,22.
Figures 5d-f concern XPS spectra of electrodeposited L-tyrosine. Three peaks in the C1s region Figure 5d are positioned at 291.6, 292 and 294.8 eV and correspond to the carbon polymer backbone, C-N and C=O. The N1s region has two peaks at 399 and 401 eV related to nitrogen atoms in the amide band and NH3+, respectively. Two peaks in the O1s region are centered at 538 and 542.5 eV for oxygen in each C=O and –OH groups Figure (5f) 23,24.
CONCLUSION
Electropolymerization usually includes a specific interaction between the monomer and the electrode surface, leading to good coating adhesion. The electrochemically active species submit a heterogeneous surface reaction on the electrode during the reaction. Poly-L-amino acids were produced. The produced polymers are thin films used as pH sensors.
REFERENCES
1. M. Rodnina, M. Beringer, W. Wintermeyer, Trends in biochem. Sci. 32,2007; 20.
2. R. Faurie, J. Thommel "Microbial Production of L-Amino-Acids" 2003; Springer-Verlag Berlin Heidelberg, Germany.
3. R Lund, M. Baizer "Organic Electrochemistry" 3rd ed. 1991; Marcel Dekker, New York, U.S.A.
4. A. De Bruyne, J. Delplancke, R. Winand, J. Appl. Electrochem. 25,1995; 284.
5. G. Herlem, S. Monney,V. Patissier, B. Fays and T. Gharbi, Electrochimica Acta 54, 2009; 6797.
6. Handhal , N. A.; Ahmaed, A. S. . A Survey Study To Isolate Some Pathogenic Bacteria For Cooked Rice At Baghdad City. Journal of Life Science and Applied Research. 2020, 1, 54-59..
7. Y. Gu, S. Sun, S. Chen, C. Zhen, Z. Zhou, Langmuir 19, 2003; 9823.
8. A. Sandoval, J. Orts, A. Rodes, J. Feliu, Electrochimica acta 89, 2013; 72.
9. X. Xiao, S. Sun, J. Yao, Q. Wu, Z. Tian, Langmuir 18, 2002; 6274.
10. B. Malfoy and J. Reynaud, J. Electroanal. Chem. 114, 1980; 213.
11. Alrseetmiwe, D. S. .; Almayah, A. A. .; Nasser, A. A. .; Alnussairi, M.; Zadeh, H. A.; Mehrzi, F. A. Escherichia Coli Strain Bl21: Cloning And Expression Of An Optimized Interferon Alpha 2B (DE3). Journal of Life Science and Applied Research. 2020, 1, 40-44.
12. S. Chitravathi, B. Swamy, G. Mamatha, B. Chandrashekar, J. Mol. Liq. 172, 2012; 130.
13. J. Song, J. Yang, X. Hu, J. Appl. Electrochem. 38 , 2008; 833.
14. Y. Wei, L. Luo, Y. Ding, X. Si, Y. Ning, Bioelectrochemistry 98, 2014; 70.
15. S. M. Abdulateef, O. K. Atalla1, M. Q. A L-Ani, TH. T Mohammed, F M Abdulateef And O. M. Abdulmajeed. Impact of the electric shock on the embryonic development and physiological traits in chicks embryo. Indian Journal of Animal Sciences.2021, 90 (11): 1541–1545.
16. A. Pron, P. Rannou, Prog. Poly. Sci., 27, 2002; 135.
17. Yehya, W. A. . Seasonal Monumental Insects Accompanying Euphrates Poplar Leaves. Journal of Life Science and Applied Research.2020, 1, 45-53..
18. Othman Ghazi Najeeb Alani , Yassen Taha Abdul-Rahaman and Thafer Thabit Mohammed. Effect Of Vêo® Premium and Vitamin C Supplementation on Lipid Profile Before and During Pregnancy in Some Local Iraqi Ewes During Heat Stress. Iraqi Journal of Science.2021, Vol. 62, No. 7, pp: 2122-2130.
19. Y. Wang, C. Bi, J. Mol. Liq. 177, 2013; 26.
20. C. Zinola, J. Rodriguez, M. Carmen Arevalo, E. Pastor, J. Electroanal. Chem. 585, 2005; 230.
21. P. Pandey, A. Pramanik, T. Chakraborty, Chemical physics 389, 2011; 88.
22. Y. Zubavichus, M. Zharnikov, A. Shaporenko, O. Fuchs, L. Weinhardt, C. Heske, E. Umbach, J. Denlinger, M. Grunze, J. Phys. Chem. A 108, 2004; 4557.
23. Y. Zubavichus, M. Zharnikov, A. Shaporenko, O. Fuchs, L. Weinhardt, C. Heske, E. Umbach, J. Denlinger, M. Grunze, J. Phys. Chem. A 108, 2004; 4557.
24. K. Anderson, J. Slocik, M. McConney, J. Enlow, R. Jakubiak, T. Bunning, R. Naik, V. Tsukruk, Small 5, 2009; 741.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: Hassen , T.F.; Gharbi , T.; Cattey ,H.; Herlem ,G. Electropolymerization of some amino acids on platinum electrode. Revis Bionatura 2023;8 (2) 64. http://dx.doi.org/10.21931/RB/2023.08.02.64
