2022.07.01.33
Files > Volume 7 > Vol 7 No 1 2022
Porous frameworks from Ecuadorian clays
1 Universidad de Tecnología Experimental Yachay Tech, Escuela de Ciencias Químicas e Ingenieria, Grupo de Investigación Aplicada en Materiales y Procesos (GIAMP); [email protected], [email protected] & [email protected]
* Correspondence: [email protected]; Tel.: +593995371712
Available from: http://dx.doi.org/10.21931/RB/2022.07.01.33
ABSTRACT
This research provides a literature review on several topics as a foundation to comprehend porous materials, their structure, and behavior to explore how they can be derived from clays and nanoclays. In this case, considering the several minerals present in some Ecuadorian clays, which are a potential starting material for the synthesis of porous frameworks, they constitute a solid source of metal atoms such as Silicon or Aluminum. This research presents the evaluation and characterization via XRD and AAS of clay samples collected in the southeast of Ecuador in the provinces of Azuay, Morona Santiago and Zamora Chinchipe, which present diversified soil mineralogy with many chemical and crystallographic features for suitable precursors in nanomaterials design.
Keywords. porous frameworks, clays, nanoclays, zeolites, X-ray powder diffraction, AAS.
INTRODUCTION
The greenhouse effect, global warming, and climate change have come to a state where little to nothing can be done or approached in a traditional way to mitigate or tackle their effects. Scientists have a hard job developing innovative solutions for this pollution problem. Yet, since the early 2000s, there have been advances in gases capturing and removing them from the atmosphere through chemical processes. However, these efforts are not enough, and the approach has evolved to merge these chemical processes to physical ones using nanotechnology to achieve the adsorption of different kinds of gases. The presence of acid gases in the atmosphere has had a crucial impact on the quality of life of people everywhere. Nowadays, targeting the concentration of such gases in the atmosphere is one of the biggest goals of science. One of the recent techniques is the adsorption process of such gases through porous frameworks1.
Now, what can one understand how porous framework materials are? To answer that, one can see at these materials' nano or picoscopic scale that if the constituents are not densely stacked but form voids, the material is defined as porous material2. To exemplify this better, it is easy to picture a bee panel where there are blocks formed, leaving voids to be filled with honey. In materials science, those voids are to be filled with substances such as acid gases that are chemically and structurally compatible and trapped in the frameworks. Therefore, one can say that porous frameworks materials are becoming all those with voids and a structure with framework form.
Porous frameworks synthesis can imply a wide range of processes. Materials with permanently porous structures made either entirely from organic building blocks or a combination of organic ligands and inorganic nodes have been at the forefront of chemistry for two decades3.
Decades of painstaking observational and empirical synthetic advances have made it possible to predict, with a relatively high degree of confidence, which structures might result if certain building blocks are joined together4. Porous frameworks, whether they are organic or metal-organic frameworks or zeolites, have emerged as advanced materials with a wide range of applications such as chemical catalysis, gas adsorption, ion exchange, and advanced nanotechnology applications, as will be discussed later.
In the adsorption process, the molecules or ions are to be adhered to a surface rather than penetrate the framework. Particularly in nanostructured materials, the applications field for porous frameworks become a powerful tool since they present large surface areas, high stability, and small size5. For the adsorption process, the most remarkable feature is the large surface-volume ratio since it represents more binding sites.
In Chemistry, the applications of interest are generally removing undesired compounds, molecules, and impurities from different matrixes that may contain contaminants traces or subproducts. Therefore, the porous frameworks may act as sieves, binding layers, or regular adsorbents with an appropriate chemical reactivity, improving their efficiency when using nanostructured materials6.
Nowadays, nanomaterials constitute a wide range variety of materials. One of the emerging types is nanoclays, which are naturally occurring or synthetic clays treated and scaled to nanostructures7. Nanoclays, are of great interest since they represent an opportunity for industrial and technological applications. Nanostructures display enhanced functional features that are not found in larger dimensions materials8.
Consequently, the field also thrives because it has a relatively low barrier to entry: it does not generally require sophisticated apparatus or complicated synthetic techniques. This allows contributions from synthetic chemists and engineers, spectroscopists, and physicists3. All of whom are spurred by the increasing availability of these materials without necessarily relying on collaborators to supply samples.
The hydrothermal synthesis method of porous frameworks has become of consistent and urgent interest for material scientists due to its easy access when one refers to equipment and reagents used in the laboratory as vital factors for the manufacture of the monomers producing the framework afterward. Performing a hydrothermal method for the synthesis, there is a higher chance of successfully modifying the framework. It is well known that clays display many exciting components to produce multiple porous frameworks3 since they contain silicon, iron, and aluminum minerals.
Theoretical Background
Porous materials and frameworks
Porous material can be defined as every solid or primarily solid material that presents pores in its structure, giving certain features relative to the system's porosity, pore size, and the fraction of pore volume concerning the total volume of the material9. Applications of porous materials occupy a varied assortment; they are commonly used as insulators, transistors, and conductors in the electronic industry as well as sieves for the water filtration system, chemical catalysis, etc10,11. Yet research in this area continues to take innovative and necessary paths.
The efforts of researchers have been reflected in the synthesis and production of materials that are being applied in processes for the elimination of polluting substances, employing the adsorption process of compounds whether they are in the liquid or gaseous phase. These materials are known as adsorbents, and these have high demand, but like any kind of material, their applications may be limited because of the precursors, specifically to the use of contaminating substances as templates, as well as due to the synthesis methods. So, the zeolites are examples of excellence since their synthesis is a greener approach.
Zeolites, silica gel, intercalated layered materials, etc., are common minerals used in such applications due to their pore dimensions9. These kinds of materials can be used in complex conditions given their ilk. The particularity of zeolites is that their structure is what gives such behaviors that can be utilized in several industrial applications like catalysis, gas adsorption, water purification, and treatment12.
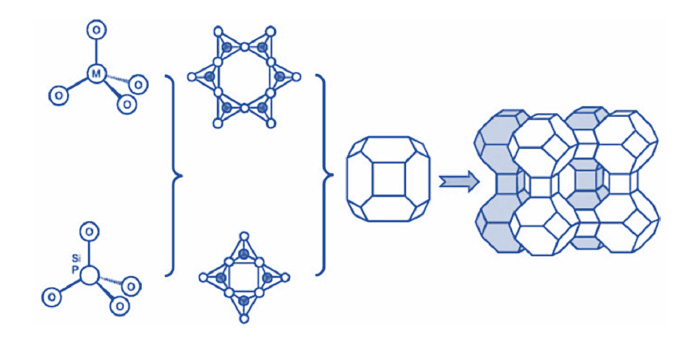
Figure 1. Formation of a zeolite, from the primary TO4 to secondary building units and further assemble to form extended zeolite13.
There is particular importance in the synthesis of the porous materials because of the specifications that they must present to be adequate and suitable for specific applications; therefore, the pore size, the porosity, the surface area, etc., must be controlled. Even in a more intricate way when the material is of nanometric scale. Engineering the design of porous material in clays is intended to change porosity, surface area14, surface content of solids, as well as thermal stability15.
Although the engineering process for the obtention of zeolites from layered silica clays has reached a high interest in the material sciences scenario, the research on three-dimensional structures (3D) from these compounds has been limited. This may be due to the limited application of pillared clay, so the effort to increase the use of coated material through pore engineering begins to transform the coating into a zeolite structure16. Now, data shows over two hundred types of zeolites based on a silica-alumina ratio17.
Clays
Clays are an extended collection of minerals, yet in chemistry, they are known as hydrous silicates18. They are found in nature from different natural sources and can be produced in their synthetic form. Their natural origin is after geological processes; whether they were physical or chemical processes, weather-depending processes, decompositions, etc., it will determine both the composition and properties of the clays. Even though they can travel because of natural phenomena, they are generally found near their origin site. Clay minerals may be divided into four major groups, mainly in terms of the layered structure variation, as shown in Table 1. These include the kaolinite group, the smectite group, the illite group, and the chlorite group19.
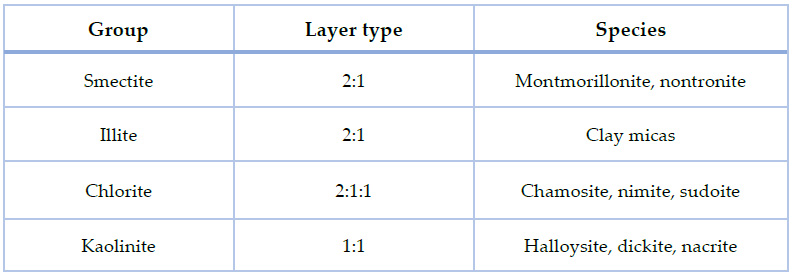
Table 1. Clay minerals classification19.
The arrangement in these crystals consists of silicate layers that coordinate two tetrahedral atoms combined to edge-shared octahedral sheets20. Thus, generally, clay structures are proposed in layers, and these layers are seen in sheets both tetrahedral and octahedral. This indicates that the tetrahedral layer is composed of silica-oxygen molecules sharing the corners to other tetrahedrons of the same type. Instead, the octahedral layer is structured by aluminum or magnesium in sixfold coordination, for instance, halloysite as seen in Figure 118,21, that displays this conformation in nature. The layers are then arranged by interactions such as van der Waals forces, hydrogen bonds, cationic or static forces, etc. Clay's ability for surface modification is what allows the dispersion of layered silicates into separate sheets20.
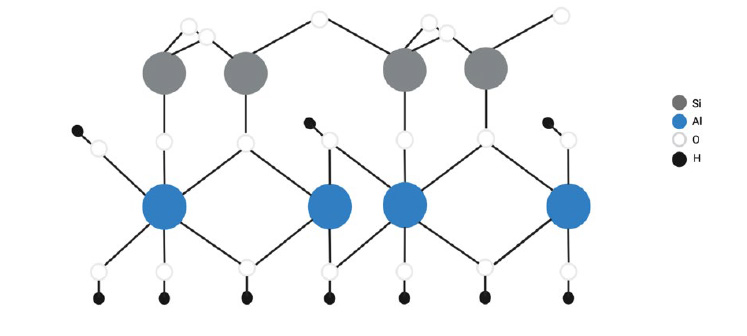
Figure 2. Chemical structure of halloysite21.
Clay particles can absorb or lose water in response to simple humidity content changes in the surrounding environment; when water is absorbed, it fills the spaces between the stacked silicate layers22.
Ecuadorian Clays
With regards to the soil mineralogy of Ecuador, there is barely any research done. Despite the large variety and existence of clays, there is still a significant lack of knowledge or information about their conformation, structure, and chemical formula.
Clays in Ecuador may have several compositions, yet they all show approximate levels of silica 60%, alumina 15%, low alkali and carbonates, and high levels of iron23. However, the lack of information opens the door to new research being done. There is a crescent interest in using clays for nanotechnology purposes, and most Ecuadorian lands provide them. As mentioned before, the clays may be found to be of various types, as shown in Table 1, even more than the few ones already discovered. And therefore, it can be used for many applications, including the development of nanomaterials and the improvement of nano-polymers.
In the same way, there is a need to discuss strategies and their implementation in nanoscale technology to take advantage of the naturally occurring resources, such as the Ecuadorian clays that can bring new and emerging materials, tackling ecological issues also industrial and economic concerns, etc. The lack of information on Ecuadorian clays is an opportunity to research them and discover and develop many other applications.
This review considered the data from the repositories of Ecuadorian Clays by the INEDITA project at Yachay Tech University, Ecuador. Under the INEDITA project, the clays were collected from Azuay, Zamora Chinchipe, and Morona Santiago provinces, located in the southeast of Ecuador, also known as the austral region of Ecuador. The total area of these provinces covers about 33000 km2, yet the samples were extracted from specific points, as shown below in Figure 3.
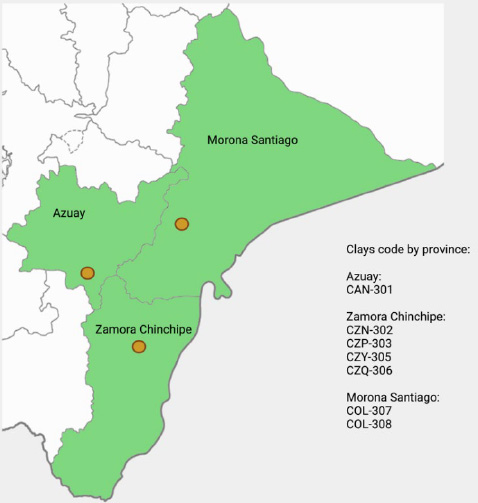
Figure 3. ap of the Ecuadorian provinces and sites where clays were collected. This sampling was performed by G.I.A.M.P (Research Group Applied in Materials and Processes).
They are labeled and enlisted in Table 2, considering the numbers used for classifying and identifying the clay series of the INEDITA-GIAMP project. The code corresponds to the origin site of the clay and the number of the sample in the set. Bouyoucos' method was used to collect the clays. Clay minerals were characterized via X-Ray Powder Diffraction. This technique is a non-destructive method that permits a collection of data about the composition of the crystallographic structure and, therefore, the physical behavior of materials24. The mentioned clays were previously used in nanocomposite research.
The physical appearance of each clay sample is shown below in Figures 4 and 5, which allow seeing how they vary in color. It could be related to the content of iron oxide or iron salts, not being this the only reason for the color variance in each of the samples, as can be observed. In Table 3 as well, it is indicated the iron concentration present in them. Still, the color of the samples will derive from other factors as the different mineralogical composition, environmental factors, organic compounds, etc. It must be said that the texture of the clays was very alike between all the samples. Between clays 301, 302, and 303 there is a notorious difference in color, while there is a similarity between 303 and 306 which were collected in the same province. It could be said that 302 and 308 also look alike, although 302 is more pinkish while 308 tends to a yellowish tone.
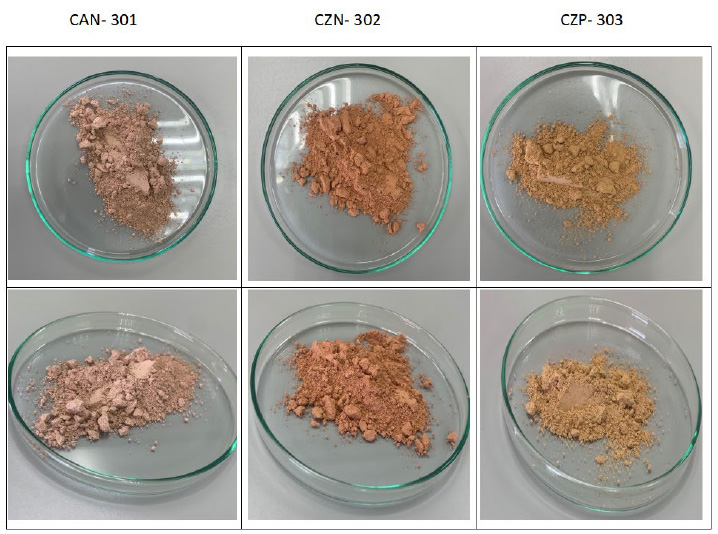
Figure 4. Samples corresponding to clays CAN-301, CZN-302 and CZP-303

Figure 5. Samples corresponding to clays CZQ-306, COL-307 and COL-308
Over 2950 phases matching the XRD data were obtained and analyzed with the software SmartLab Studio II (Rigaku Corporation, Japan), on average for all the samples. It was necessary to see what compounds were present in the analyzed samples, from which it was observed that Quartz and other forms of silicon oxides appeared in all the samples in different compositions. As it is noticeable, the spectra show similarities for data sets that correspond to the exact location of provenience, and they also are alike in composition.
The most remarkable features obtained of the analyzed samples are shown in Table 2:
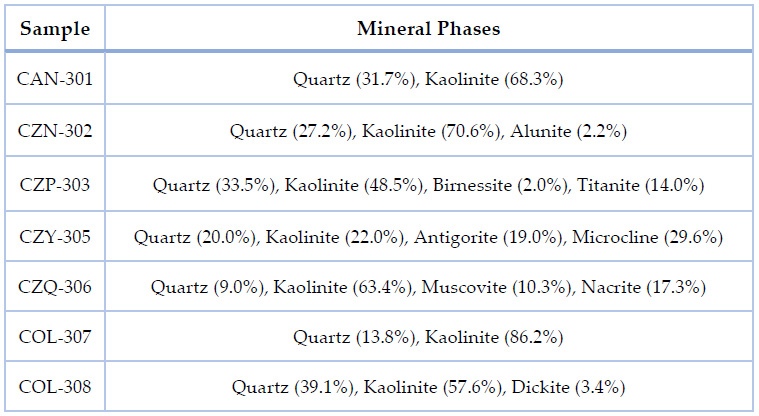
Table 2. Data corresponding to known mineral phases of each sample analyzed by XRD and treated via QUALX2.0, the values in parenthesis correspond to relative abundance (%).
Besides their mineralogical composition, the samples are also composed mainly of inorganic complexes, oxides, and long carbon chains. There is a high relative abundance of Quartz in all samples except for the CAN-306 sample, composed mainly of Kaolinite.
From the characterization of clays through Atomic Absorption Spectroscopy' AAS', during the INEDITA project activities, the iron concentration data (in parts per million) was obtained for each sample. This is shown in Table 3:
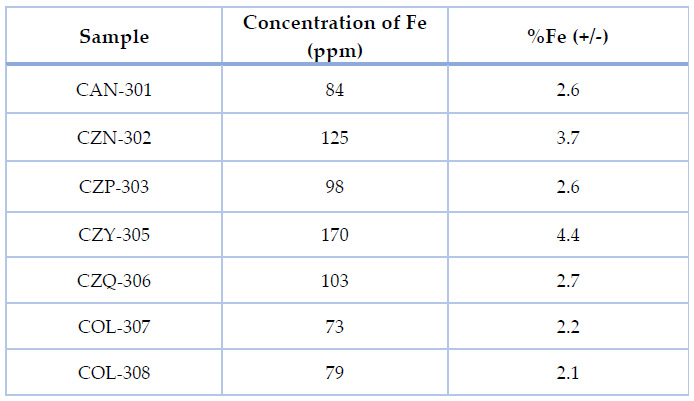
Table 3. Data corresponding to Iron (Fe) concentration and percentage present in each sample25.
The similar iron percentage in the analyzed clay samples indicates that almost all the clays share a similar element concentration. Moreover, sample CZY-305 shows a doubled concentration concerning the other clays, see Figure 6, which suggests that this sample is the most suitable precursor for porous framework synthesis designed for H2S capture.
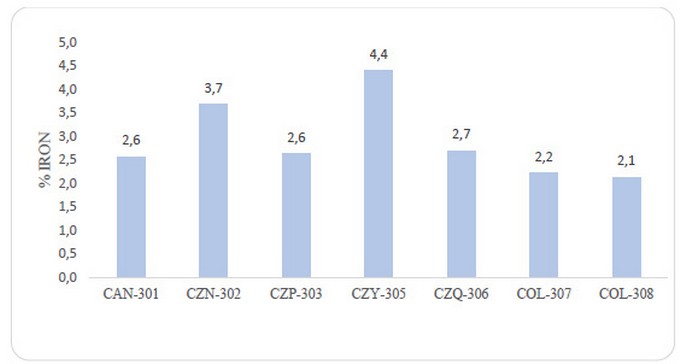
Figure 6. Percentage of iron concentration in clay samples obtained by Atomic Absorption Spectroscopy
Nanoclays
Nanomaterials, specifically nanoclays, permit and present various chemical formulas because of their modifiable structure7. For example, Kaolinite or Montmorillonite which chemical formula varies depending on the environment since its structure adapts to both water and soil18. Besides, materials like clays present characteristics such as the Cation Exchange Capacity (CEC), which allows the core of the material to create a charge imbalance that leads to a change in chemical composition. Additionally, it indicates the level of potential substitution in the core of the material, depending on which cations are used to fill the voids somewhat; thus, resulting in a promising new electronic or polymeric application. Thus, the exact theoretical formula is rarely presented in such a way in nature; rather, the material will have a certain number of waters, etc.
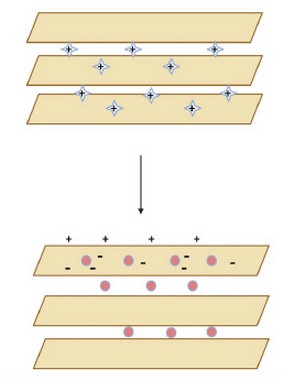
Figure 7. Adsorption of positively charged molecules on the negatively charged surface of a nanoclay26.
Research on nanoclays has made significant progress in the last years because of the urgent and rising interest in the polymer market7. These nanomaterials show many advantages as additives, coatings, etc. In clays, it is essential to notice that they present isomorphous substitution, which is why they tend to have a charge resulting from the exchange of Si4+ with Al3+ leaving a negative charge to be balanced later. This gives the matrix the space to accept positive charges, which allow the clay sheet structure to undergo modification, providing them the possibility of exciting applications as precursor material since it allows the obtention, synthesis, or manufacturing of additives for polymeric chains27. Also, nanoclays are layered mineral silicate with layered nanoparticles that form a crystalline structure after stacking. After, those stacks can be dispersed in a polymeric matrix to achieve a specific new material or feature of a polymer19.
Nanoclays heavily affect the performance and behavior of nanocomposites, although research is still to be done. An extensive set of characteristics may determine the best and most optimum usage of a specific type of nanoclays but is still little and unknown. The level of intercalation and exfoliation are some of the prior mentioned features, and they could severely change the output of the nanomaterials. Nowadays, research has turned its eyes into attaining unique and functional combinations of polymer matrix and nanoclays. Some of these so desired features go from an immiscible or intercalated structure to an exfoliated structure, or even all of them together19.
Most of the research has been directing and investing its resources in flame retardancy and thermal modification, and the remaining efforts go to organophilic clays for mechanical or barrier properties18. Nonetheless, as mentioned before, extending the field of research on nanoclays features will lead to the synthesis of new materials and could even expand technology as one knows it. For example, suppose one considers the decomposition rate of certain polymers (already of commercial use) when added a more organophilic and high exfoliating nanoclay to its matrix or as a coating. In that case, it could disintegrate much more easily48 resulting in a greener material or a vector that adsorbs toxins from the environment, etc.
Still, there is an urgency to consider the environmental impact and concerns, and the proper production of new nanoclays, as every emerging material, must be examined against its ecological cost. In this case, the extraction of clays and the subsequent nanoclays for both nature as a whole and humans are two main factors.
Although it can be a completely clean process, the extraction represents a depletion of resources and must be carefully considered, primarily when they represent a non-renewable good, which in the long term will leave a mark on its primary source.
On the other hand, it must be considered the size of the particle and the chemical composition and its effect on both living and inert beings. Will these nanoclays be safe? For example, for living beings, if the nanomaterial has some aspects in its structure, could it enter the blood circulation system and harm it? Or could they accumulate and cause disease? And so on, many questions must be considered.
Toxicity and ecological challenges
The studies and analysis of nanoparticle toxicology focus on the capacity of nanoparticles to damage or change the function of cells, genetic code, and living environments caused by shaping factors such as the very high surface area of these molecules. It aims to determine in what mechanism the damage is caused, and more importantly, it attempts to find a proper response or a bridge to change the hazardousness into a non-destructive feature.
The different modifiers and their incorporation or combination in the layered silicates can result in nanoclays very miscible in polymers but could also represent a highly dangerous when inhaled or a general direct exposure28. In the same way, the factors mentioned above can lead to toxicological profiles after random scenarios as thermal degradation and chemical composition affect the nanoclays properties and their size and shape, which provokes a variation in toxicity that can be somewhat hazardous.
It was previously discussed that the modification generally occurs via ion exchange, and this process will directly impact the resulting nanocomposite28. Despite the obtention of a high-quality polymer, the usage or consumption of them will certainly open the possibility of degradation and, therefore, the release of the nanoclays from the matrix29, which can turn into a pool of free nanocomposites and depending on the medium or vicinity it can increase the risk of toxicity.
The obtention of naturally sourced clays for the posterior manufacture of nanoclays composites represents another ecological challenge. It is not a threatening challenge, yet it is essential to consider these factors since clays are obtained from limited resources that constitute not only the ecosystem for living beings but also the inorganic portion of a wholesome environment, and like everything in nature, it provides a balance that must be taken care of. In the obtention of clays, it is fundamental to consider the alterations in the surroundings rather than in the clays themselves.
Applications
In recent years, functionalization has been the main objective of research in nanomaterials, since it seems to be, for now, the mechanism which has shown higher effectiveness for targeting many problems, such as drug delivery, surface activity for medical and mechanical purposes, etc., and so many others30.
Bioactive molecules are a new resource that has been under the loupe for many applications since it opens the possibilities of modifying both inner and outer surfaces and other types of chemical modification31. For this reason, its applicability is broad. Another essential feature is that these molecules can give multifunctionality. In nanoscale chemistry, this means the opportunity to synthesize a material, a coating, or a nanoparticle formulation that can accomplish two or more objectives or target two problems at a time. This is of particular interest for material scientists who are constantly searching for new functionalities that enable the material's mechanical properties in the recipient and can adapt to the environment it is in.
One of the approaches to solve the mentioned above is supposedly the surfactant activity of the nanoparticles and, if they lack such activity, to enhance their performance by adding or assisting them via bio-templates. However, this method continues to present certain unwanted behaviors and properties, which, although not inevitable, represent a particular difficulty and hinder the exploitation of its potentials, such as the creation of micro-voids in the matrix or the existence of agglomeration32. Despite, when compared to other surface treatments, introducing a multi-scale synthesis via bio-templates is a current and promising technique to be more investigated.
There are many chemical and physical procedures to functionalize materials, as enumerated in Figure 8.
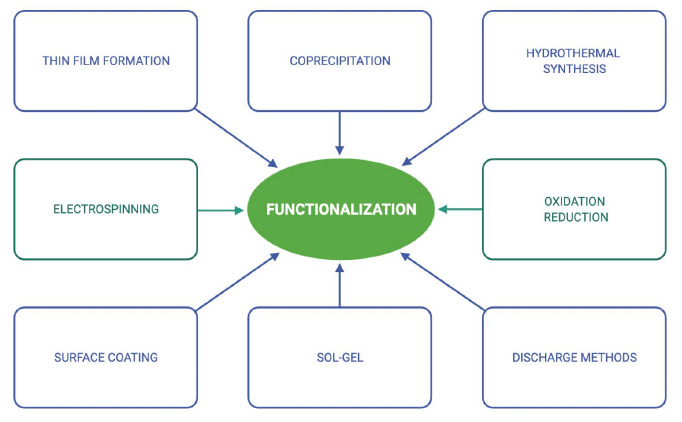
Figure 8. Common techniques used for synthesis and functionalization of nanomaterials33.
Clays and nanoclays had acquired particular interest for the applicability; they present for functionalization because of their large surface areas. Clays are characterized for their mechanical polymeric properties and one of those features is the large surface area; this remains after nano scaling them and this behavior makes them a special nanocomposite which more likely will present more necessary properties, such as favorable kinetics in adsorption terms, the desired pore size, and expected crystal arrangement, among others.
Nanoclays can provide the chance not only to be functionalized inherently but also to accept, or interact with templates, resulting in the formation of a functionalized bilayer
nanomaterial. When the template comes from a naturally occurring source, such as plants, it widens the sustainability in the process of fabricating a new material, responsibly made, which tackles several green chemistry principles, some of them being:
• Prevent waste
• Less hazardous synthesis
• Design of benign chemicals
• Use of renewable feedstocks
• Reduce derivatives
• Design for degradation
From: The Twelve Green Chemistry Principles34.
There are several studies in recent years corresponding to the research of functionalization of materials and their applications. This so-called functionalization takes advantage of a wide range of available resources, whether they are naturally occurring or synthetic. The branch of study they can apply is an open window to futuristic materials, devices, and medicine. An assorted list of articles is shown below om Table 4, which shows the research that has been done between 2020 and 2021 with functionalized materials.
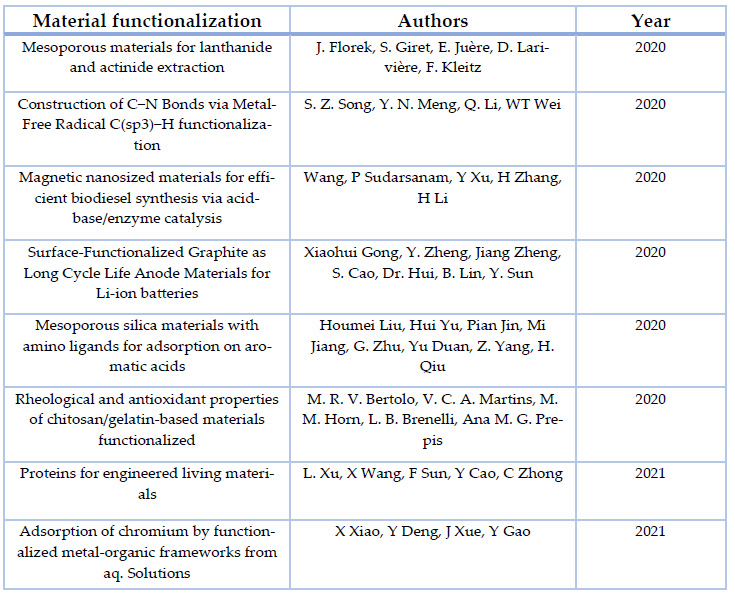
Table 4. Assorted list of the recent studies on functionalized materials, corresponding to years 2020 & 2021
Gas adsorption membranes
Previously, it was mentioned that nanocomposites in being widely used to revert the damage caused by the past wave of plastics mass production. Nowadays, there is a particular interest in producing new materials that are more ecological and a real option of biodegradable polymers but also in the adsorption of gases causing a greenhouse effect35.
Porous frameworks can work for both liquid and gas systems since their design permit to catch of molecules depending on their size rather than any other characteristics. The benefits of a functionalized nanoclay go beyond acting as a sieve. For example, in this case, where they are placed as a gas adsorption membrane, they would trap molecules and a physisorption process. Another feature is chemical selectivity, which means that these nanoclays and their chemical makeup must also act as, in a certain way, a selector, which allows specific other molecules to pass or not36.
The synthesis of nano-porous materials faces several challenges. Luckily, they are successfully overcome daily because of the increasing interest in this research field. Mao, et al., designed a hierarchical membrane that can undergo volatile organic adsorption and hydrogen gas storage36, demonstrating the many advantages that nanomaterials have to offer. The hierarchical nano-porous membranes in the mentioned study were synthesized from wood cellulose nanocomposites with graphene oxide and carbon spheres.
Industrial treatment for both gas separation and hydrogen storage have a long and robust relationship with zeolites and porous materials for the removal of volatile organic compounds36,37. Still, in this era, nanotechnology offers the chance to turn those conventional techniques into brand new innovative paths to solve the same problems with a sustainable approach. Nanoclays offer large surface areas, which is of consideration when scaling membranes to industrial use. Another remarkable feature is that they are lightweight materials that improve transport, installation, replacement, etc., procedures of high cost in the industry.
Moreover, reducing the negative impact that fossil fuel footprint from the environment is always a massive triumph for green chemistry, material science, the planet, and humans. Individuals have been suffering the consequences of high concentrations of carbon dioxide in the air. By August 4, 2021, there was a 415.16 ppm presence of atmospheric CO2 on the planet, showing a 0.31% increase concerning the same date in 2020, when the concentration was 413,88 ppm, according to co2.earth, a web page dedicated to recording daily the levels of carbon dioxide reached.
Under this perspective, it is easy to interpret that separation of carbon dioxide is a strategic and practical factor when it comes to energy storage and a wholesome ecological development37. New nanomaterials, especially those functionalized, hope to ease environmental problems.
Polymeric research aims to synthesize membranes that have high flux and selectivity; however, there are many significant obstacles, such as an increased permeability when selectivity decreases or the contrary. Also, the elasticity of the desired material may vary depending on the pore size and the permeability it presents, etc.
Development of membranes with high flux and selectivity is desirable, but one of the major problems of polymeric membranes is that as their permeability increases, their selectivity decreases significantly or vice versa. Various methods have been investigated to overcome this problem thus far; these include the synthesis of new polymers, mixing common polymers with organic and inorganic nanoparticles, etc.38, which allow the formation of a membrane with high permeability, and the nanoclays should also allow a high selectivity.
Hydrogen Sulfide (H2S) capture by porous frameworks obtained from nanoclays containing iron (Fe)
Hydrogen sulfide H2S can represent a harmful environmental factor even at low concentration levels39. It is generally the byproduct of industrial processes as it is formed after the fermentation of several organic residues. This gas is toxic and highly corrosive and deactivates catalysts, making it extremely necessary to remove it before any biogas treatment40.
The current and most common methods for H2S capture are wet desulfurization and dry desulfurization41. The research aims to develop new innovative and sustainable processes for the removal of H2S increase day by day, especially considering the challenge it means to remove it while having a good sulfur recovery42.
The structures that are achievable with porous materials and nanoclays, provide another solution for the adsorption of toxic particles, such as H2S. The pore size acts as a molecular sieve at a nanometric scale, and the high surface area of these frameworks can adsorb these molecules, depending on the design of the material, such as metal-exchange zeolites and other porous materials metal-organic frameworks which are highly effective adsorbents39.
Many materials scientists have already proved that there is an increased efficiency when the iron is present in zeolites, silicates, etc. Lee et al. showed that the H2S adsorption capacity of zeolites is directly proportional to the iron concentration, under a very important condition as it is room temperature43. Bentonite loaded with iron oxide was prepared via the hydrothermal method by Long and Loc, which showed to decrease the H2S concentration after the removal processes in a fixed bed column44.
Therefore, if clays contain iron, then they can be used as a source for the synthesis of nanoclays. This will help and enhance hydrogen sulfide removal by any porous framework derived from such starting reagents, such as the clays samples discussed previously (refer to Table 3, 4, and Figure 6).
Water Treatment
Potabilization of water
The introduction of clays for sustainable water treatment has been long researched and implemented worldwide. Commonly, most water filtration systems include at least one ceramic piece mainly composed out of clays in their structural design. Typically, ceramic filters are made with a high percentage of clays (up to 80-85%) and combustible material, which form a homogeneous mixture after a fine sieving process45.
It is reported that polymeric Fe & Al modified clays are highly effective in heavy metals adsorption since it takes the clays' adsorbent properties and their ion exchange capacity. Clays, such as Montmorillonite and Kaolinite, had been provided suitable features for such processes because of the large surface area, which was previously discussed. It is demonstrated that these Al/Fe polymeric species are most efficient for coagulation/adsorption purposes like chemicals removal or emulsion for clean water treatment. Both natural and modified clays exhibit the potential to produce new sorbent materials45. The combination of both organic and crystalline materials derived from clays might result in an optimum sorbent material of such properties.
In the past, an approach for achieving this has been the use of carbon in the form of charcoal10. Yet, granular-activated carbon, a microporous structure, fails to remove large molecules46 or waste-water residues, which are generally oil and grease already emulsified. This lack of efficiency from charcoal. Large molecules plug the exposed macro-porous layer; hence the inner microporous layer becomes ineffective.
Dyes removal from water residues
It has been long studied that clays are an effective method for removing organic dyes from water residues, yet in nano dimensions, the effectiveness of these clays is still under the scientific eye47. The applicability of these processes is of high potential. The removal of these dyes from aqueous solutions, considering three crucial conditions: temperature, pH, and ionic strength11.
Nanoclays are a very innovative approach to remove some sort of residues from water samples or aqueous solutions since their large surface area provides the space to accept a larger quantity of molecules. When these nanoclays have been functionalized for improvement, they can be provoked the process of dye removal47. Several conditions must be studied to determine the efficacy of specific clays for these kinds of processes. One, for instance, is the pH measurements before and after, these giving the chance to track the changes in the solution while the dye is being adsorbed. Another method for determining the amount of dye is measuring the difference in dye concentrations at certain points during the experiment11. A mass-balance equation must be used to obtain accurate and precise data for these. It is given below,
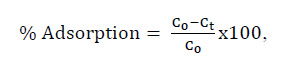
with Co as the initial concentration and Ct as the concentration different from the initial. Any Ct can be considered, yet is highly recommended to consider the final time to have a wider window of a full adsorption process. Under the same considerations, the above equation can also be used to study gas adsorption; however, many factors would change because gas molecules' behavior is much different from liquids.
Handling and treatment of nanoclays: suggestions on their toxicity
The nanoscale size of nanoclays allows them to play an innovative role in new biological and environmental functions. Yet, they can also become a significant problem since they also can interfere with cellular processes8. The complexity of nanoclays effects and behavior requires putting up very detailed and systematic handling and precautions to reduce the risks of exposure.
Despite the several useful biological and environmental applications of nanoclays, their nanoscale size enables them to penetrate the cell membranes and interfere with cellular processes. Given the complex and uncertain effects of nanoclays on biological environments, systemic management, analysis, and precautions are required to assess and minimize the exposures during their use, handling, and discarding.
More research advances in this specific topic are required to comprehend the dynamics and behavior of nanoclays in living environments and assess the possible damages and side effects caused by the toxicity of the mentioned molecules. It is of primary importance to achieve a safe production of nanocomposites and nanomaterials as they represent and play a fundamental role in the next significant generation of polymers and their application.
Nowadays, there is no clear nor solid explanation of the exact mechanism of how these molecules can be prevented to cause adverse effects on cells, yet the most researched motive seems to be its large and augmented surface area, which also is what gives such excellent properties they present also allowing the surface functionalization, becoming a double edge sword.
CONCLUSIONS
Porous frameworks are a suitable approach to tackle contamination in many forms. Therefore, they provide the door to chemical modification and the opportunity to create novel materials from a wide range of precursors, as clays since they constitute a primary and naturally occurring source of starting materials such as Silicon or Aluminum. The soil mineralogy of Ecuador allows the obtention of such nanoclays, that can lead to the synthesis of nano-porous materials. This idea derived the characterization of Ecuadorian clays from the INEDITA project as a plus contribution to a repository and research compendium of the soils they came from. This review also displays the usefulness and possible applications such as adsorption of the greenhouse effect, water cleaning, and purification, dyes removal, etc., as a starting point of what can be achieved with nanoclays and porous frameworks resulting from the proposed synthesis while considering late and new research on the topic.
Acknowledgments: The INEDITA project and the Research Group Applied in Materials and Processes' GIAMP' are deeply acknowledged for supporting this research, providing data and materials. In the same way, Yachay Tech University for the materials used for the characterization of clays.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Jeon, M. Y., Kim, D., Kumar, P., Lee, P. S., Rangnekar, N., Bai, P., Shete, M., Elyassi, B., Lee, H. S., Narasimharao, K., Basahel, S. N., Al-Thabaiti, S., Xu, W., Cho, H. J., Fetisov, E. O., Thyagarajan, R., DeJaco, R. F., Fan, W., Mkhoyan, K. A., … Tsapatsis, M. (2017). Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature, 543(7647), 690–694. https://doi.org/10.1038/nature21421
2. Chen, Y., Wang, N., Ola, O., Xia, Y., & Zhu, Y. (2021). Porous ceramics: Light in weight but heavy in energy and Environment Technologies. Materials Science and Engineering: R: Reports, 143, 100589. https://doi.org/10.1016/j.mser.2020.100589
3. Dincă, M., & Long, J. R. (2020). Introduction: Porous framework chemistry. Chemical Reviews, 120(16), 8037–8038. https://doi.org/10.1021/acs.chemrev.0c00836
4. Rosi, N. L., Kim, J., Eddaoudi, M., Chen, B., O'Keeffe, M., & Yaghi, O. M. (2005). Rod Packings and metal−organic frameworks constructed from rod-shaped secondary building units. Journal of the American Chemical Society, 127(5), 1504–1518. https://doi.org/10.1021/ja045123o
5. Kecili, R., & Hussain, C. M. (2018). Mechanism of adsorption on nanomaterials. Nanomaterials in Chromatography, 89–115. https://doi.org/10.1016/b978-0-12-812792-6.00004-2
6. Azodi-Deilami, S., Najafabadi, A. H., Asadi, E., Abdouss, M., & Kordestani, D. (2014). Magnetic molecularly imprinted polymer nanoparticles for the solid-phase extraction of paracetamol from plasma samples, followed its determination by HPLC. Microchimica Acta, 181(15-16), 1823–1832. https://doi.org/10.1007/s00604-014-1230-9
7. Huczko, A. (2000). Template-based synthesis of nanomaterials. Applied Physics A: Materials Science & Processing, 70(4), 365–376. https://doi.org/10.1007/s003390051050
8. Zanjani, J. S., Oğuz, O., Okan, B. S., Yildiz, M., & Menceloğlu, Y. Z. (2019). Polymer composites containing functionalized nanoparticles and the environment. Polymer Composites with Functionalized Nanoparticles, 437–466. https://doi.org/10.1016/b978-0-12-814064-2.00014-7
9. Ishizaki, K., Komarneni, S., & Nanko, M. (1998). Porous materials. Materials Technology Series. https://doi.org/10.1007/978-1-4615-5811-8
10. Beall, G. (2003). The use of organo-clays in water treatment. Applied Clay Science, 24(1-2), 11–20. https://doi.org/10.1016/j.clay.2003.07.006
11. Salam, M. A., Kosa, S. A., & Al-Beladi, A. A. (2017). Application of nanoclay for the adsorptive removal of Orange G dye from aqueous solution. Journal of Molecular Liquids, 241, 469–477. https://doi.org/10.1016/j.molliq.2017.06.055
12. Sabarish, R., & Unnikrishnan, G. (2019). Synthesis, characterization and evaluations of micro/mesoporous ZSM-5 zeolite using starch as Bio Template. SN Applied Sciences, 1(9). https://doi.org/10.1007/s42452-019-1036-9
13. Zhu, G., & Ren, H. (2015). Porous organic frameworks design, synthesis and their advanced applications. Springer Berlin Heidelberg.
14. Aguado, S., Bergeret, G., Daniel, C., & Farrusseng, D. (2012). Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite a. Journal of the American Chemical Society, 134(36), 14635–14637. https://doi.org/10.1021/ja305663k
15. Greer, H. F., Zhou, W., Zhang, G., & Ménard, H. (2017). Nanocone decorated zno microspheres exposing the (0001) plane and enhanced photocatalytic properties. Advanced Materials Interfaces, 4(13), 1601238. https://doi.org/10.1002/admi.201601238
16. Rahman, A., Purwanto, A., Endah, A., Handoko, E., Kusrini, E., & Prasetyanto, E. A. (2019). Synthesis and characterization of LTA zeolite from Kaolin Bangka. Journal of Physics: Conference Series, 1402, 055057. https://doi.org/10.1088/1742-6596/1402/5/055057
17. Khaleque, A., Alam, M. M., Hoque, M., Mondal, S., Haider, J. B., Xu, B., Johir, M. A. H., Karmakar, A. K., Zhou, J. L., Ahmed, M. B., & Moni, M. A. (2020). Zeolite synthesis from low-cost materials and environmental applications: A Review. Environmental Advances, 2, 100019. https://doi.org/10.1016/j.envadv.2020.100019
18. Uddin, F. (2008). Clays, nanoclays, and Montmorillonite Minerals. Metallurgical and Materials Transactions A, 39(12), 2804–2814. https://doi.org/10.1007/s11661-008-9603-5
19. Guo, F., Aryana, S., Han, Y., & Jiao, Y. (2018). A review of the synthesis and applications of polymer–nanoclay composites. Applied Sciences, 8(9), 1696. https://doi.org/10.3390/app8091696
20. Rajeswari, A., Jackcina Stobel Christy, E., Gopi, S., Jayaraj, K., & Pius, A. (2020). Characterization studies of polymer-based composites related to functionalized filler-matrix interface. Interfaces in Particle and Fibre Reinforced Composites, 219–250. https://doi.org/10.1016/b978-0-08-102665-6.00009-1
21. Na Nazir, M.S., Kassim, M.H., Mohapatra, L., Gilani, M.A., Raza, M.R., & Majeed, K. (2016). Characteristic Properties of Nanoclays and Characterization of Nanoparticulates and Nanocomposites.
22. The Clay Mineral Group. THE CLAY GROUP. (n.d.) from http://www.galleries.com/Clays_Group
23. Cárdenas, J.L., Paredes, C.A., Pacheco, J., 2003. La industria cerámica en el Ecuador. Rev. Tecnol. 16, 19–23.
24. Sima, F., Ristoscu, C., Duta, L., Gallet, O., Anselme, K., & Mihailescu, I. N. (2016). Laser thin films deposition and characterization for biomedical applications. Laser Surface Modification of Biomaterials, 77–125. https://doi.org/10.1016/b978-0-08-100883-6.00003-4
25. Vera, D. (2020). Characterization of Ecuadorian Ferruginous and Titaniferous Sands for Hydrogen Sulfide Capture (thesis).
26. Hosseini, F., Hosseini, F., Jafari, S. M., & Taheri, A. (2018). Bentonite nanoclay-based drug-delivery systems for treating melanoma. Clay Minerals, 53(01), 53–63. doi:10.1180/clm.2018.4
27. Wagner, A., White, A. P., Stueckle, T. A., Banerjee, D., Sierros, K. A., Rojanasakul, Y., Agarwal, S., Gupta, R. K., & Dinu, C. Z. (2017). Early assessment and correlations of Nanoclay's toxicity to their physical and chemical properties. ACS Applied Materials & Interfaces, 9(37), 32323–32335. https://doi.org/10.1021/acsami.7b06657
28. Saleh Alghamdi, S., John, S., Roy Choudhury, N., & Dutta, N. K. (2021). Additive Manufacturing of Polymer Materials: Progress, promise and challenges. Polymers, 13(5), 753. https://doi.org/10.3390/polym13050753
29. Roes, L., Patel, M. K., Worrell, E., & Ludwig, C. (2012). Preliminary evaluation of risks related to waste incineration of polymer nanocomposites. Science of The Total Environment, 417-418, 76–86. https://doi.org/10.1016/j.scitotenv.2011.12.030
30. Araújo, F., das Neves, J., Martins, J. P., Granja, P. L., Santos, H. A., & Sarmento, B. (2017). Functionalized materials for multistage platforms in the oral delivery of biopharmaceuticals. Progress in Materials Science, 89, 306–344. https://doi.org/10.1016/j.pmatsci.2017.05.001
31. Patra, J. K., Das, G., Fraceto, L. F., Campos, E. V., Rodriguez-Torres, M. del, Acosta-Torres, L. S., Diaz-Torres, L. A., Grillo, R., Swamy, M. K., Sharma, S., Habtemariam, S., & Shin, H.-S. (2018). Nano based drug delivery systems: Recent developments and future prospects. Journal of Nanobiotechnology, 16(1). https://doi.org/10.1186/s12951-018-0392-8
32. Blasco, E., Sims, M. B., Goldmann, A. S., Sumerlin, B. S., & Barner-Kowollik, C. (2017). 50th anniversary perspective: Polymer functionalization. Macromolecules, 50(14), 5215–5252. https://doi.org/10.1021/acs.macromol.7b00465
33. Ozkantar, N., Yilmaz, E., Soylak, M., & Tuzen, M. (2020). Pyrocatechol violet impregnated magnetic graphene oxide for magnetic solid phase microextraction of copper in water, black tea and diet supplements. Food Chemistry, 321, 126737. https://doi.org/10.1016/j.foodchem.2020.126737
34. American Chemical Society. (n.d.). Retrieved February 3, 2022, from https://www.acs.org/content/acs/en.html
35. Khajeh, M., & Ghaemi, A. (2020). Exploiting response surface methodology for experimental modeling and optimization of CO2 adsorption onto NaOH-modified Nanoclay Montmorillonite. Journal of Environmental Chemical Engineering, 8(2), 103663. https://doi.org/10.1016/j.jece.2020.103663
36. Mao, H., Tang, J., Chen, J., Wan, J., Hou, K., Peng, Y., Halat, D. M., Xiao, L., Zhang, R., Lv, X., Yang, A., Cui, Y., & Reimer, J. A. (2020). Designing hierarchical nanoporous membranes for highly efficient gas adsorption and storage. Science Advances, 6(41). https://doi.org/10.1126/sciadv.abb0694
37. Amini, Z., & Asghari, M. (2018). Preparation and characterization of ultra-thin poly ether block amide/nanoclay nanocomposite membrane for gas separation. Applied Clay Science, 166, 230–241. https://doi.org/10.1016/j.clay.2018.09.025
38. Dehghani Kiadehi, A., Rahimpour, A., Jahanshahi, M., & Ghoreyshi, A. A. (2015). Novel carbon nano-fibers (cnf)/polysulfone (PSF) mixed matrix membranes for gas separation. Journal of Industrial and Engineering Chemistry, 22, 199–207. https://doi.org/10.1016/j.jiec.2014.07.011
39. Khabazipour, M., & Anbia, M. (2019). Removal of hydrogen sulfide from gas streams using porous materials: A Review. Industrial & Engineering Chemistry Research, 58(49), 22133–22164. https://doi.org/10.1021/acs.iecr.9b03800
40. Liu, D., Li, B., Wu, J., & Liu, Y. (2019). Sorbents for hydrogen sulfide capture from biogas at low temperature: A Review. Environmental Chemistry Letters, 18(1), 113–128. https://doi.org/10.1007/s10311-019-00925-6
41. Wang, F., Zhong, H.-H., Chen, W.-K., Liu, Q.-P., Li, C.-Y., Zheng, Y.-F., & Peng, G.-P. (2016). Potential hypoglycaemic activity phenolic glycosides from moringa oleifera seeds. Natural Product Research, 31(16), 1869–1874. https://doi.org/10.1080/14786419.2016.1263846
42. Chiappe C., Pomelli C.S. (2017) Hydrogen Sulfide and Ionic Liquids: Absorption, Separation, and Oxidation. In: Kirchner B., Perlt E. (eds) Ionic Liquids II. Topics in Current Chemistry Collections. Springer, Cham. https://doi.org/10.1007/978-3-319-89794-3_10
43. Lee, E. Y., & Suh, M. P. (2004). A robust porous material constructed of linear coordination polymer chains: Reversible single-crystal to single-crystal transformations upon dehydration and rehydration. Angewandte Chemie International Edition, 43(21), 2798–2801. https://doi.org/10.1002/anie.200353494
44. Long, N.Q., Loc, T.X. Experimental and modeling study on room-temperature removal of hydrogen sulfide using a low-cost extruded Fe2O3-based adsorbent. Adsorption 22, 397–408 (2016). https://doi.org/10.1007/s10450-016-9790-0
45. Zereffa, E. A., & Bekalo, T. B. (2017). Clay ceramic filter for water treatment. Materials Science and Applied Chemistry, 34(1). https://doi.org/10.1515/msac-2017-0011
46. Jjagwe, J., Olupot, P. W., Menya, E., & Kalibbala, H. M. (2021). Synthesis and application of granular activated carbon from biomass waste materials for water treatment: A Review. Journal of Bioresources and Bioproducts, 6(4), 292–322. https://doi.org/10.1016/j.jobab.2021.03.003
47. Stagnaro, S. M., Volzone, C., & Huck, L. (2015). Nanoclay as adsorbent: Evaluation for removing dyes used in the textile industry. Procedia Materials Science, 8, 586–591. https://doi.org/10.1016/j.mspro.2015.04.112
48. Fu, X., & Qutubuddin, S. (2000, September 28). Clay nanocomposites: Exfoliation of organophilic montmorillonite nanolayers in polystyrene. Polymer. Retrieved February 3, 2022, from https://www.sciencedirect.com/science/article/abs/pii/S0032386100003852
Received: 25 December 2021 / Accepted: 25 January 2021 / Published: date. 15 February 2022
Citation: Calle Luzuriaga M, . Ávila E E, Alfredo Viloria D. Porous frameworks from Ecuadorian clays. Revis Bionatura 2022;7(1). 33. http://dx.doi.org/10.21931/RB/2022.07.01.33
