2020.05.03.18
Files > Volume 5 > Vol 5 No 3 2020
REVISION/REVIEW
Antiviral and healing potential of Sambucus nigra extracts

Michalina Bartak1, Agata Lange2, Anna Słońska1, Joanna Cymerys*1
Available from: http://dx.doi.org/10.21931/RB/2020.05.03.18
ABSTRACT
Nowadays, the application of alternative methods instead of clinical treatment creates a new possibility to prevent the development of diseases. Medicinal plants such as Sambucus nigra have been well known due to their extraordinary properties. The similarity to synthetic substances makes it potentially dependable; however, a high concentration of cyanogenic glycosides may exert detrimental consequences. It has been documented that Sambucus nigra extracts are used against both human and animal viruses, like influenza A and B viruses, human immunodeficiency virus (HIV), dengue virus (DENV-2), human herpesvirus type 1 (HSV-1) and human coronavirus NL63 (HCoV-NL63). Such reports are notably valuable especially considering the widespread usage of commercial drugs, which could be ineffective. This review provides insight on recent research on the health properties of plant Sambucus nigra as an antiviral medication that may help propose new therapy.
Keywords. Sambucus nigra, extracts, antiviral, healing
INTRODUCTION
One of the fundamental issues of general biology and present knowledge of the philosophy of nature is the problem of demarcation of nonliving and living matter. The definition of viruses is similar - they can be categorized as a connection between mortuus et vivus materia 1.
Coping with viral diseases using commercial drugs is difficult. They penetrate living cells and alter their metabolism using specific enzymes. It creates a possibility to replicate. The majority of synthetic drugs is precisely directed against the replication process. The main difficulty of antiviral cure is a high potential of getting synthetic substances resistance.
What is more, our knowledge of healing with antiviral drugs is not up to the standard level yet. As a result, finding alternative treatment methods became common 2. Using plants as medicine have long been known 3. Nowadays, there is a widespread increase in using natural pharmaceutics, which is defined as a less expensive source of healing 4. Worldwide estimates prove that 70-80% of people rely on natural medicine because of its sufficient actions in healthcare. However, the topic of plant medicine is not flawless. Sometimes it may come from access to plants’ material, including proper living conditions and some other dependencies 5.
This study is aimed to better understand the health properties of plant Sambucus nigra as an antiviral medication that may help propose new therapy.
Sambuca nigra
Sambucus nigra L. is a part of Adoxaceae family (following Integrated Taxonomic Information System) 6 in the northern hemisphere, especially in Europe, Northern America, and Western Asia 7,8. There are three subspecies of Sambucus nigra L.: S. nigra L. ssp. nigra, S. nigra L. ssp. canadensis, S. nigra L. ssp. Cerulean 9.
Sambucus nigra is a 7- 10 meters high bush or rarely a small tree with a small white flower gathered in cyme (Figure. 1) 7. The flowering season falls in June and July, but it usually happens in its third or fourth year. The leaves with total length in 30 cm are accumulated in 5-7 pair 10. Both flowers and leaves (during rubbing) have a specific aroma, which can be found as an unpleasant 11. The elders’ fruits are firstly green and elongated, but during ripening, they become roundish black and shiny berrylike drupes. Each drupe contains 3-5 seeds inside. The seeds’ maturation is connected with the mean October temperature, which is required to be approximately 7°C 12.
Black elder prefers soil based on nitrogen and calcium compounds. Despite its predilection for alkaline soil, it is also able to grow on a different type of soil even with a pH ranging between 4,2 and 8,0 10,12.
Figure 1. Sambucus nigra – flowers, berries, and leaves (Source: Amedee Masclef – Atlas des Plantes de France, 1891.
Constituents
Sambucus nigra’s chemical composition is associated with plenty of factors - climate and manner of agriculture alike 12. Both extracts from fruits and flowers have shown a high possibility of reducing viral infections symptoms 13.
Crucial constituents of black elder are RIPs (ribosomal inactivating proteins), which (beside lectins) are part of S. nigra agglutinins (SNAs). RIPs found in S. nigra fruits aim for specific cells or substances which many pathogens bind. They show higher potential than RIPs from other plants. Other antiviral substances present in black elders are peptic polysaccharides through their availability to activate Macrophages 13. The main substances are present in Table 1.
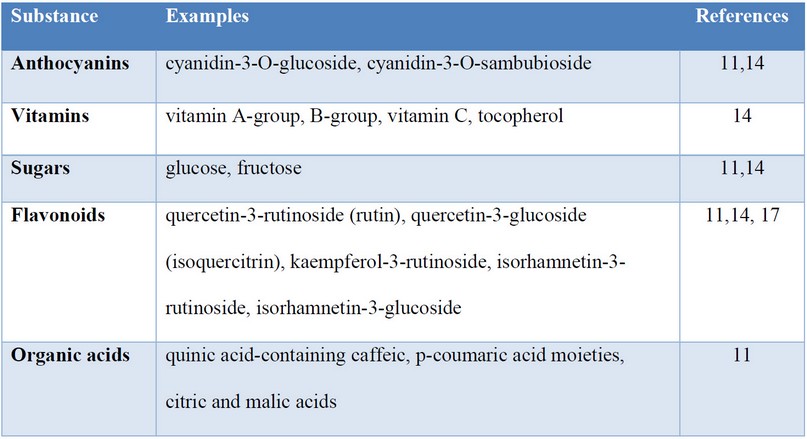
Table 1. The most abundant substances in Sambucus nigra (Source: as listed in table).
Flowers
The flowers’ extract is full of bioactive flavonoids and phenolic acids 14. Through the Schmitzer et al. study 11, there were identified major flavonoids: flavonol glycosides rutin, kaempferol-3-rutinoside, and isorhamnetin-3-rutinoside.
Listed substances constitute 90% of total flavonoid concentration in elderflowers. In the case of phenolic acids, 70% of them were 5-caffeoylquinnic acid and 1,5-di-caffeoylquinnic. Using products from the black elder as medicine is supported by its electrochemical activity, which comparable to 21g ascorbic acid per kg dry flowers 11.
Listed substances constitute 90% of total flavonoid concentration in elderflowers. In the case of phenolic acids, 70% of them were 5-caffeoylquinnic acid and 1,5-di-caffeoylquinnic. Using products from the black elder as medicine is supported by its electrochemical activity, which comparable to 21g ascorbic acid per kg dry flowers 11.
Fruits
The main products from black elders show the same properties, such as ones used in medical aid. Most of them are anthocyanins that are known for their health-related features, including antiviral and antibacterial activity, reducing oxidative stress and, as a result – free radicals. Among anthocyanins, cyanidin-3-O-glucoside and cyanidin-3-O-sambubioside were identified. S. nigra fruits consist of different variety of sugars with a total content from 68.53 to 104.16 g per kg fresh weight. The central part of this group is occupied by glucose and fructose. Furthermore, organic acids, such as critic and malic acid, are also established in berries. Their concentration in S. nigra berries has been observed six times higher than in apples 11.
Application of Sambucus nigra
There are plenty of purposes of using S. nigra even in everyday life, beginning in nutrition. The extracts from fruits are standard in the production of juice, jellies, or wine. However, there are a lot of other properties used in medicine (Figure. 2). Tea from fruits is intended for the treatment of colds and its’ symptoms such as high temperature 11. Abundant in tannins and polyphenols extracted from the S. nigra shows antioxidant properties, resistance to U.V. radiation, and high biological activity, which can be a base of users in cosmetology 15,16.
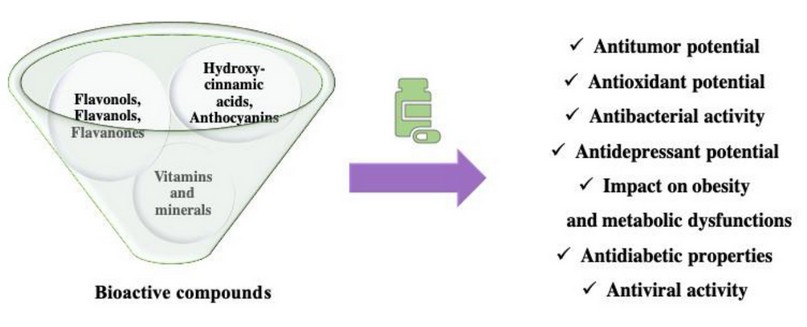
Figure 2. The bioactive compounds found in elderberry (own work based on 9)
What is more, the blackberries are valued in folk medicine. The infusion of blossom is popular for diaphoretic and diuretic properties7,8,17. The black elder is associated with the immune system; it modules the production of proinflammatory cytokines such as IL-6, TNF-α 15,18.
S. nigra extracts are also useful in healing diabetics or obesity. It is related to glucose metabolism in the human organism and insulin uptake. Additionally, S. nigra decreases cholesterol and lipids levels which may cause a weight loss 13,19.
Disadvantages of usage Sambucus nigra
As we mentioned in the introduction, using plant extract can have disadvantages. The main disadvantage in the case of S. nigra is the risk of allergenicity. That effect is due to the high amount of cyanogenic glycosides (CGG), which are present in all parts of S. nigra. Among them, sambunigrin and prunasin occur the most frequently (Fig. 3). The content of cyanogenic compounds might have health consequences manifested in poisoning 12. The mechanism of cyanide’s toxicity relies on inhibition of oxidative phosphorylation caused by cytochrome oxidase, which stops electron transport. As a result, adenosine triphosphate (ATP) synthesis cannot proceed, and cellular respiration has to be halted 20.
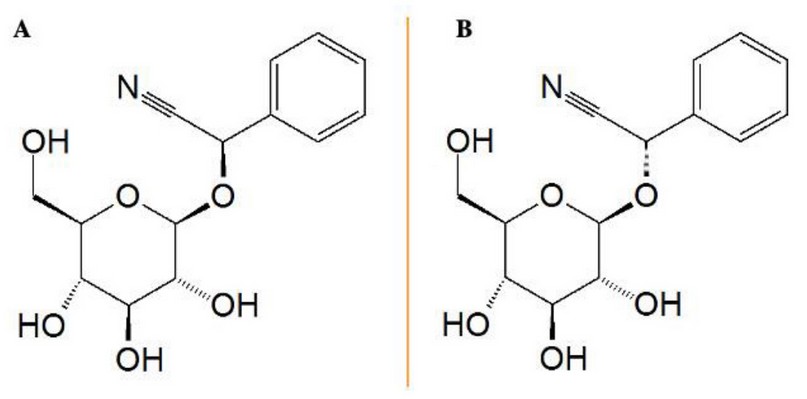
Figure 3. (A) Chemical structure of Prunasin [(R)-Prunasin], and (B) Sambunigrin [(S)-Prunasin] own elaboration based on PubChem. (Source: https://pubchem.ncbi.nlm.nih.gov/compound/Sambunigrin#section=2D-Structure,
https://pubchem.ncbi.nlm.nih.gov/compound/Prunasin#section=Structures).
Cyanogenic glycosides may be harmful to animals and humans when implemented between 0,5 and 3,5 mg per kg body weight. It is also known that there exists a possibility to reduce the high level of cyanide during boiling, fermentation, and drying. Despite processing methods that cause a decrease of the cyanide, the human body can only detoxicate it when the low level is present 20.
What is more, Forster-Waldl et al.21 in clinical trials showed detection of a protein of 33.2 kDa by IgE in patients’ sera. That is why S. nigra is supposed to contain the allergen Sam n1 which is a representative of RIP (ribosomal inactivating protein)21.
Summarizing, excessive ingestion of black elders may result in the digestive system’s diseases such as vomiting and diarrhea 13,22. The solution to the toxicity problem of the black elder is appropriate preparation, which may minimize its harmful effects 13.
Antiviral properties of Sambucus nigra extracts
As we described in the introduction, the history of usage of plant extracts is well known. The therapeutic use of plants as a remedy is dated back to 5000 B.C 5. Nowadays, a significant amount of S. nigra extracts are adopted as homeopathy medicines as well as pharmaceuticals. It is embedded in Slavic and European countries’ culture and history 23, 24. There are many documented examples of therapeutic use on several human and animal viruses 25.
Influenza virus
Influenza viruses, the members of the Orthomyxoviridae family, are enveloped negative-strand RNA viruses with segmented genomes containing seven to eight gene segments. They are divided into three types (A, B, and C), which differ in host range and pathogenicity. Influenza A infects a wide variety of hosts, including birds, swine, horses, humans, and other mammals, and it causes a seasonal epidemic. In humans, the virus causes an acute respiratory disease characterized by the sudden onset of high fever, cough, headache, and inflammation of the upper respiratory tract 26. Influenza A is classified based on surface glycoproteins: hemagglutinin (H.A.), which binds to host cell sialic acid conjugated glycoproteins and neuraminidase (N.A.), essential for viral release and propagation. There are 16 types of hemagglutinin (H1-H16) and nine types of neuraminidase (N1-N9) 27.
Even though S. nigra extract has already been used repeatedly for treating colds and influenza, the antiviral mechanisms of the elderberry extract are still under investigation 28,29,30,31. The clinical study presented by Kong 28 showed that administration of the elderberry extract to patients presenting flu symptoms significantly relieve influenza‐like symptoms within 24 hours from the administration of the first dose. According to recent studies, it can be assumed that flavonoids in elderberry can inhibit the H1N1 influenza virus infection in vitro by binding to the surface of the virus28. S. nigra constituents cause the deactivation of hemagglutinin and thus prevent the virus from entering and replicating in the host cell. The flavonoids in elderberry can also bind to neuraminidase and cause its inactivation; however, confirmation of this hypothesis requires additional research 28.
In a recent paper, Ulbericht et al. 25, provides the Natural Standard Evidence-Based Validated Grading Rationale™ of the clinical bottom line of S. nigra. The grading scale presents levels from A (strong scientific evidence) to F (strong contrary scientific evidence). Clinical trials on the impact of S. nigra for influenza virus have proven an excellent scientific basis for the use of S. nigra extracts to combat the disease caused by the virus, classifying it on level B (based on the criteria) 25.
In monography S. nigra by Throne Research (Biotech & Pharma; USA) 9, authors provided the example of Zakay-Rones 31 research, in which two randomized, placebo-controlled, double-blind studies, demonstrated the extract (Sambucol) that effectively inhibited influenza A and B strains after 48 hours post-infection symptoms. In the earlier study, 27 individuals experiencing common early flu symptoms were given Sambucol or placebo daily for three days – 2 tablespoons (children) or four tablespoons (adults). Patients were followed for six days, and symptoms were monitored. Serum from all subjects was analyzed for antibodies to influenza type A and B at the initial dose and during the convalescent phase. In the treatment group, significant improvement in flu symptoms was observed in 93.3 percent of subjects within two days after initial dosing, while 91.7 percent of the control group demonstrated improvement after six days. A complete resolution was achieved in the treatment group in 90 percent of patients after 2-3 days, while the placebo group yielded similar results after six days. Of these 27 patients, 23 had laboratory confirmation of influenza type B 31. The mechanism is believed to be rendering viruses nonfunctional by staining and coating them. According to a review, in vitro as well as animal research reported that elderberry fruit (Sambucci fructus) affected influenza, other viral infections, and increased antibody titers 30.
Other studies have proved that elderberry extract may affect the immune system by enhancing the production of cytokines by monocytes 32,33,34. Torabian et al.35, in their recent research, indicated that the immunomodulatory property of elderberry extracts manifested by increased expression of IL-6, IL-8, and TNF 35.
S. nigra has also inhibiting properties against avian influenza virus H9N2. Research presented by Karimi et al. .36 showed antiviral properties of the mixture of Echinacea and elderberry extract on the avian influenza virus. The extracts tested caused a reduction of the load of the virus and thus could be used as a valuable aid to control avian influenza in poultry fields 36.
In conclusion, elderberry extract shows multiple modes of therapeutic action against influenza infection, mainly by suppressing viral entry, affecting the post-infection phase, and viral transmission from cell to cell. Further research is still needed to define the antiviral activity of each of the active ingredients contained in the S. nigra. Only then will the treatment be fully effective 13.
Human immunodeficiency virus
Human immunodeficiency virus (HIV) belongs to the Lentivirus genus of the family of Retroviridae. The genome of the virus is composed of two copies of positive-sense single-stranded RNA that codes for the nine genes, including the reverse transcriptase. HIV infects a variety of immune cells such as CD4+ T cells, macrophages, and dendritic cells and causes acquired immunodeficiency syndrome (AIDS) 37.
Recent studies indicate that the elderberry extract also has activity against HIV, and it can effectively block the ability of HIV virions to infect host cells 37,38. Antiviral effectiveness show flavonoids and A-type proanthocyanidins (PACs) contained in S. nigra extract. These compounds bind to viral envelope glycoproteins, likely to the gp120, and block HIV entry into and infection of host cells likely through the CD4/CCR5 receptor system 39.
Interestingly in other cases, after application of the boiled extract of Sambucol (with Glucosamine sulfate, chondroitin, and commercial product-Thymate), a decrease of viral particles was observed from 39,000 particles/mL to an undetectable amount in ten days after the administration of a mixture 9. Moreover, S. nigra extract (Sambucol) was tested for the potential to inhibit the infectivity of HIV isolates in CD4+ cell lines, peripheral blood lymphocytes, and laboratory HIV strains. S. nigra extract, prepared in two different dilutions, was incubated with the virus before adding to cells. A significant decrease in virus infectivity was observed, and no virus load could be detected after four- or nine-days post-incubation 40. Other studies show that the combination of elderberry extract and thymus extract has reduced the viral load in HIV patients. Unfortunately, studies do not sufficiently confirm the effect of this treatment method 9.
The identified compounds of elderberry extract, mainly flavonoids, which inhibit HIV infection 41, could be a new therapeutic target for HIV-1/AIDS, which shows promise for co-therapy uses with existing HIV-1 antiviral agents.
Herpes simplex type 1
Herpes simplex virus type 1 (HSV-1), the member of the Alphaherpesviridae subfamily, causes a contagious infection that affects approximately 1/3 population of the world population 42. HSV-1 has a double-stranded linear DNA genome that is approximately 152 kbp in length, and it is an etiological agent of orofacial blisters, keratitis, pneumonia, or encephalitis. Following primary infection in the mucosal epithelia, virions migrate to the trigeminal ganglion neurons, where the latent infection is established.
The influence of Sambucol against HSV-1 was examined in the cell line of human diploid fibroblasts. In their research, Morag et al. .42 have used four HSV-1 strains – a reference strain, two acyclovir-resistant strains, and a strain isolated from a patient. Complete inhibition of viral replication was observed in all utilized strains, whether the cells were pre-incubated with the extract, simultaneously incubated with extract, or the extract was added 30 minutes after viral adsorption to cells. The complete inhibition of four strains of HSV-1 in vitro by elderberry extract warrants further clinical trials in humans 42. A formula of S. nigra (flower extract) in combination with Hypericum perforatum and Saponaria officinalis was also found to inhibit the replication of HSV-1 in vitro 29. Among the described flavonoids of S. nigra, the kaempferol and quercetin show the most promising activity against HSV- 1 43.
Dengue virus
Another virus that is claimed to be cured with S. nigra extract is the dengue virus. Dengue virus (DENV) is a single positive-stranded RNA virus, which belongs to the family Flaviviridae and genus Flavivirus. DENV is a mosquito-borne, and it causes a wide range of clinical manifestations, from mild fever to potentially fatal dengue shock syndrome 44. Effective anti-dengue therapeutic drugs, as well as protective vaccines, have still not been developed 45.
The antiviral effect of various flavonoids obtained from various plants on the dengue virus has already been repeatedly confirmed 46,47,48. S. nigra methanolic extracts apart from flavonoids also contain alkaloids and small amounts of coumarins which exhibit anti-DENV-2 activity. In their research, Castillo-Maldonado et al. 45. revealed that methanolic extracts of leaves and flowers of S. nigra. have anti-DENV-2 properties when added to Vero and BHK-21 cell lines. The best results were obtained when DENV-2 was pre-incubated with the extracts for 1 hour and then added to the cell cultures. They additionally confirmed this result by analyzing the synthesis of NS-1 and the extent of intracellular DENV-2 45.
Human coronavirus NL63
Human coronavirus NL63 (HCoV-NL63) is one of the common HCoVs species that occur worldwide. HCoV-NL63 belongs to the genus of Alphacoronavirus in the Coronaviridae family. The viral genome is positive-sense, single-stranded RNA. HCoV-NL63 infects the upper respiratory tract causing runny nose, cough, and sore throat and also infects the lower respiratory tract (pneumonia, bronchiolitis). Considering this, HCoV-NL63 is a significant pathogen, which is an etiological factor of mild and severe respiratory diseases and even acute undifferentiated febrile illness (AUFI). Preclinical studies by Weng et al. 49 show promising results by demonstrating that elderberry ethanol extract inhibits replication and attachment of HCoV-NL63. Researchers investigated that among phenolic acid constituents in plant ethanol stem extract (chlorogenic acid, caffeic acid, coumaric acid, ferulic acid) caffeic acid in Sambucus spp. Inhibits replication and attachment in human airway epithelial cells. Other phenolic acid components that showed prominent antiviral action was chlorogenic acid and gallic acid 49. More importantly, caffeic acid affects the binding of HCoV-NL63 to co-receptors (such as heparan sulfate proteoglycans) and the receptor (ACE2), the same that novel pathogenic SARS-coronavirus 2 utilizes 49,50. SARS-CoV-2 appeared in 2019 in Wuhan, China.
In contrast to several members of the coronaviruses that continuously circulate in the human population, SARS-CoV-2 has very high infectivity and causes the severe acute respiratory syndrome. Recent research indicates that SARS-CoV-2 also uses the ACE2 receptor to enter cell 51. It is worth noting that unraveling which viruses use cellular factors during the replication cycle, including entry, can be used in antiviral therapy. Currently, there is no effective vaccination or therapy that could be used to treat COVID-19. For that reason, the finding presented by Weng et al. could help develop antivirals against human coronaviruses.
Infectious bronchitis virus
Infectious bronchitis virus (IBV) is of the species avian Coronavirus, which belongs to the family Coronaviridae. The viral genome is positive-sense single-stranded RNA 52. IBV infects the respiratory tract of chickens and causes the deformation of produced eggshell, thus causing economic losses to the poultry industry 53. Due to the highly recombinant nature of the virus, current vaccination strategies are not sufficient against new infections, and for that reason, new methods defending against IBV are needed. In their work, Chen et al. 54 investigated the influence of three plant species: Rhodiola rosea, Nigella sativa, and Sambucus nigra on avian IBV replication. Among these plants, S. nigra presented the best results of inhibition in the early step of the infection cycle. More precisely, the results from these experiments revealed that combining pre-V (the only virus was treated before infection) treatment with post-treatment worked together to inhibit IBV replication fully. The pre-C (only cells were treated with extract before infection) treatment was not necessary for full virus inhibition, nor did it impact the viral load of the supernatant. However, it did work synergistically with pre-V treatment to reduce viral load in the cells an additional three orders of magnitude, as compared to pre-V treatment alone. Probably, the bioactive compounds are lectins (Elder bark agglutinin I (SNA-I), Elder bark agglutinin II (SNA-II), Sambucus nigra agglutinin-III (SNA-III), from Sambucus nigra that could bind directly to viral proteins and inhibit infection. What is more, authors suspect that two flavonols isolated from Sambucus nigra fruits (same, which affect influenza: 5,7,3’,4’-tetra-O-methylquercetin and 5,7-dihydroxy-4-oxo-2-(3,4,5-trihydroxyphenyl)chroman-3-yl-3,4,5 trihydroxycyclohexanecarboxylate 55) could also have an inhibitory impact on IBV replication 54.
CONCLUSION
Ancient knowledge of the antimicrobial properties of various plants’ extracts provides an authoritative source for new antiviral drugs, which can be successfully used instead of synthetic medicine. Because of its chemical composition, S. nigra is a plant that finds its contribution in widespread use. S. nigra is rich in phenolic acids, flavonoids, catechins, and proanthocyanidins, and these compounds in addition to antiviral properties, they also show anti-cancer, immune-stimulating, antibacterial activity, antioxidant and antidepressant potential. The increasing resistance of viruses to commonly used drugs forces the development of new therapeutic methods that are not based on synthetic chemical compounds. In the present review, we provided insight on recent research on the health properties of plant S. nigra is an antiviral medication that may help propose new therapy.
The search for new antiviral therapies is especially important nowadays when humanity is struggling with the SARS-CoV-2 pandemic. Both influenza virus and Sars-CoV-2 have an RNA genome and cause respiratory disease. Moreover, there is preclinical research showing that S. nigra inhibits replication and viral attachment of Human coronavirus NL63 (HCoV-NL63), which similar to SARS-CoV-2, belongs to the coronavirus family 49. Among the above examples of antiviral applications, most reports refer to the use of S. nigra extracts in the treatment of diseases caused by the influenza A and B viruses. This is probably related to the high variability of influenza virus, as a result of which the flu vaccine is reformulated each season for a few specific influenza strains and is usually effective against three or four types of influenza activity in the world during that season. Based on evidence that S. nigra could be used in the treatment of influenza, it is tempting to speculate that it could also be applicable in the treatment of COVID-19. However, this hypothesis requires thorough research and confirmation.
The antiviral effect of S. nigra has also been documented against herpes simplex virus, human immunodeficiency virus, dengue virus, or infectious bronchitis virus. The possibility of full-value application of extracts S. nigra is not currently used. Despite this, it is worth noting that it has excellent potential for antiviral use due to its good tolerability and low extraction costs in contrast to synthetic drugs.
Conflict of interest
No conflict of interest is declared.
REFERENCES
1. Ślaga SW. Odrębność Żywej Materii Na Przykładzie Wirusów. Roczniki Filozoficzne, Filozofia Przyrody 1963; 87-108.
2. Sohail MN, Rasul F, Karim A, Kanwal U, Attitalla IH. Plant as a source of Natural Antiviral Agents. Asian Journal of Animal and Veterinary Advances 2011;6(12):1125-1152.
3. Sornpet B, Potha T, Tragoolpua Y, Pringproa K. Antiviral activity of five Asian medicinal pant crude extracts against highly pathogenic H5N1 avian influenza virus. Asian Pacific Journal of Tropical Medicine 2017;10(9):871–876.
4. Hafidh RR, Abdulamir AS, Jahanshiri F, Abas F, Abu Bakar F, Sekawi Z. Asia is the Mine of Natural Antiviral Products for Public Health. The Open Complementary Medicine Journal 2009;1:58-68.
5. Pushpa R, Nishant R, Navin K, Pankaj G. Antiviral Potential of Medicinal Plants: An Overview. International Research Journal of Pharmacy 2013;4(6):8-16.
6. Retrieved [June 3, 2020], from the Integrated Taxonomic Information System on-line database, http://www.itis.gov. https://www.itis.gov/servlet/SingleRpt/SingleRpt?search_topic=TSN&search_value=525081#null
7. Novak FA. Wielki Atlas Roślin. Polskie wydawnictwo rolnicze i leśne. Warszawa, 1979;394-395
8. Tundis R, Ursino C, Bonesi M, Loizzo MR, Sicari V, Pellicanò T, Manfredi IL, Figoli A, Cassano A. Flower and Leaf Extracts of Sambucus nigra L. Application of Membrane Processes to Obtain Fractions with Antioxidant and Antityrosinase Properties. Membranes 2019;9:127.
9. Monography’ Sambucus nigra (Elderberry). Alternative Medicine Review. 2005;10(1):51-55.
10. Szweykowska A, Szweykowski J. Słownik botaniczny, Wiedza Powszechna, Warszawa, 2003;69.
11. Schmitzer V, Veberic R, Stampar F. European elderberry (Sambucus nigra L.) and American Elderberry (Sambucus canadensis L.). Botanical, chemical and health properties of flowers, berries and their products. Berries: Properties, Consumption and Nutrition 2012;127-148.
12. Charlebois D, Byers PL, Finn CE, Thomas AL. Elderberry: Botany, Horticulture, Potential. Horticultural Reviews 2010;37:213-280.
13. Porter R, Bode R. A Review of the Antiviral Properties of Black Elder (Sambucus nigra L.). Products: Antiviral Properties of Black Elder (Sambucus nigra L.). Phytotherapy Research 2017;31. DOI: 10.1002/ptr.5782
14. Kaack K, Christensen LP. Phenolic acids and flavonoids in tea processed from flowers of black elder (Sambucus nigra) stored in different packing materials. European Journal of Horticultural Science 2010;75(5): 214-220.
15. Sidor A, Gramza-Michałowska A.Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food – a review. Journal of Functional Foods 2014. DOI: 10.1016/j.jff.2014.07.012
16. Stoilova I, Wilker M, Stoyanova A, Krastanov A, Stanchev V.Antioxidant activity of extract from elder flower (Sambucus nigra L.). Herba Polonica Jounral 2007;53.
17. Mikulic-Petkovsek M, Samoticha J, Eler K, Stampar F, Vebe R. Traditional Elderflower Beverages: A Rich Source of Phenolic Compounds with High Antioxidant Activity. Journal of Agriculture and Food Chemistry 2015, DOI: 10.1021/jf506005b.
18. Barak V, Halperin T, Kalickman I. The effect of Sambucol, a black elderberry-based natural product, on the production of human cytokines: I. Inflammatory cytokines. European Cytokine Network 2001;12(2): 290-296.
19. Knudsen BF, Kaack KV. A Review of Traditional Herbal Medicinal Products with Disease Claims for Elder (Sambucus nigra) Flower. Acta Horticulturae 2015;1061:109-120.
20. Bolarinwa IF, Oke MO, Olaniyan SA, Ajala AS. A Review of Cyanogenic Glycosides in Edible Plants. Toxicology - New Aspects to This Scientific Conundrum 2016. DOI:10.5772/64886.
21. Forster-Waldl E, Marchetti M, Scholl I, Focke M, Radauer C, Kinaciyan T, Jensen-Jarolim E. Type I allergy to elderberry (Sambucus nigra) is elicited by a 33.2 kDa allergen with significant homology to ribosomal inactivating proteins. Clinical & Experimental Allergy 2003; 33(12):1703–171
22. Senica M, Stampar F, Veberic R, Mikulic-Petkovsek M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. Journal of Science Food and Agriculture 2016;97(8):2623–2632.
23. Luczaj L, Szymanski WM. Wild vascular plants gathered for consumption in the Polish countryside: a review. Journal of Ethnobiology and Ethnomedicine 2007;3:17.
24. Tizio A, Luczaj LJ, Quave CL, Redzic S, Pieroni A. Traditional food and herbal uses of wild plants in the ancient South-Slavic diaspora of Mundimitar/Montemitro (Southern Italy). Journal of Ethnobiology and Ethnomedicine 2012;8:21.
25. Ulbricht C, Basch E, Cheung L, Goldberg H, Hammerness P, Isaac R, Khalsa K, Romm A, Mills E, Rychlik I, Varghese M, Weissner W, Windsor R, Wortley J.An Evidence-Based Systematic Review of Elderberry and Elderflower (Sambucus nigra) by the Natural Standard Research Collaboration. Journal of Dietary Supplements 2014;11:10.3109.
26. Palese P, Shah ML. Orthomyxoviridae: the viruses and their replication. In Fields Virology, ed. DM Knipe, PM Howley, Philadelphia: Lippincott, Williams &Wilkins 5th ed., 2007;1647–90.
27. Obenauer JC, Denson J, Mehta PK, Su X, Mukatira S, Finkelstein DB, Xu X, Wang J, Ma J, Fan Y, Rakestraw KM, Webster RG, Hoffmann E, Krauss S, Zheng J, Zhang Z, Naeve CW. Large-scale sequence analysis of avian influenza isolates. Science 2006; 311:1576–80.
28. Kong F. Pilot Clinical Study on a Proprietary Elderberry Extract: Efficacy in Addressing Influenza Symptoms. Online Journal of Pharmacology and PharmacoKinetics 2009;5:32-43.
29. Serkedjieva J, Manolova N, Zgorniak-Wowosielska I, et al. Antiviral activity of the infusion (SHS-174) from flowers of Sambucus nigra L., aerial parts of Hypericum perforatum L., and roots of Saponaria officinalis L. against influenza and herpes simplex viruses. Phototherapy Research 1990;4:97-100.
30. Vlachojannis JE, Cameron M, Chrubasik S. A systematic review on the sambuci fructus effect and efficacy profiles. Phytotherapy Research 2010;24(1):1–8.
31. Zakay-Rones Z., Varsano N, Zlotnik M, Manor O, Regev L, Schlesinger M, Mumcuoglu M. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. Journal of Alternative & Complementary Medicine 1995;1:361-369.
32. Ho GT, Ahmed A, Zou YF, Aslaksen TH, Wangensteen G, Barsett H. Structure–activity relationship of immunomodulating pectin’s from elderberries. Carbohydrate Polymers 2015;125: 314–322.
33. Ho GT, Zou YF, Aslaksen TH, Wangensteen G, Barsett H. Structural characterization of bioactive pectic polysaccharides from elderflowers (Sambuci flos). Carbohydrate Polymers 2016; 135:128–137.
34. Kinoshita E, Hayashi K, Katayama H, Hayashi T, Obata A. Anti-influenza virus effects of elderberry juice and its fractions. Bioscience, Biotechnology and Biochemistry 2012;76(9):1633–1638.
35. Torabian G, Valtchev P, Adil Q, Dehghania F. Anti-influenza activity of elderberry (Sambucus nigra). Journal of Functional Foods 2019;54:353-360.
36. Karimi S, Dadras H, Mohammadi A. The effect of the extracts of Echinacea purpurea and Sambucus nigra (black elderberry) on virus shedding in H9N2 avian influenza infected chickens. Iranian Journal of Veterinary Research 2014;3:256-261.
37. Gao F, Bailes E, Robertson DL, Chen Y, Rodenburg CM, Michael SF, Cummins LB, Arthur LO, Peeters M, Shaw GM, Sharp PM, Hahn BH. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 1999; 397: 436–441.
38. Fink R, Roschek B, Alberte RS. HIV type-1 entry inhibitors with a new mode of action. Antiviral Chemistry & Chemotherapy 2009;19:243–255.
39. Sahpira-Nahor O, Zakay-Rones Z, Mumcuoglu M. The effects of Sambucol® on HIV infection in vitro. Annual Israel Congress of Microbiology 1995; 6-7.
40. Mahmood N, Pizza C, Aquino R, De Tommasi N, Piacente S, Colman S, Burke A, Hay AJ. Inhibition of HIV infection by flavanoids. Antiviral Research 1993; 22(2–3):189–199.
41. Rechenchoski DZ, Faccin-Galhardi LC, Linhares REC, Nozawa C. Herpesvirus: an underestimated virus. Folia Microbiologica 2017; 62(2):151-156.
42. Morag AM, Mumcuoglu M, Baybikov T, et al. Inhibition of sensitive and acyclovir-resistant HSV-1 strains by an elderberry extract in vitro. Z Phytotheraphy 1997; 25:97-98.
43. Amoros M, Simões CM, Girre L, Sauvager F, Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. Journal of Natural Products 1992; 55(12):1732–1740.
44. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. The global distribution and burden of dengue. Nature 2013; 496:504-507.
45. Castillo-Maldonado I, Moreno-Altamirano MMB, Serrano-Gallardo LB. Anti-dengue serotype-2 activity effect of Sambucus nigra leaves-and flowers-derived compounds. Virology Research & Reviews 2017. DOI:10.15761/VRR.1000117.
46. Muliawan SY, Kit LS, Devi S, Hashim O, Yusof R. Inhibitory potential of Quercus lusitanica extract on dengue virus type 2 replication. Southeast Asian Journal of Tropical Medicine. Public Health 2006;37(3):132-135.
47. Sánchez I, Gómez-Garibay F, Taboada J, Ruiz BH. Antiviral effect of flavonoids on the dengue virus. Phototherapy Research 2000;14:89-92.
48. Tang LI, Ling AP, Koh RY, Chye S.M., Voon K.G. Screening of anti-dengue activity in methanolic extracts of medicinal plants. BMC Complementary Medicine and Therapies. 2012;12:3.
49. Weng JR, Lin CS, Lai HC, et al. Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Research 2019; 273:197767.
50. Sungnak W, Huang N, Bécavin C et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine 2020; 26:681–687.
51. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger, Nadine HT, Erichsen S, Schiergens T, Herrler G, Wu Nai-Huei & Nitsche A, Müller M, Drosten C, Pöhlmann S. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181:271–280.
52. Franzo G, Legnardi M, Tucciarone CM, Drigo M, Martini M, Cecchinato M. Evolution of infectious bronchitis virus in the field after homologous vaccination introduction. Veterinary Research 2019;50,92.
53. Sevoian M, Levine PP. Effects of infectious bronchitis on the reproductive tracts, egg production, and egg quality of laying chickens. Avian Diseases 1957; 1:136.
54. Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E., Pendleton A.R.: Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Veterinary Research 2014;10:24.
55. Roschek B, Fink RC, McMichael MD, Li D, Alberte R S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009; 70(10):1255–1261.
Received: 9 june 2020
Acepted: 12 july 2020
Michalina Bartak1 , Agata Lange2, Anna Słońska3, Joanna Cymerys*1
1Division of Microbiology, Department of Preclinical Sciences, Institute of Veterinary Medicine, Warsaw University of Life Sciences, Warsaw, Poland https://orcid.org/0000-0002-9872-3017
2Department of Nanobiotechnology and Experimental Ecology, Institute of Biology, Warsaw University of Life Sciences, Warsaw, Poland
3Division of Microbiology, Department of Preclinical Sciences, Institute of Veterinary Medicine, Warsaw University of Life Sciences, Warsaw, Poland https://orcid.org/0000-0002-9872-3017
*1Corresponding author: Joanna Cymerys Ph.D., Division of Microbiology, Department of Preclinical Sciences, Institute of Veterinary Medicine, Warsaw University of Life Sciences, Warsaw, Poland, Ciszewskiego 8, 02-768, Warszawa;
e-mail: [email protected] https://orcid.org/0000-0002-5273-3930
Phone number: +48 22 593 60 55
