2023.08.01.16
Files > Volume 8 > Vol 8 No 1 2023
Propagation of the Colombian genotype of cacao (Theobroma cacao L.) CNCh-12 by somatic embryogenesis
1 Agrobiotechnological Development Center for Innovation – CEDAIT, Universidad de Antioquia, Km. 1.7 vía San Antonio de Pereira - Carmen de Viboral, A.A 054048, Colombia;
2 Agrobiotechnological Development Center for Innovation – CEDAIT, Universidad de Antioquia, Km. 1.7 vía San Antonio de Pereira - Carmen de Viboral, A.A 054048, Colombia; X
3 Biology Institute, Universidad de Antioquia, Calle 70 No. 52-21, Medellín, A. A 050010, Colombia;
* Correspondence: [email protected]; Tel.: +57 3192652633
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.16
ABSTRACT
Cocoa production (Theobroma cacao L.) is essential globally and constitutes one of the leading export products for Colombia. Understanding the limitations faced by this crop in Latin American countries, it is required, among other aspects, to contribute to strengthening the first link in the production chain through efficient propagation methods and genetic improvement. Knowing that somatic embryogenesis is an alternative to conventional propagation and constitutes an obligatory step in a breeding platform, the objective of this work was to establish a somatic embryogenesis protocol until the plantlet acclimatization in the nursery for the regional genotype CNCh-12, a promising material with productivities higher than 2,000 kg/ha. Different protocols were evaluated, from callogenesis induction, through the expression of primary somatic embryos (PSE) followed by maturation and subsequent conversion to plantlet two types of explants (petal and staminode) and culture time (according to the stage). Additionally, the induction of secondary somatic embryos (SSE) was evaluated in two culture media (L and F). For CNCh-12, the petal was found as an appropriate explant, with a minimum time of 15 days in induction for PSE formation, without difference between the culture media F and L (22 average embryos). Embryo maturation was achieved in medium F after 30 days, followed by an additional 30 days for conversion to plantlet (52.83%). The concentration of salts to increase the conversion and development of the embryos was 1/5 of that used in F. The highest number of SSE was in the L medium. Finally, the ex-vitro adaptation was achieved when the plants were planted in 50:50 sand-coconut fiber and moistened weekly with Hoagland's solution (1:10).
Keywords: Cacao, petals, in vitro propagation, plant growth regulators, somatic embryogenesis.
INTRODUCTION
The Theobroma cacao L. is a diploid tree species (2N), native to South America and domesticated approximately 3000 years ago in Central America1; it belongs to the class Magnoliopsida, order Malvales and family Malvaceae2. The genus Theobroma has 22 species; the only ones that represent high commercial value are T. Grandiflorum and mainly T. cacao3.
Cocoa cultivation is essential as it is a fundamental part of the economy of many underdeveloped countries4. According to The International Cocoa Organization (ICCO), most of the production is concentrated on the African continent in countries such as Côte d'Ivoire and Ghana5. Although traditional cocoa production figure at worldwide, in average the yield is low, per example, world production of fine cocoa is less than 5%. In general, low productivity is due to the incidence of diseases, poor technological infrastructure, lack of training of farmers, etc. that affect the production of the crop and obtain fine flavor cocoa1. In Colombia, cocoa is grown in 29 of the 32 departments of the country; for the year 2020, 188 thousand hectares of planted areas were registered, distributed in the departments of Santander (41%), Antioquia (9%), Arauca and Huila (8%), Tolima (7%) and Nariño (5%)6. The national average production in the last ten years was 46 thousand tons. However, for the year 2020, it was close to 63 thousand, distributed mainly in the departments of Santander (26 thousand tons), Antioquia (5 thousand tons), and Arauca (5 thousand tons), which on average register annual productivity of 500 kg/ha/year6.
Currently, the Colombian market is seeking to increase the areas of cocoa crops and the efficient production of cocoa since there are many unproductive trees, mainly due to inadequate crop management, deterioration, old age and sexual incompatibility7. Some of the methods used for the propagation of T. cacao, such as planting from seeds, have as a disadvantage the germination times, the rapid loss of viability of the seed after being extracted and the high genetic variability characteristic of the crop, so it has been necessary to implement asexual propagation methods such as grafting (conventional procedure) and somatic embryogenesis (biotechnological method). The induction of embryogenic structures in somatic tissue permits us to keep the same genetic information as the parental plant from which the explant was taken8. To this end, effective propagation protocols have been developed following the guidelines of somatic embryogenesis in different cocoa genotypes9–13.
Traditionally, new cocoa genotypes have been developed that can improve crop yields or add value due to their chemical, organoleptic and aroma characteristics, among others. Currently, despite having these new genotypes, they are not having a sufficient impact on the country's production chain due, among other causes to the lack of sufficient production of plant material7. An example of these genotypes is the regional elite genotype CNCh-12. This genotype has high polyphenol content, with approximately 76.23 mg GAE g-114 and high productivity of 2.000kg/ha; these characteristics make it a candidate for mass propagation by somatic embryogenesis. There is only one research work about somatic embryogenesis with CNCh-12, in which it was possible to induce the formation of embryos until the globular stage. Still, the somatic embryos did not continue their development until the complete plant15. Considering the importance of CNCh-12 and intending to contribute to strengthening the cocoa production chain, the objective of this research was to establish a protocol for propagation by somatic embryogenesis of this regional genotype.
For this purpose, we studied the embryogenic response of CNCh-12 to different culture protocols that vary in growth regulators and nutrient concentrations in the different stages of the embryogenic process: induction, expression, multiplication, maturation, and germination to plantlet.
MATERIALS AND METHODS
Plant material
For the explant type, the response of petals and staminodes to the embryogenic process was studied. Unopened flower buds of the cocoa genotype CNCh-12 were provided by the Compañía Nacional de Chocolates (CNCh) at the Yariguies farm, located in the department of Santander, Colombia; the flower buds were collected and duly stored in FalconTM tubes containing a solution of DKW salts16,17 at half concentration. Shipment was carried out in thermal coolers with cold gel packs.
For the disinfection of plant material, the protocol proposed by Henao et al. (2018)15 was followed with some modifications. Initially, flower buds are immersed in a solution of Belico® fungicide; the active ingredient is carbendazim at a concentration of 2 g/l for 40 minutes, followed by washing with sterile distilled water. Subsequently, they are immersed in a 0.5% hypochlorite solution for 5 minutes. They were washed with sterile distilled water, and finally, they were engaged in a streptomycin solution at 250 ppm for 20 minutes, renewing the solution after 10 minutes. Finish then with rinses in sterile water. All steps are performed in sterile FalconTM tubes and constant agitation in a rotator. After the disinfection process, the flower buds were transferred to sterile Petri dishes, where they were dissected to extract the petals and staminodes to be sown in the respective culture media. The disinfection process and all stages of culture and subculture were carried out in a laminar flow chamber to maintain the required sterile conditions.
Protocols
To address the embryogenic process, the developmental stages of somatic embryogenesis were taken as a reference: induction, expression, multiplication, maturation, germination, and plant development. The induction stage comprises the induction of callogenesis and embryogenic callus formation; in the expression stage, the formation of primary somatic embryos occurs; in multiplication, the formation of secondary embryos or repetitive embryogenesis is induced; in maturation, the embryo continues with the development of its apical and root poles with the extension of the hypocotyl; in germination, the conversion to a plantlet with photosynthetically active tissues is achieved.
To induce the embryogenic process and evaluate the development of the different stages, the protocols selected were those proposed by Li, Traore, Maximova, & Guiltinan (1998)18 (Protocol L), Maximova et al. (2002)19 (Protocol M), Fontanel et al. (2002)20 (Protocol F) and Henao et al. (2018)15 (Protocol H).
Induction stage
In the induction stage, the effect of the culture media (protocols) was evaluated during a culture time of 15 days in each medium for protocols L and M and 15, 30, 45 and 60 days for protocols F and H, on callogenesis like the number of explants with granular, filamentous, friable, and nodular callus. The culture media used were as follows: PCG [micronutrients, macronutrients and vitamins DKW, 2,4-D (1.98 mg/L), TDZ (0.005 mg/L), L-glutamine (250 mg/L), glucose (20000 mg/L) and Gellex (2700 mg/L)], SCG-1 [Gamborg Vitamins, 2,4-D (1.98 mg/L), kinetin (0.3 mg/L), coconut water (5%), Mc Cowns salts, glucose (20000 mg/L) and Gellex (2700 mg/L)], SCG-2 [Gamborg vitamins, 2,4-D (1.98 mg/L), BA (0, 3 mg/L), Mc Cowns salts, glucose (20000 mg/L) and Gellex (2700 mg/L)], INDI [micronutrients, macronutrients and vitamins DKW, 2,4-D (1 mg/L), kinetin (0.25 mg/L), glucose (30000 mg/L) and Gellex (3000 mg/L)] and INDILab [same as INDI with the addition of arginine (0.4355 g/L), glycine (0.1876 g/L), leucine (0.328g/L), lysine (0.4565 g/L), tryptophan (0.5105 g/L)].
Expression stage
In the expression stage, the culture medium, explant type and time in the induction medium were evaluated on (1) the total number of somatic embryos; (2) the number of embryos between globular and torpedo and the number of cotyledonary embryos; (3) the number of roots and (4) the number of necrotic explants at 15, 30, 45 and 60 days of culture. The following culture media were used: ED [micronutrients, macronutrients and vitamins DKW, sucrose (30000 mg/L), glucose (20000 mg/L), Gellex (2700 mg/L)] and INDIExp [micronutrients, macronutrients and vitamins DKW, glucose (30000 mg/L), Gellex (3000 mg/L)]. For the evaluation of repetitive embryogenesis at the expression stage, EM2 medium [MS macronutrients, DKW micronutrients and vitamins, sucrose (40000 mg/L) and Gellex (3000 mg/L)] was used.
Multiplication stage.
The repetitive embryogenic response of the CNCh-12 genotype was evaluated based on the protocols of Fontanel et al. (2002)20 and Li et al. (1998)18, which presented the best significant results after statistical analysis in primary embryogenesis in terms of embryos formed number. Regarding repetitive embryogenesis, a stage where a greater number of embryos per explant is obtained, primary embryos in the early cotyledonary stage were taken from culture media at the expression stage, ED and INDIexp media for the L and F protocols, respectively. The embryos were cut transversely into fragments and placed in CM2 repetitive embryogenesis induction medium in the case of protocol F. They were subsequently transferred to EM2 maturation medium and MM6 germination medium. In the case of protocol L, the embryo fragments are placed in a PCG induction medium for the induction of repetitive embryogenesis. They subsequently follow the process and media used in primary embryogenesis to finish MM6 maturation and germination medium.
For the induction of repetitive embryogenesis, CM2 medium [MS macronutrients, micronutrients and DKW vitamins, 2,4,5-T (1 mg/L), adenine (0.25 mg/L), glucose (30000 mg/L) and Gellex (3000 mg/L)] was used.
Maturation and germination stage.
In the embryo maturation and germination stage, the culture media PR and MM6 of protocols L and F were first evaluated on embryos with the renewal of the culture media every 30 days. In the second step, the response of embryos as stem height and root length to different concentrations of macronutrients was studied. Three different concentrations of macro MS salts were evaluated, ½ of concentration (½ C) = 2.115 g/l (Fontanel et al., 2002), ¼ of concentration (¼ C) = 1.0575 g/l and ⅕ of concentration (⅕ C) = 0.846 g/l. To continue with the conversion of embryos to plantlets, the effect of macronutrient concentration on stem and root length and the number of leaves formed on plantlets at 45 days was evaluated in embryos from EM2-MM6 (after remaining 15 days in MM6 medium).
The culture media used were: MM6Lab [equal to MM6 with different concentrations of NAA (0.1 mg/L)], MM6-1 (1) [MS macronutrients at 1/5 concentration, micronutrients and DKW vitamins, NAA (0.01 mg/L), GA3 (0.02 mg/L), activated carbon (1000 mg/L), glucose (40000 mg/L) and Gellex (3000 mg/L)], MM6-2 (2) [DM macronutrients at 1/4 concentration, DKW micronutrients and vitamins, NAA (0.01 mg/L), GA3 (0.02 mg/L), GA3 (0.02 mg/L), activated carbon (1000 mg/L), glucose (40000 mg/L) and Gellex (3000 mg/L)], MM6-3 (3) [DM macronutrients at 1/2 concentration, DKW micronutrients and vitamins, NAA (0.01 mg/L), GA3 (0.02 mg/L), GA3 (0.02 mg/L), activated carbon (1000 mg/L), glucose (40000 mg/L) and Gellex (3000 mg/L)] and PR [micronutrients, macronutrients and vitamins DKW, KNO3 (200 mg/L), sucrose (5000 mg/L), glucose (10000 mg/L), Gellex (2700 mg/L)].
For all experiments performed, the culture media were pH adjusted to 5.8 and autoclaved for 20 minutes at 121ºC.
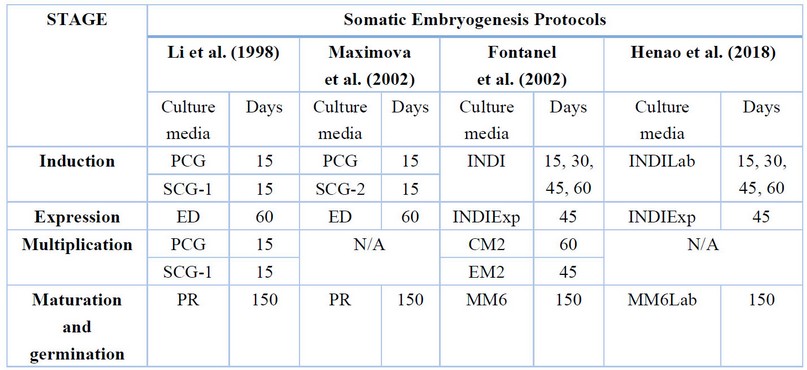
Table 1. Different culture media are used in each stage of the somatic embryogenesis process and the permanence of explants.
Culture conditions
All the cultures were randomly placed in a growth chamber at an average temperature of 28°C ± 2°C and 70% relative humidity and continuous darkness in induction, expression, and multiplication stages. In the maturation stage and germination, the early cotyledonary embryos and plantlets were cultured in 500 ml vessels and placed in a growth chamber under light with a 12-hour photoperiod and a photosynthetic photon flux density (PPFD) of 50 μmol m − 2 per second, at an average temperature of 28°C ± 2°C and 70% relative humidity.
Statistical analysis
For each of the protocols (treatments) evaluated, 5 replicates per explant (petal and staminode) were considered; the experimental unit is a Petri dish, in each of which 25 explants were sown. There were 10 Petri dishes per treatment, 1000 explants were planted per replicate, 8 replicates were performed for induction and expression, and 3 replicates to evaluate multiplication. In maturation, there were 3 replicates to assess the incidence of macronutrient concentration conversion with 5 replicates, each with 5 plants.
The data obtained from the experiments carried out to determine the effect of culture medium, explant type and culture time on the formation of embryogenic structures (factors) were analyzed using the R project statistical software. Compliance with the assumptions of normality and homoscedasticity of variance was verified; in cases where this was not achieved, nonparametric tests, Friedman's test, Wilcoxon's test and Welch's test were applied as appropriate. All tests were tested with 95% confidence intervals and a significance value of 0.05.
RESULTS
Induction stage
In this initial phase of the culture, it was found that the explants began to increase in size after one week, mainly the staminodes. From day 15 of the culture, callus growth was observed on the surface of the explants in all the protocols evaluated. The 4 most common callus types of the embryogenic process in cocoa were recorded: granular (GC), nodular (NC), filamentous (FC) and friable (FR), described by Henao et al. (2018) (Fig. 1A-1D).
Between INDI (Protocol F) and INDILab (Protocol H) culture media, there were no significant differences in callus formation. However, for the type of explants, callus formation was achieved in the staminodes at 15 days (p=0.030, p=0.006 and p=0.831) (Fig.S1). On the other hand, for SCG-1 (Protocol L) and SCG-2 (Protocol M) culture media, the result was similar; there were no significant differences between media, but important differences between explants (p<0.001), being higher callus formation in staminodes (Fig.S2).
Regarding the type of callus developed, in all protocols and in both explants, the formation of granular callus was similar between culture media and days of evaluation. Staminodes in INDI (Protocol F) and INDILab (Protocol H) media showed higher granular callus development concerning petals at days 30 and 45 (p=0.002 and p=0.001) (Fig.S3).
In the induction media of protocols L and M, the amount of nodular callus was not higher than 10%, no significant differences were found between them, but between explants, favoring the production of this type of callus in petals (p<0.001) (Fig.S4). The percentage obtained of filamentous and friable callus in protocols F and H did not exceed 0.5% and 5.3%, respectively; for protocols L and M it was no more than 0.07% and 0.22%, respectively. With these values, in some cases, it was not possible to perform an analysis of variance.
Expression stage
In the culture media used in the expression stage, normal and abnormal embryos were obtained. The external appearance of normal embryos agrees with that described by Li et al. (1998)18 and Henao et al. (2018)15 for other genotypes, with characteristics such as yellowish, matte white and translucent white color, with pink or white cotyledons and with defined embryonic axis, sometimes, the growth of embryos occurred in groupings or clusters (Fig. 1E-1G). Among the abnormalities found, trichotyledonary embryos were the most abundant, followed by multi-cotyledonary, mono-cotyledonary embryos (Fig. 1M-1O), without hypocotyl and without a defined embryonic axis. Trichome-like structures were also present on the cotyledons, as described by Maximova et al. (2002)19 (Fig. 1P). It should be emphasized that all embryos, normal and abnormal, were transferred to maturation media. In addition, the number of embryos obtained in the expression media of protocols F and H was analyzed considering the time the explants remained in the induction media.

Figure 1. Different callus types and embryonic stages were observed in the process of somatic embryogenesis for the CHCh-12 genotype. Granular callus (A), filamentous callus (B), friable callus (C), and nodular callus (D). Clusters of embryos in different callus types, granular from staminode, mixed and friable from petals (E-G). Different stages of embryo development globular and heart (H), torpedo (I), and early cotyledonary (J-K). Growth of somatic embryos on embryos with mature cotyledons (L). Abnormalities are presented by some somatic embryos, tricotyledonary (M), monocotyledonary (N), and multicotyledonary (O). Abnormal cotyledons with overgrowth and abundant trichomes in their structure (P).
For explants with 15 days in the induction medium and subculture in expression media, the medium of protocol F (p=0.016) has the highest production, an average of 22 embryos per explant compared to an average of 2 embryos obtained with protocol H. The difference between petals and staminodes was significant (p=0.005), obtaining with petals a higher formation of embryos (22 on average per explant), 11 times more than in staminodes (Fig. 2A). For the explants maintained for 30 days in the induction medium, the difference in total embryos formation was not significant. However, in the explant type, staminodes have a higher average number of embryos. 35 embryos (p=0.009) (Fig.S5A-B). This indicates that both petal and staminode are suitable explants for embryo expression in this genotype.
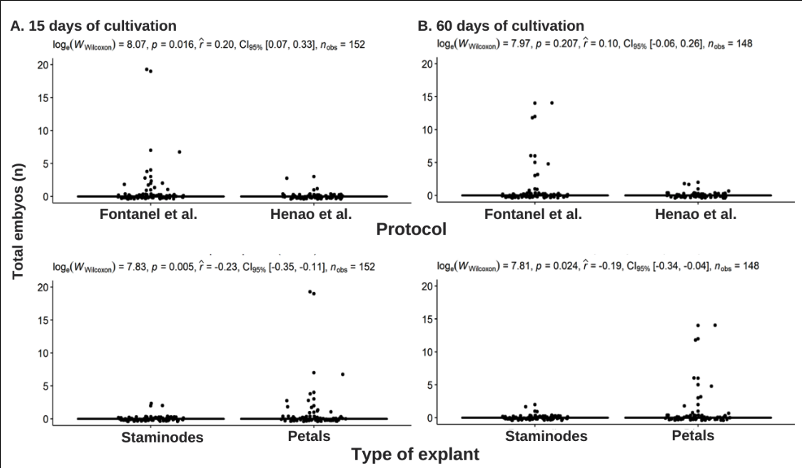
Figure 2. Effect of culture medium expression stage, explant type and time in induction medium on the total number of somatic embryos obtained in the Fontanel et al. (2002) (F) and Henao et al. (2018) (H) protocols. (A) 15 days of culture, (B) 60 days of culture.
In explants maintained for 45 days in the induction medium, total embryo production did not show significant differences between culture media or explants (Fig.S5C). The explants maintained for 60 days in induction did not show substantial differences in the total number of embryos formed in the expression media. Regarding the type of explant, callus from petals formed an average of 22 somatic embryos, with significant differences compared to those obtained from staminodes, with an average of one embryo (p=0.0024) (Fig. 2B).
Multiplication stage
The embryogenic response of the CNCh-12 genotype to repetitive embryogenesis was positive for both the F and L protocols, being statistically significant in embryo production (p-value = 0.011 and 0.002, respectively). A higher embryo production was obtained in repetitive embryogenesis concerning primary embryogenesis. In the case of repetitive embryogenesis, 4.7 embryos were obtained using protocol L, compared to 6.4 obtained with protocol F. Also, with protocol L the embryo formation was uniform per explant, while with protocol F embryo production was discontinuous over time (Fig. 3).
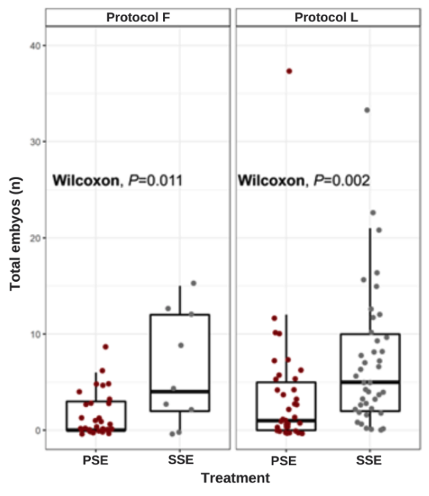
Figure 3. Effect of culture protocol F (Fontanel et al. (2002)) and protocol L (Li et al. (1998) on the induction of primary and repeat or secondary embryogenesis measured as a total number of somatic embryos.
Maturation and germination stage
Initially, after one month of being transferred to the maturation media, MM6 (Protocol F) and MM6Lab (Protocol H), the embryos continued with the development of the cotyledons and elongated, some reaching 1 cm in height. By the second month, the formation of the main root, the consolidation of the cotyledons and the growth in the height of the embryos were evident. In the third month, for both protocols, the development of leaf primordia and cotyledon fell, defined apices, and developed root systems were observed in some embryos (Fig. 4B), with secondary roots and root hairs. By the fourth month, true leaves were present, with plantlets up to 6 cm in protocol F (Fig. 4A) and 2 cm in protocol H. In both protocols, the persistence of cotyledons was present in some plantlets.
Complete plants were obtained in vitro in protocols F (Fig. 4A-C) and H with a germination percentage in the fifth month of 52.83% and 40.42%, respectively. Regarding explant type, both petal and staminode complete plants were obtained with a conversion percentage of up to 54% and 21%, respectively. Between protocols and between explant types, no significant differences were found in the number of embryos advancing in their development. Some of the embryos with abnormalities continued to develop to full plant, and abnormal germinated embryos were also obtained with characteristics such as overgrowth of the cotyledons, atrophied apices, or absence of roots (data not shown).
Embryos at the maturation stage on PR medium (Protocols L and M), did not show root development or true leaves and cotyledon growth was slow. By the fourth month on maturing medium, embryos had not exceeded 1 - 3 cm in height (Fig. 4E) and some with true leaves (Fig. 4D). Embryos from both protocols were completely necrotic (Fig. 4F).

Figure 4. Maturation stage. Plants on MM6 maturation medium (A-C) from protocol F (Fontanel et al. (2002)). Single embryo with true leaf growth on PR maturation medium belonging to protocol M (Maximova et al. (2002)) (D). Growing embryos in PR medium from protocol L (Li et al. (1998) (E). Necrosis of explants from protocols M (Maximova et al. (2002)) and L (Li et al. (1998)) in PR medium after four months (F).
On the other hand, for the growth and development of the plantlets, two types of embryos were considered, embryos coming from the EM2 medium and embryos coming from the transition of the EM2-MM6 medium. In embryos from EM2 medium, the effect of the salt concentration of the MS medium on embryo development was found to be significant after 45 days of culture (p<0.0001) (Fig.S6). The highest growth 2.33 cm in length, was obtained in the culture medium 1/5 C salts. When the culture medium contained ¼ C the length was 1.64 cm and with ½ C was 1.43 cm. Regarding root length, significant differences were found in growth after 45 days (p<0.0001) (Fig.S7), 3.64 cm of root length was the highest root growth with ¼ C, with 1/5 C was 2.87 cm and 1.40 cm with ½ C was.
In treatment 2, embryos from the transition between EM2-MM6 media, there was no significant difference in elongation between the 3 concentrations evaluated after 45 days of culture (p=0.75) (Fig.S8). The average measurements for 1/5 C, ¼ C and ½ C were 1.86 cm, 2.27 cm and 1.91 cm, respectively. Root growth was evident in the three treatments at this stage, having significant differences among them (p<0.0001) (Fig.S9), with ½ C having the highest growth with an average of 3.77 cm, followed by 3.71 cm for ¼ C and 3.49 cm for the ½ C. For the number of true leaves, there were significant differences between treatments after 45 days (p=0.00058) (Fig.S10)., having plants with an average of 2.34 leaves in 1/5 C, 1.69 in ¼ C and 1.57 in ½ C. Therefore, embryo growth is favored in the mentioned parameters in the MS medium at 1/5 C.
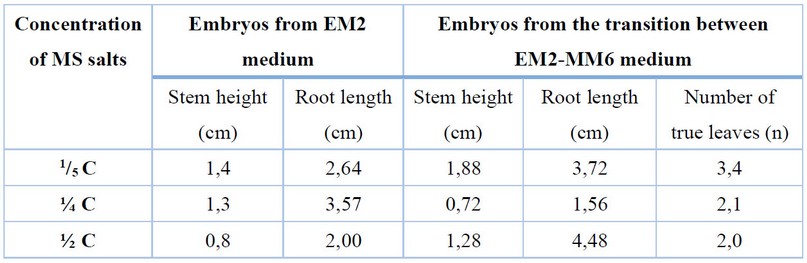
Table 2. Effect of salt concentration of MS medium (1/5 C, ¼ C, ½ C) on plantlet development after 45 days of culture.
Plantlets with ³2 cm of height with the root system (primary and secondary roots) and more than one true leaf were adapted to ex vitro conditions (Fig. 5A). They were initially sown in magentas containing a 1:3 sand: coconut fiber mixture, moistened with Hoagland solution 1:10 and with the addition of 1 g of mycorrhiza per planting well (Fig. 5B). Subsequently, they were covered and placed in a climatic chamber (conditions previously mentioned) for 40 days, with irrigation every 2 days and fertilization once a week (Fig. 5C) and were uncovered after 20 days. The plants were then transferred to the Yariguies farm in Barrancabermeja, Santander, where the adaptation process continued (Fig. 5D-E). After 1 month of adaptation, the survival rate was 89.4%, and after 2 months, it was 71.2%.

Figure 5. Ex vitro adaptation process. Plants in MM6 medium with desirable characteristics initiate the adaptation process (A). Newly planted plants of the CNCh-12 genotype after remaining in ideal conditions in the climatic chamber and maturation medium (B-C). Plants recently transferred to the Yariguies farm and were fully exposed to the environment (D) and two months after the transfer (E).
DISCUSSION
Staminodes excelled in their response to callus formation, mainly because of the short time they took to form. This behavior was observed in all the protocols studied and was repetitive for the culture times. This result agrees with that described by Díaz, Sánchez, Valera-Leal, & García (2015)21, where more excellent callus formation is reported in staminodes in Venezuelan cocoa genotypes. It is possible that the structure of the petals, being concave, does not allow total contact with the culture medium, and this undoubtedly influences the response to the callusing process, initiating the development on the surfaces in direct interaction with the culture medium, taking longer to develop completely, the opposite happens in the staminodes, where most of it is in contact with the medium. This result in the callogenesis stage could lead to suggest the exclusive use of cocoa staminodes as described by Guillou et al. (2018)10, Li et al. (1998)18 and Maximova et al. (2002)19; however, the embryogenic response of explants with slow callus growth, such as petals, should not be ruled out, as some genotypes have developed a greater number of embryos in petals with respect to staminodes22.
In protocols, F and H, contrary to what was described by Henao et al. (2018)15, complete regeneration of embryos of the CNCh-12 genotype was achieved, and the average number of embryos at 15 days exceeded those reported at 30 days of evaluation. The embryogenic tissues in the present study were not dedifferentiated like the previously reported ones. This response is because cocoa crops are subject to changing climatic conditions, and some variables, such as the stages of development of flower buds, tree age, and environmental factors, among others, were not evaluated in this study. It is not excluded that these variables had a different incidence on the results of both studies, even if they were of the same genotype.
The protocol developed by Li et al. (1998)18 and the modifications made by Maximova et al. (2002)19 have been widely used in the primary and secondary somatic embryogenesis of different cocoa genotypes. With these protocols, it has been possible to evaluate parameters such as in vitro culture efficiency, response to other exogenous agents such as antioxidants, optimal concentrations of growth regulators and their effects, among others. CNCh-12 genotype with the L and M protocols formed somatic embryos on the second week in the expression medium, a result that does not coincide with that described by Li et al. (1998)18 and Maximova et al. (2002)19. Most embryos originated from white granular callus, against the results described by Boutchouang et al. (2016)23 and Kone et al. (2019)24, who found that most embryogenic structures came from friable callus. The difference obtained in embryo formation between these two protocols has as a possible explanation for the use of kinetin in the SCG-1 medium; this is supported by the results of Kouassi, Kahia, Kouame, Tahi, and Koffi (2017)25, who evaluated four elite genotypes of Ivory Coast and found that in culture media enriched with kinetin, more embryogenic callus formation was presented than in those supplemented with 6-Benzylaminopurine (BAP). In this same sense, García et al. (2016)26 suggest that depending on the genotype evaluated, kinetin or BAP can be used in this stage of the embryogenic process.
The abnormalities presented by some of the embryos can be attributed to the presence in the induction media of the auxin 2,4-dichloro phenoxy acetic acid (2,4D) and the thidiazuron (TDZ). These have been considered to cause increased proportions of abnormal embryos in different species27,28, particularly in cocoa, Garcia et al. (2016) describe that depending on the genotype evaluated and the concentration of 9.05 uM of 2,4D it is possible to induce the formation of abnormal embryos.
Endogenous signals, such as phytohormones present in the tissue, and exogenous signals, such as added growth regulators, stress (temperature, light, mechanical damage), explant type-dependent tissue sensitivity or combinations of several of these factors, can result in tissue dedifferentiation and callus formation, root formation or somatic embryo development. In this study, the root was obtained on the explant, and a similar result was described by Botero et al. (2015)29, who obtained root formation in embryogenic callus induced under dark conditions for the species Psychotria ipecacuana. These responses may obey the same principle explained by Fehér (2014)30, who, in his compilation of several studies using Arabidopsis tissues, demonstrated that auxin and cytokinin-enriched media induce the formation of a particular callus characterized by a gene expression pattern similar to root meristems and also that the early stages of callus development are similar to those of lateral root development even when the process are induced in explants from aerial organs.
A problem in vitro cocoa culture is the browning of callus and embryos by exudation of phenolic compounds, and autocatalytic and light-driven processes 31. Activated charcoal has been widely used in somatic embryo production and germination processes by various researchers for its ability to adsorb growth inhibitory substances in culture media, it has been shown that in liquid or semi-solid media, it decreases the concentration of phenolic compounds and oxidants32. Phenolic compounds are abundant in the genotype studied, CNCh-12; taking this into account, the results obtained lead us to think that at this stage of development, phenolic substances accumulate in greater proportion, inhibiting or weakening the normal development of the embryos.
Furthermore, sucrose is used as a carbon source in the PR medium; Thomas (2008)32 also states that the autoclaving of this carbohydrate results in a growth inhibitor compound called 5-hydroxymethyl-furfural, which is eliminated by absorption thanks to the activated carbon. Due to the above, apart from the concentrations of phenolic compounds in the PR medium, there could be the formation of other inhibitory compounds in the medium that do not allow the development of somatic embryos. On the other hand, the MM6 and MM6Lab media have GA3 (gibberellic acid) in their composition, which in species such as Sesamum indicum and Panax ginseng, favors the conversion of somatic embryos to plantlets (Gaj 2004)33. The slow growth observed in the cotyledons in the PR medium could be the effect of the lack of growth regulators in the culture medium.
Embryos with high presence of trichomes on mature cotyledons (Fig. 1P) did not develop complete plants, similar to that reported by Iracheta-Donjuan et al. (2019)34, who describes that globular embryos with the presence of trichomes did not continue development to the cotyledonary stage. In the present study, the formation of embryos from other embryos (Fig. 1L and 1N) in the maturation medium reflects repetitive embryogenesis, as described by Maximova et al. (2002)19.
The most plausible causes of the difference between our results with those of other researchers may lie first in the contrast of the genotypes evaluated, reiterating that the genetic information of each one of them is decisive in the embryogenic response26,35. Second, not all explants nor all culture media are suitable to induce embryo formation; for this to happen, each genotype has different requirements of carbon sources, growth regulators, vitamins, and minerals, among others and differential sensitivity of their tissues25,36–38.
CONCLUSIONS
This study describes for the first time the induction of primary and repetitive embryogenesis up to the production of nursery-adapted plantlets of the Colombian regional elite genotype CNCh-12. To produce CNCh-12 plantlets, the protocol of Li et al. (1998)18 is effective up to the germination stage, where the culture media of the protocol of Fontanel et al. (2002)20 allows obtaining plantlets with better characteristics for the subsequent process of growth in nursery and planting in the field. It was validated that the optimal explant for the embryogenic process is the staminodium for shorter response time and the complete culture cycle until obtaining plantlets is at least 4 months, and it was found that, for this genotype, as in other cases, the total number of somatic embryos obtained in the process of secondary or repetitive embryogenesis is greater than that obtained in primary embryogenesis. In addition, the ex vitro adaptation process is successfully carried out with high survival percentages for plants with desirable characteristics.
Supplementary Materials: The following are available online, Figure S1: Effect on induction stage of culture medium, explant type and time in induction medium on the total number of callus obtained in Fontanel et al. (2002) (F) and Henao et al. (2018) (H) protocols at 15, 30 and 45 days of culture, Figure S2: Effect on induction stage of culture medium, explant type and time in induction medium on the total number of callus obtained in Maximova et al. (2002) (M) and Li et al. (1998) (L) protocols at 15, 20, 25 and 30 days of culture, Figure S3: Effect on induction stage of culture medium, explant type and time in induction medium on the total number of granular callus obtained in Fontanel et al. (2002) (F) and Henao et al. (2018) (H) protocols at 15, 30, 25 and 45 days of culture, Figure S4: Effect on induction stage of culture medium and explant type on the total number of nodular callus obtained in Maximova et al. (2002) (M) and Li et al. (1998) (L) protocols, Figure S5: Effect on the stage of expression of the culture medium, type of explant and time spent in the induction medium on the total number of somatic embryos obtained in treatments F and H. Where S4-S8 corresponds to 15 days (A), S6-S7 corresponds to 30 days (B), S2-S5 corresponds to 45 days (C) and S1-S3 to 60 days (D), Figure S6: Stage I for genotype CNCh-12. Embryo length from EM2 medium in the different treatments. T1: ⅕ MS salts, T2: ¼ MS salts, T3: ½ MS salts, after 45 days of culture on MM6 medium, Figure S7: Stage I for genotype CNCh-12. Root length of embryo from EM2 medium in the different treatments. T1: ⅕ MS salts, T2: ¼ MS salts, T3: ½ MS salts, after 45 days of culture on MM6 medium, Figure S8: Stage II for genotype CNCh-12. Embryo length from EM2-MM6 medium in the different treatments. T1: ⅕ MS salts, T2: ¼ MS salts, T3: ½ MS salts, after 45 days of culture on MM6 medium., Figure S9: Stage II for genotype CNCh-12. Root length of embryo from EM2-MM6 medium in the different treatments. T1: ⅕ MS salts, T2: ¼ MS salts, T3: ½ MS salts, after 45 days of culture on MM6 medium, Figure S10: Stage II for genotype CNCh-12. Number of true leaves of the embryo from EM2-MM6 medium in the different treatments. T1: ⅕ MS salts, T2: ¼ MS salts, T3: ½ MS salts, after 45 days of culture on MM6 medium.
Author Contributions: Conceptualization, Sandra Marcela Macias Naranjo; formal analysis, Sandra Marcela Macias Naranjo, Ana Maria Henao Ramirez; methodology, software, investigation, resources, data curation, writing—original draft preparation, Sandra Marcela Macias Naranjo; writing—review, editing, and supervition, Ana Maria Henao Ramirez, Aura Inés Urrea Trujillo. All authors have read and agreed to the published version of the manuscript.
Funding: This research was funded by General Royalties System - Science, Technology, and Innovation Fund with the Center of Agrobiotechnological Development and Innovation– CEDAIT- BPIN 2016000100060, National Planning Department, Office of the Governor of Antioquia, Universidad de Antioquia, Universidad Católica de Oriente and Compañía Nacional de Chocolates.
Acknowledgments: We would like to thank David Borrego for his statical and advice, as well as laboratory of Plant Physiology and Plant Tissue Culture of the Universidad de Antioquia. Granja Yariguíes – Compañia Nacional de Chocolates.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Argout X, Salse J, Aury JM, et al. The genome of Theobroma cacao. Nature Genetics. 2011;43(2):101-108. doi:10.1038/ng.736
2. Nair KPP. Cocoa (Theobroma Cacao L.).; 2010. doi:10.1016/b978-0-12-384677-8.00005-9
3. Diby L, Kahia J, Kouamé C, Aynekulu E. Tea, Coffee, and Cocoa. Encyclopedia of Applied Plant Sciences. 2016;3:420-425. doi:10.1016/B978-0-12-394807-6.00179-9
4. Rizzuto L, Díaz K. El mercado mundial de cacao. Revista agroalimentaria, ISSN 1316-0354, No 18, 2004, pags 48-60. 2004;10.
5. ICCO. Quarterly Bulletin of Cocoa Statistics, Vol. XLIV, No. 3, Cocoa Year 2017/18.; 2018.
6. Ministerio de Agricultura y Desarrollo Rural (MADR) -Dirección de Cadenas Agrícolas y Forestales. Cadena de Cacao.; 2021. Accessed September 30, 2022. https://sioc.minagricultura.gov.co/Cacao/Documentos/2020-03-31 Cifras Sectoriales.pdf
7. Abbott P, Benjamin T, Burniske G, et al. Análisis de La Cadena Productiva Del Cacao En Colombia.; 2019. doi:10.13140/RG.2.2.10934.14400
8. Winkelmann T. Somatic Versus Zygotic Embryogenesis: Learning from Seeds BT - In Vitro Embryogenesis in Higher Plants. In: Germana MA, Lambardi M, eds. Springer New York; 2016:25-46. doi:10.1007/978-1-4939-3061-6_2
9. Ajijah N, Syafaruddin, Inoue E. Cacao (Theobroma cacao L.) somatic embryos development under heat stress condition. IOP Conference Series: Earth and Environmental Science. 2020;418:12012. doi:10.1088/1755-1315/418/1/012012
10. Guillou C, Fillodeau A, Brulard E, et al. Indirect somatic embryogenesis of Theobroma cacao L. in liquid medium and improvement of embryo-to-plantlet conversion rate. In Vitro Cellular & Developmental Biology - Plant. 2018;54(4):377-391. doi:10.1007/s11627-018-9909-y
11. Henao-Ramírez AM, Urrea-Trujillo AI. Somatic Embryogenesis for Clonal Propagation and Associated Molecular Studies in Cacao (Theobroma cacao L.). Agricultural, Forestry and Bioindustry Biotechnology and Biodiscovery. Published online 2020:63-102. doi:10.1007/978-3-030-51358-0_5
12. Urrea Trujillo AI, Atehortúa Garcés L, Gallego Rúa AM. Regeneración vía embriogénesis somática de una variedad colombiana élite de Theobroma cacao L. Revista Colombiana de Biotecnología; Vol 13, Núm 2 (2011). Published online 2011. https://revistas.unal.edu.co/index.php/biotecnologia/article/view/27916
13. Garcia C, Marelli JP, Motamayor JC, Villela C. Somatic Embryogenesis in Theobroma cacao L. Methods in Molecular Biology. 2018;1815:227-245. doi:10.1007/978-1-4939-8594-4_15
14. Rojas L, Rúa A, Gil A, Londoño J, Atehortúa L. Monitoring accumulation of bioactive compounds in seeds and cell culture of Theobroma cacao at different stages of development. In Vitro Cellular & Developmental Biology - Plant. 2015;51:174-184. doi:10.1007/s11627-015-9684-y
15. Henao Ramírez AM, de la Hoz Vasquez T, Ospina Osorio TM, Garcés LA, Urrea Trujillo AI. Evaluation of the potential of regeneration of different Colombian and commercial genotypes of cocoa (Theobroma cacao L.) via somatic embryogenesis. Scientia Horticulturae. 2018;229(October):148-156. doi:10.1016/j.scienta.2017.10.040
16. Driver J, Kuniyuki AH. In vitro propagation of Paradox Walnut root stock. HortScience: a publication of the American Society for Horticultural Science. 1984;18:506-509.
17. Mcgranahan G, Driver J, W T. Tissue Culture of Juglans. In: Vol 26. ; 1987:261-271. doi:10.1007/978-94-017-0992-7_19
18. Li Z, Traore A, Maximova S, Guiltinan MJ. Somatic embryogenesis and plant regeneration from floral explants of cacao (Theobroma cacao L.) using thidiazuron. In Vitro Cellular & Developmental Biology - Plant. 1998;34(4):293-299. doi:10.1007/BF02822737
19. Maximova S, Alemanno L, Young A, Ferriere N, Traore A, Guiltinan M. Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis of Theobroma Cacao L. In Vitro Cellular & Developmental Biology - Plant. 2002;38:252-259. doi:10.1079/IVP2001257
20. Fontanel A, Gire-Bobin S, Labbé G, et al. In vitro multiplication and plant regeneration of Theobroma cacao L. via stable embryogenic calli. 10 IAPTC Congress. Plant Biotechnology. Published online 2002:23-28.
21. Díaz A, Sánchez D, Valera-Leal J, García A. Formación de embriones somáticos en cinco cultivares de Theobroma cacao L. cultivados en Venezuela. Biotecnología Vegetal. 2015;15:27-34.
22. Kouassi M, Koffi K, Oumar S, Tahi M, Toure M, Konan E. Comparison of systems combining auxins with thidiazuron or kinetin supplemented with polyvinylpirrolidone during embryogenic callus induction in three Theobroma cacao L. genotypes. International Journal of Biological and Chemical Sciences. 2018;12:804. doi:10.4314/ijbcs.v12i2.15
23. Boutchouang R, Olive F, Tchouatcheu GA, Niemenak N. Influence of the position of flowers buds on the tree on somatic embryogenesis of cocoa (Theobroma cacao L.). International Journal of Plant Physiology and Biochemistry. 2016;8:7-16. doi:10.5897/IJGMB2016.0247
24. Kone D, Kouassi M, N'Nan-Alla O. Induction of somatic embryos of recalcitrant genotypes of Theobroma cacao L. Journal of Applied Biosciences. 2019;133:13552. doi:10.4314/jab.v133i1.7
25. Kouassi M, Kahia J, Kouame C, Tahi M, Koffi K. Comparing the Effect of Plant Growth Regulators on Callus and Somatic Embryogenesis Induction in Four Elite Theobroma cacao L. Genotypes. HortScience. 2017;52:142-145. doi:10.21273/HORTSCI11092-16
26. Garcia C, Corrêa F, Findley S, et al. Optimization of somatic embryogenesis procedure for commercial clones of Theobroma cacao L. African Journal of Biotechnology. 2016;15(36):1936-1951. doi:10.5897/ajb2016.15513
27. Dewir YH, Nurmansyah, Naidoo Y, Teixeira da Silva JA. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Reports. 2018;37(11):1451-1470. doi:10.1007/s00299-018-2326-1
28. Garcia C, Furtado de Almeida AA, Costa M, et al. Abnormalities in somatic embryogenesis caused by 2,4-D: an overview. Plant Cell, Tissue and Organ Culture. 2019;137(2):193-212. doi:10.1007/s11240-019-01569-8
29. Botero Giraldo C, Urrea Trujillo AI, Naranjo Gómez EJ. Potencial de regeneración de Psychotria ipecacuanha (Rubiaceae) a partir de capas delgadas de células. Acta Biológica Colombiana. 2015;20:181-192.
30. Fehér A. Somatic embryogenesis — Stress-induced remodeling of plant cell fate. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms. 2014;1849. doi:10.1016/j.bbagrm.2014.07.005
31. Pancaningtyas S. Study on the presence and influence of phenolic compounds in callogenesis and somatic embryo development of cocoa (Theobroma cacao L.). Pelita Perkebunan (a Coffee and Cocoa Research Journal). 2015;31:14-20. doi:10.22302/iccri.jur.pelitaperkebunan.v31i1.81
32. Thomas TD. The role of activated charcoal in plant tissue culture. Biotechnology advances. 2008;26(6):618-631. doi:10.1016/j.biotechadv.2008.08.003
33. Gaj MD. Factors Influencing Somatic Embryogenesis Induction and Plant Regeneration with Particular Reference to Arabidopsis thaliana (L.) Heynh. Plant Growth Regulation. 2004;43(1):27-47. doi:10.1023/B:GROW.0000038275.29262.fb
34. Iracheta-Donjuan L, Cruz-López LA, López-Gómez P, Avendaño-Arrazate CH, Ortíz-Curiel S. 2iP Y BRASINOSTEROIDES PROMUEVEN LA INDUCCIÓN DE LA EMBRIOGÉNESIS SOMÁTICA EN Theobroma cacao L. AGROProductividad. 2019;12:65+.
35. Ajijah N, Hartati RS, Rubiyo R, Sukma D, Sudarsono S. EFFECTIVE CACAO SOMATIC EMBRYO REGENERATION ON KINETIN SUPPLEMENTED DKW MEDIUM AND SOMACLONAL VARIATION ASSESSMENT USING SSRs MARKERS. AGRIVITA, Journal of Agricultural Science; Vol 38, No 1 (2016): FEBRUARYDO - 1017503/agrivita.v38i1619 . Published online January 8, 2016. http://agrivita.ub.ac.id/index.php/agrivita/article/view/619
36. Ajijah N, Hartati R. PRIMARY AND SECONDARY SOMATIC EMBRYOGENESIS OF CACAO: THE EFFECT OF EXPLANT TYPES AND PLANT GROWTH REGULATORS. Indonesian Journal of Agricultural Science. 2020;20:69. doi:10.21082/ijas.v20n2.2019.p69-76
37. Issali A, Abdoulaye T, Koffi K, N'goran J, Sangaré A. Characterization of Callogenic and Embryogenic Abilities of Some Genotypes of Cocoa ( Theobroma cocoa L.) Under Selection in Cote d`Ivoire. Biotechnology. 2008;7. doi:10.3923/biotech.2008.51.58
38. Quainoo A, Dwomo A. The Effect of TDZ and 2, 4-D Concentrations on the Induction of Somatic Embryo and Embryogenesis in Different Cocoa Genotypes. Journal of Plant Studies. 2012;1. doi:10.5539/jps.v1n1p72
Received: October 23, 2022 / Accepted: January 15, 2023 / Published:15 February 2023
Citation: Macias-Naranjo, SM.; Henao-Ramírez, AM.; Urrea-Trujillo, AI. Propagation of the Colombian genotype of cacao (Theobroma cacao L.) CNCh-12 by somatic embryogenesis. Revis Bionatura 2023;8 (1)26. http://dx.doi.org/10.21931/RB/2023.08.01.16
