2023.08.04.77
Files > Volume 8 > Vol 8 no 4 2023
Some histological effects of Bisphenol-A on some reproductive organs in male adult rabbits (Oryctolagus cuniculus)
Manar Al-Murshidi 1, Walaa salih Hassan 2, Wurood Hamza Muttaleb 3, *
1 College of Science for Women/ University of Babylon. Iraq
2 College of Science/ University of Babylon. Iraq
3 College of Science for Women/ University of Babylon Iraq.
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.77
ABSTRACT
Bisphenol-A (BPA) is a broadly used substance in our environment. The current study was established to investigate the potentially toxic effects of BPA in the histology of adult male albino rabbits' reproductive organs. 45 adult male rabbits were subdivided into three groups. Group 1 orally received standard saline solution daily (proposed as a negative control); Group 2 received (0.5 ml/day) of olive oil orally daily (offered as a positive control); and the third Group, 3 rabbits, were treated orally with a dose of 25mg\Kg BW of the BP-A which dissolved in olive oil for 30 days. Tissue specimens from epididymis, testis, seminal vesicles and prostate were collected for histological examination. Blood samples were collected for serum hormone level evaluation. Results showed that BPA caused a significant decrease in testosterone, Luteinizing hormone, Follicle stimulating hormone, and a significant increase in estradiol and prolactin, along with some histopathological alterations in the epithelial and connective tissues of all the organs of studied animals. In conclusion, BPA induced hormonal disturbances in some hormones of the reproductive pituitary axis and histologic and toxic effects on the histology of all reproductive organs.
Keywords: Bis-Phenol A; reproductive hormones; testis; epididymis; accessory sex glands; histology.
INTRODUCTION
Endocrine-disrupting chemicals (EDCs) could affect human and animal endocrine coordination, comprising their effects on the synthesis of hormones, secretion, and metabolism in the living bodies. One such chemical was Bisphenol -A (BP-A), which has more and more concerns in the scientific communities due to its widespread usage, daily exposure, and potentially harmful effects on human health 1,2. BP-A is constituted of polycarbonate and epoxy resins, which may be used as the lacquer lining of food and beverage cans, drink containers, baby bottles, and some composites and dental sealants. Despite their lifestyles, many people were diagnosed as having BP-A metabolites in their urine 2. Bisphenol A (BPA) is an estrogenic endocrine disrupter substance through its direct effects on binding to classic cell membrane estrogen receptors and activating it 3. It has been shown that bisphenol A induces different pathological problems such as cancers, neurological disorders, behavioral defects, abnormalities in liver enzymes, cardiovascular diseases, and type 2 diabetes. 4. Bisphenol A was associated with male reproductive abnormalities in sperm morphology and motility, sperm count reduction, and testicular lesions 5.
MATERIALS AND METHODS
Materials used in the present study were Bisphenol -A [CAS No. 80-05-7], prepared from Sigma–Aldrich Inc. German, in the form of crystalline white powder. hormones were assayed by (ELIZA kit); an enzyme-linked immune-sorbent assay kit [IB79174, IBL-America., Minneapolis, Minnesota., USA].
Experimental design
Forty percent healthy male adult rabbits with an average weight of 2500±250 grams at eight months old. They were obtained from the laboratory of the animal house. Animals were kept in wide-ventilated stainless-steel animal cages under the prevalent environmental circumstances at room temperature of (25±2ºC) and an illumination period of 12 h-light 12 h-dark, with free pathogens conditions. Rabbits' balanced diet comprises carrots, vegetables, pellets, and water. Before the experiment, rabbits were acclimatized to the experimental conditions for at least one week. All rabbits were cared for according to the standard guides and ethics for the care and use of laboratory animals. Rabbits were equally divided into three groups, each containing fifteen animals. In the first Group, 1 rabbit received the usual food [without any additives] and received normal saline via intragastric tube daily (negative control); Group 2 orally received olive oil via intragastric tube daily (0.5 ml/day), it is proposed as positive control, while third Group, 3 rabbits were received bisphenol A in a dose of 25 mg/kg of the body weight for 30 days orally, that dissolved in olive oil via intragastric tube 5.
Biochemical study
Blood samples were obtained from the experimental animals and centrifuged for 15 minutes at 3000 rpm to estimate hormone levels in serum. Levels were assessed by [ELIZA enzyme-linked immunosorbent assay kit], [IB79174, IBL-America, Minneapolis, Minnesota, USA].
Histological study
Experimental rabbits were anesthetized by the use of diethyl ether through inhalation. Testis, seminal vesicles, prostate, and epididymis were excluded, fixed in 10% formalin buffer for 6 hours, then processed at 5 μm paraffin stained with Hematoxylin and Eosin stain(H&E) 6.
Statistical analysis
Levels of serum testosterone hormone were assessed and statistically analyzed using (one-way analysis of variance test) ANOVA and LSD. The data were expressed as Mean ± SD at a significant P value of < 0.05.
Ethical approval
The experiment was carried out according to the guidelines laid down by the International Animal Ethics Committee or Institutional Ethics Committee and by local laws and regulations; approval was obtained on 17/8/2022 with number 332.
RESULTS
Biochemical results
Results exposed a non-significant difference (P >0.05) in serum testosterone, LH, FSH, Prolactin and Estradiol levels of group 1 (negative control) as compared to group 2(positive control). The levels of serum testosterone, LH, and FSH of the experimental Group 3 animals that treated with BPA were showed a significant decrease (P<0.001) when compared with the first treated Group 1 and Group 2, as elucidated in [Table1].
Levels of prolactin and estradiol were inversely increased due to Bis-PA administration.
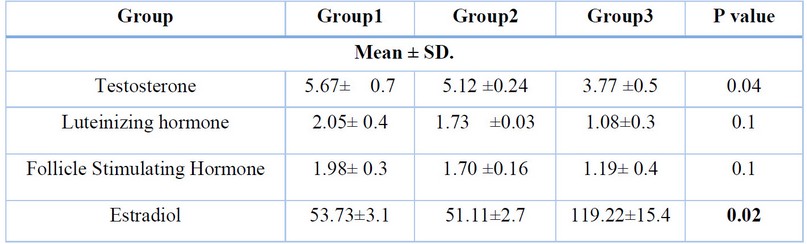
Table 1. Serum hormone levels of the experimental and control groups.
Histological results
The histological examination of the animals of groups 1 and 2 indicated the similar histological structures of all the examined reproductive organs studied: the prostate, epididymis, testis, and seminal vesicles. Testis of the control groups (1 & 2) of the adult male rabbits showed an intact, well-developed interstitium between the regularly arranged seminiferous tubules, with sperm-filled lumens. The lumens were lined by stratified epithelial tissue that was built from sperm line cells, beginning with spermatogonial cells that appeared as round small cells that had round nuclei. Primary spermatocytes were looked at as large, round nuclei organized as single or double layers. Spermatids were rounded, small, and had pale nuclei. Then, mature sperms were located inside the tubule's lumens. Sertoli cells were found with their triangular nuclei attached to the basement membranes of the testicular tubules. The testis of the rabbits treated with Bisphenol- A [group 3] was explained by a widening in the tissue of the interstitium along with a germinal epithelium sloughing, causing a loss in the typical arrangement of seminiferous tubules. Blood vessels of the interstitial spaces were congested and dilated, invaded with inflammatory monocellular cells. The seminiferous tubule lumens appeared with tiny debris of spermatids and degenerated Sertoli cells containing pyknotic nuclei. The germinal epithelium was degenerated, spaced, and separated from the basement membrane. The cauda of the epididymis of both control groups (1 and 2) were circular tubules -lined with pseudostratified epithelium of three cell types; basal, principle, and clear cells - separated from each other by the interstitial tissues with some cells of connective tissue and smooth muscles fibers along with a large number of sperms in their lumen [Fig. 3B].
The histological observations of the caudal portion of the epididymis of the BP-A- A treated rabbits [group 3] showed flared interstitial spaces with thickness in smooth muscle layers and connective tissue, almost with congestion of the blood vessels altogether with inflammatory cells. Histological results also showed a loss of the tubule's circular shape and irregularity, with altered epithelium from columnar to flat hypertrophic cells toward the lumen; with vacuolated light cytoplasm, principal cells showed degeneration of their stereocilia.
The prostate of control groups (1 & 2) of the adult male rabbits were formed by acini filled with prostatic secretions and embedded in the fibromuscular stroma that contains blood capillaries. The prostatic acini were an arrangement of simple columnar epithelial cells, the height of the lining epithelium was varied, inconsistent with the prostate functional state. The histological examination of BPA-treated animal prostates (group 3) showed hyperplasia in the prostatic stroma and acini lining epithelium. The prostatic acini also showed infiltration with small dark blue inflammatory mononuclear cells inside the lumen and around the acini, and there were papillary folds. Blood vessels of the capsule of the prostate were congested and hemorrhaged.
Seminal vesicles of the rabbits of control groups (1 & 2) showed branched acini with pseudostratified columnar epithelium and smooth muscles between the branches. Meanwhile, animals of group 3 exhibited a thickening in the acinar epithelium and hyperplastic nodules. The blood vessels of the tissue between the branches were congested with edema and inflammatory cells.
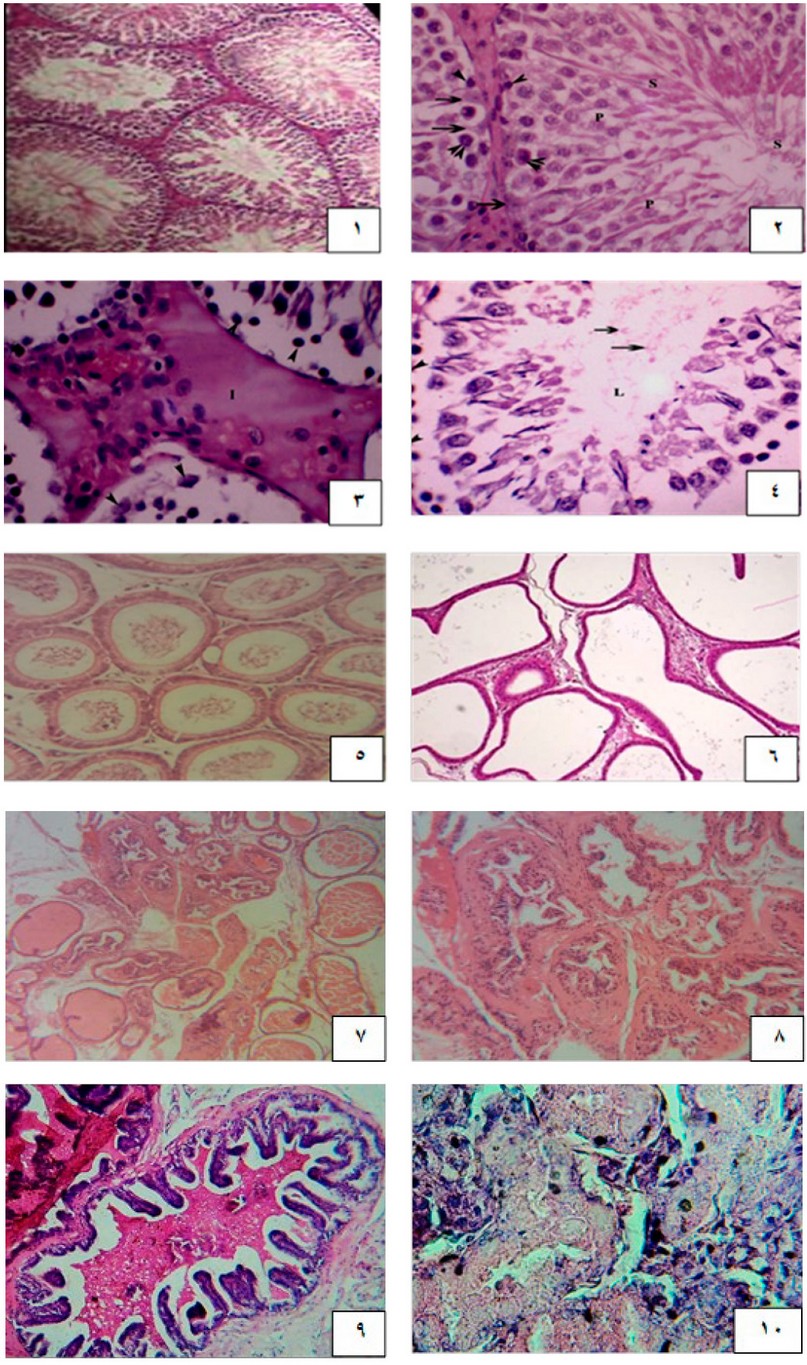
Figure 1. A cross-section of the control rabbit testis shows intact seminiferous tubule architecture with intact spermatogenesis. (2): Cross section of control rabbit testis showing the seminiferous tubules lumen, with spermatogonia resting on the basal membrane, appeared as round small cells (arrowheads), primary spermatocyte (two arrowheads). spermatids (P), Sertoli cells and spermatozoa (s). (3): Cross section of BP-A treated rabbit testis showing the expansion of the interstitium (I) between the testicular tubules and sloughing of the germinal epithelium (arrowheads). Congested blood vessels (star) and inflammatory cells (arrow). (4): Cross section of Bisphenol-A treated rabbit testis showing few remains of spermatids (arrows) in the lumen with disintegrated Sertoli cells exhibiting pyknotic nucleus, (arrowheads (5): Cross section of control rabbit epididymis showing the intact tubules separated by interstitium. with lumen filled with sperms. Figure (6): Cross section of Bisphenol-A treated rabbit testis showing irregularity in the shape of the tubules with widening of the interstitial spaces and thickening of epithelium and smooth muscle tissue with increased fibrotic tissues, interstitial spaces with infiltration of connective tissues and inflammatory cells with low epithelium. (7): Cross section of control rabbit prostate with a regular size acinus, with secretions in lumen (8): Cross section of BP -A treated rabbits showing hyperplasia. There is a thick stroma surrounding the acini with fibrosis and hypertrophy, monocellular inflammatory cells infiltration, and disassembled stroma (9): Cross section of the seminal vesicle from control rabbits displaying the branched acini with secretion in the lumen. (10): Cross section of the seminal vesicle from BP-A treated rabbit exhibiting coiled hyperplastic nodules with congested blood vessels and some inflammatory cells.
DISCUSSION
Several factors, such as endocrine disrupters and toxic elements, can affect spermatogenesis 7. The decreased testosterone levels might be caused by the reducing effect of Bis-PA on Leydig cells; actually, their count is 8,9, where the appropriate cause is the effect of Bis-PA on the enzyme a-reductase which converted testosterone into BHT. The current results show that the rabbits treated with Bisphenol A significantly decreased testosterone hormone levels. These findings were confirmed by 15,16. However, this diverges with the findings of 17, who explained that serum testosterone levels do not alter, although BP-A could affect fertility. The decrease in LH levels might be caused by the reduced sensitivity of pituitary gonadotropene cells 10, or low levels of testosterone may cause it via the antiandrogenic action of Bis-PA 8. The decreased FSH level could be caused by 17 B-estradiol that inhibits the secretion of gonadotropins from the pituitary due to the estrogen mimic action, as documented by 11. Going ahead with estradiol Bis-PA could cause hyperprolactinemia through its binding with estrogen receptors in both the anterior and posterior pituitary. It could also cause an increase in Prolactine gene expression and release, 12,13.
The recent study exhibited the disorganization of seminiferous tubules of BP- Treated rabbits with germinal epithelium sloughing; those outcomes were granted with 14 findings attributed to the increasing smooth muscle movement because of the inflammation that affects the epithelial tissue responses and then causing disproportionate stretch of the epithelial lining, through the release of chemotactic substances 8. In the current study, results explained a dis-organization of the epididymal tubules with cellular hypertrophy of the BP-A- A treated rabbits, the shape of the epithelial layer was conformed from columnar to flat; the results were in contract with 18 results, who related the cause to the defense mechanisms toward the exposure to the BP- A forging substance. Similarly, 19 were referred to the phagocytosis activity of the epididymal principal cells against the rubbish of any forging substance. The hallo cells were presumed to be lymphocytes that acted as an immunological function of the male reproductive system 20. Clear cells were responsible for hunting the damaged immature sperms and absorbing droplets of sperm cytoplasm 21.
Histological examination of the prostate sections of BPA treated Group (3) revealed a presence of hyperplasia with hemorrhage in the capsule of the prostate, which was elucidated by 22,24,25, who stated that the prostate was sensitivity to the synthetic and natural estrogen hormones and altered the prostate development and differentiation. Seminal vesicles of the Group (3) that were treated with BPA showed thickness in the acini epithelium with congested blood vessels as elucidated by 8, who stated that any injury of the male reproductive system can cause differentiation of cells by mitosis, and then growth of the new hyperplastic epithelium. These findings agreed with 23, who stated that the oxidative stress in tissues also could be seen because of the exposure to BP- A through its changing the iNOS expression, which downregulated the expression of testicular androgens receptors.
CONCLUSIONS
The study investigated the effects of bisphenol A (BPA) on the reproductive system of male rabbits. The results showed that BPA exposure significantly reduced testosterone levels, sperm count, and sperm motility. It also caused disorganization of the seminiferous tubules, epididymal tubules, and prostate and increased the size of the seminal vesicles. These findings suggest that BPA is a potential endocrine disruptor that can adversely affect male reproductive health. Overall, the study provides strong evidence that BPA exposure can have a significant impact on male reproductive health.
Further research is needed to fully understand the mechanisms by which BPA affects male reproductive health. Public awareness campaigns should be undertaken to educate people about the potential dangers of BPA exposure. Regulations should be implemented to reduce the use of BPA in products that come into contact with food and beverages.
Author Contributions: methodology, Manar Al-Murshidi, Walaa salih Hassan; Conceptualization, 2, Wurood mutual;
Funding: This research received no external funding
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Babylon.
Acknowledgments: The authors thanked the College of Sciences for Women for the materials used for experiments).
Conflicts of Interest: The authors declare no conflict of interest.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the College of Sciences for Women
REFERENCES
1. Calafat A.M., Ye X., Wong L.-Y., Reidy J.A., Needham L.L. Exposure of the us population to Bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008,116:39-44.
2. Li D.k, Zhou Z, Miao M, He Y, Wang J, Ferber J, Herrinton LJ, Gao E, Yuan W .Urine bi-sphenol-A (BPA) level in relation to semen quality. Fertil steril. 2011, 95: 625-630
3. Quesada I, Fuentes E, Visoleon MC, Ripoll C, Nadal A . Low doses of the endocrine disruptor bisphenol-A and the native hormone 17β-estradiol rapidly activate the transcription factor CREB. FASEB J, 2002, 16: 1671-1673.
4. Richter C.A, Birnbaum LS, Farabollini F, New-Bold R.R, Rubin B.S, Talnness C.E, Vandenbergh J.G, Walserkuntz, Vom Saal F.S . In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol, 2007 ,24: 199-224.
5.Wisniewski P, Romano R.M, Kizys M.M, Oliviera K.C, Kasamatsu T, Giannocco G, Chiamolera M.I, Diasdasilva M.R, Romano MA. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicol, 2015 , 2 : 1-9.
6. Shawkat, S. S.; Mohammed, A. I.; Rashid, S. A. .; Abdulateef, S. M. Effect Of Some Unfavorable Behavioral Traits On The Behavior Of Broiler Chicks. JLSAR 2023, 4, 1-8.
7.Vandenberg L.N, Chahoud I, Heindel J.J, Padmanabhan V, Paumgartten F.R, Schoen-Felder G .Urinary, circulating, and tissue bio-monitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect, 2010 ,118: 1055-1070.
8. Khudai M Y, Abdulateef S M, Mouhammed T Th, Alamili H S. Use of modern geometric design of fish ponds to increase welfare and blood parameters. Revis Bionatura 2023;8 (2) 82. http://dx.doi.org/10.21931/RB/2023.08.02.82.
9.Chitra, K.C.; Latchoumycandane, C. and Mathur, P. P. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicol., 2003, 185 ,: 119-127.
10.Benson ,B ; Deborah S., Mark R. S., Alfred M. C., Alvaro T. F., Patrick J. F., Monika K. K., Jean M., Pamela M.A., Eric V. C., Melissa B., Manya C., Daniel G. H.. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004, 15: 3408-19.
11.Catherine A.R. , Linda S.B., Retha R.N., Beverly S.R.,Chris E, John C.V. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol, 2007, 24:199–224
12.Brotons J.A, Olea-Serrano M.F., Villalobos M, Pedraza V, Olea N . Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect , 1995,103:608–612.
13.Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogencity of resin-based composites and sealants used in dentistry. Environ Health Perspect., 1996, 104:298–305
14.Zhang Hang C, Wang A, Sun X, Li X, Zhao X, Li S, MA A .Protective effects of lycium barbarum polysac-charides on testis spermatogenic injury induced by bisphenol a in mice. Evid Based Complement Alternat Med, 2013
15. Kurmar S.G., Narayana K., Bairy KL, . Docabazine induces gene toxic and cyto toxic germ cell damage withconcomitant decrease in testosterone and increase in lactate dehydrogenase concentration in the testis . Mutant Res. 2006 ,607: 240- 52
16. Zang Z, Suyun J.I, Tingting Xia, Huang S . Effects of bisphenol A on testosterone levels and sexual behaviors of male mice. Adv Sexual Medicine, 2016 , 6: 41-49.
17. Kim H.S., Han S.Y., Kwack S.J., Lee R.D., Kim I.Y., Seok J.H., Lee B.M., Yoo S.D., Park K.L . Androgenic/antiandrogenic effects of bisphenol-A in Hershberger assay using immature castrated rats. Toxicol Lett,2002 ,135 : 111-123.
18. EL Ghazzawy I.F., Meleis A.E., Farghaly E.F., Solaiman A . Histological study of the possible protective effect of pomegranate juice on bisphenol-A induced changes of the caput epididymal epithelium and sperms of adult albino rats. Alex J Med, 2011 ,47: 125-137.
19. Ahmed M, Sabry S, Zaki S, EL-Sadik A . Histological, immunohistochemical and ultrastructural study of the epididymis in the adult albino rat. Aust J Basic Appl Sci, 2009 ,3: 2278-2289.
20. Palacios J, Regardera J, Paniagua R, Gamallo C, Nistal M .Immunohistochemistry of the human ductus epididymis. Anat Rec, 1993 ,235: 560-566.
21. Sh. H. Fayyad, L., A. Sh. Al Shaheen, M. Effect Of Root Treatment With Mycorrhiza And Foliar Application With Moringa Leaf Extract On Npk Elements In Citrullus Colocynthis Leaves. Anbar Journal Of Agricultural Sciences, 2023; 21(1): 124-132. doi: 10.32649/ajas.2023.179722.
22.Huang L, Pu Y, Alam S, Birch L, Prins G.S . Estrogenic regulation of signaling pathways and home-obox genes during rat prostate development. J Androl, 2004 ,25: 330-337.
23. Chouhan S, Yadav S.K., Prakash J, Westfall S, Ghosh A, Agarwal N.K., Singh S .Increase in the expression of inducible nitric oxide synthase on exposure to bisphenol A: a possible cause for decline in steroidogenesis in male mice. Environ Toxicol Phar-macol, 2015,39: 405-416.
24. Ramos J.G., Varayoud J, Sonnenschein C, Soto A.M., Munoz D.E., Toro M, Luque E.H.. Prenatal exposure to low doses of bisphenol A alters the periductal stroma and glandular cell function in the rat ventral prostate. Biol Reprod, 2001. 65: 1271-1277.
25.Manar Mohammad Hasan AL-Murshidi. Histological study of testis tissue of male golden hamster (Mesocricetus auratus) in different time points of Diabetes. Annals of Tropical Medicine & Public Health, 2019, 22:50-56
Received: 26 September 2023 / Accepted: 15 April 2023 / Published:15 December 2023
Citation: Al-Murshidi, M.; Salih Hassan, W.; Hamza muttaleb, W. Some histological effects of Bisphenol-A on some reproductive organs in male adult rabbits (Oryctolagus cuniculus). Revis Bionatura 2023;8 (4) 77. http://dx.doi.org/10.21931/RB/2023.08.04.77
Publisher's Note: Bionatura stays neutral concerning jurisdictional claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. Submitted for possible open-access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
