2021.06.02.21
Files > Volume 6 > Vol 6 No 4 2021 > Vol 6 No 2 2021
Fermentation study of Cassava bagasse starch hydrolyzed's using INIAP 650 and INIAP 651 varieties and a strain of Lactobacillus leichmannii for the lactic acid production

Shirley Inguillaya, Felipe Jadánb and Pedro Maldonado-Alvaradoa*
Available from: http://dx.doi.org/10.21931/RB/2021.06.02.21
ABSTRACT
Ecuador is an agricultural country, which has an actual production of starch obtained from cassava. Tuber processing residues do not have an economic impact; for example, the cassava bagasse, only used for plant fertilization and animal feeding. This project aimed to study the influence of the fermentation variables (pH and agitation), on the lactic acid production. Enzymatic hydrolysis of cassava bagasse starch for the varieties INIAP 650 and INIAP 651 was performed using α-amylase and glucoamylase. Then, glucose was fermented by Lactobacillus leichmannii ATCC 7830 strains, varying conditions such as agitation (150 rpm and absence) and pH (4.5, 5.0, and 5.5). Finally, the determination of lactic acid was performed by potentiometric and FTIR analysis. Conversions of cassava bagasse to reduced sugars were 71.66 and 85.05 % for INIAP 650 and INIAP 651 varieties. The best lactic acid concentrations were 27.62 and 33.48 g/L, obtained at pH 5.5 and agitation, for INIAP 650 and INIAP 651 varieties. Qualitative analysis conducted by FTIR spectrophotometry confirmed the presence of lactic acid in the reacted products. Lactic acid production from cassava bagasse starch could contribute to the Manabí and Esmeraldas provinces of Ecuador's economic development.
Keywords: Cassava, Ecuador, bagasse starch, fermentation, Lactobacillus leichmannii, lactic acid.
INTRODUCTION
In 2016, the global market required 1 220 kton of lactic acid, with growing demand at a rate of 16.2 % per year. It is estimated that by 2025 these requirements will reach 2 000 kton, this would represent approximately 9.8 billion dollars of revenue for the global lactic acid-producing market1.
Nowadays, green methods take advantage of residues, or by-products of agroindustry, for their conversion to fuels or industrial interest products is on the rise. Lactic acid is used in food, pharmaceutical, chemical, and cosmetic industries 2. The production of lactic acid obtained from biomass rich in sugars, starch, or lignocellulosic material is relevant throughout the world due to its low production cost since the raw material used as a substrate comes from abundant and renewable resources 3,4. The production of biofuels and compounds of industrial interest, such as lactic acid, presents a limitation, the recovery of first-generation biomass to obtain these products, which is why it seeks to take advantage of the second generation biomass of various food species such as cassava, potato, corn, among others 2.
Starch is a polymer made up of two types of molecules, amylose, and amylopectin. Amylose is a linear polymer made up of about 500 - 2 000 glucose units assembled by α 1-4 glucosidic bonds. Amylopectin is a branched polymer made up of approximately 1 000 000 glucose units, grouped by α 1-4 glucosidic bonds and 5 % α 1-6 bonds in the branches. The proportion of each of these polymers depends on the starch's origin; for corn and cassava the amylose values range from 20 to 30 % and for amylopectin from 80 to 70 %, respectively 5,6.
Lactic fermentation is an anaerobic metabolic process that involves the use of lactic microorganisms. This process can be batch or fed-batch types. In fermentation, the temperature and pH conditions are specific for each microorganism. However, lactic acid production is not affected by the initial pH due to neutralizing agents such as calcium carbonate and ammonium nitrate.
The fungi and bacteria that are used in the fermentation of sugars and production of lactic acid are strains of homofermentative microorganisms that belong to the genera Kluyveromyces, Saccharomyces, Lactobacillus, and Rhizopus 7. Another study reports that Lactobacillus-type bacteria are appropriate for fermenting substrates rich in glucose, lactose, sucrose, and maltose 8. Lactobacillus delbrueckii is a homofermentative bacterium that does not develop spores, has tolerance to acidity and its fermentative metabolic route is strictly directed to the formation of D (-) lactic acid. The optimal growth conditions for the Lactobacillus delbrueckii strain, contained in Lactobacillus leichmannii ATCC 7832, are 37 ºC and a pH of 4 to 6 9. For processes with homofermentative bacteria, the conversion is 1 to 1 in g/L, respectively. The fermentation efficiency also depends on the initial concentration of carbohydrates, concentration of nitrogenous material, and the fermentation medium's agitation with ranges of 100 - 200 rpm 1,7.
Lactic acid or 2-hydroxypropanoic acid is a compound that has alcohol and carboxyl groups in its structure; it is obtained by chemical or biotechnological means 10. To chemically obtain the acid, a reaction occurs between acetaldehyde and hydrocyanic acid 11. There are two optical isomers: L (+) lactic acid, which is harmful because its consumption generates decalcification in living beings but is used for the production of polylactic acid, and D (-) lactic acid, which is considered as GRAS (food additive recognized as safe by the FDA (Food and Drug Administration) 12. Titration methods with detection carry out the quantification of lactic acid ranges from 8.10 to 20.83 g/L, by colorimetry analysis with detection thresholds from 0.3 to 10 g/L and gas chromatography with detection limits of 0.02 - 1.37 g/kg 13.
Studies have been carried out to obtain lactic acid with bakery waste using T. aotearoense, with yields of 78.4 g/L 14. Likewise, with the use of whey and L. delbrueckii and S. thermophillus, a production of 36.7 g/L of this acid was obtained 15. On the other hand, a lactic acid production of 19.17 g/L was achieved with sugarcane bagasse and L. pentosus 16. In another study, a production of 97.6 g/L of lactic acid was obtained using corn stubble and B. coagulans 17. Finally, with coffee mucilage, a fermentation efficiency of 68 % of this compound was obtained using a strain of L. bulgaricus 18.
Cassava (Manihot esculenta Crantz) is a tuber rich in complex carbohydrates, which grows mainly in tropical climates. In Ecuador, it is grown in the provinces of Manabí, Esmeraldas, Santo Domingo de los Tsáchilas, among others. For 2018, Manabí produced 13 819 000 kg of cassava of the INIAP 650 and INIAP 651 varieties 19. In Ecuador cassava is consumed fresh and in the form of starch 20. The root is 80 % made up of the tuber and 20 % of the cassava bagasse made up of the husk and bark. Cassava bagasse has a starch content of 50 to 60 %, cellulose 34 %, hemicellulose 15 %, and lignin 7 % 21–23.
Studies carried out in Colombia analyzed lactic acid production with 120 g/L of cassava starch and Lactobacillus strains grown from yogurt. In 3 days of fermentation at 37 ºC with the agitation of 100 rpm and pH of 5.0, was reached 51.15 g/L of lactic acid 24.
In April 2016, Ecuador suffered one of the strongest earthquakes in its history, with 7.6 on the Ritcher scale. The Technical Committee for the Reconstruction of the Manabí and Esmeraldas provinces was formed, focused on the productive reactivation of these areas (Unidad Digital de Pública FM, 2019) 25. PREDU 2016-015 project "Sustainable development of modified starches and products of industrial interest from cassava as an alternative for the productive reactivation of the Manabí and Esmeraldas provinces", contemplates the production of lactic acid from a hydrolyzed cassava bagasse starch of the varieties INIAP 650 and INIAP 651, with the use of a strain of Lactobacillus leichmannii ATCC 7830 in the fermentation process. For the first time in Ecuador, it seeks to generate residues from the benefit of cassava, for the production of lactic acid by a biotechnological way.
METHODS
Evaluation of the effect of pH and agitation in the fermentation process of starch hydrolyzate from cassava bagasse varieties INIAP 650 and INIAP 651
Raw material
The obtaining of cassava bagasse starch was carried out at the artisanal "Rallandería Bijahual" in the "Las Balsas" sector, located in Manabí. 90 kg of roots of the INIAP 650 and INIAP 651 varieties were extracted from the INIAP Portoviejo Experimental Station. The INIAP 650 variety comes from the MCol 2215 clone; it has a dry matter content of 37 % with a yield of 17 to 35 t/ha of fresh roots and a conversion rate from fresh to dry matter of 2 - 2.5 : 1 26. The variety INIAP 651 comes from the clone CM 1335-4; it has a dry matter content of 35.5% with a yield of 29 to 40 t/ha, and a conversion rate of 6 - 7.5 : 1 27.
The cassava root barks were separated and placed in a needle rack for grinding. With the help of a canvas-type cloth, the ground material was washed until the starch was released. The precipitate was collected and sun-dried at the extraction site's temperature (29 ºC) for 24 h 28.
Characterization of cassava bagasse starch
The properties of bagasse starch were determined employing a proximal analysis. The standardized norms indicated in Table 1. were used.
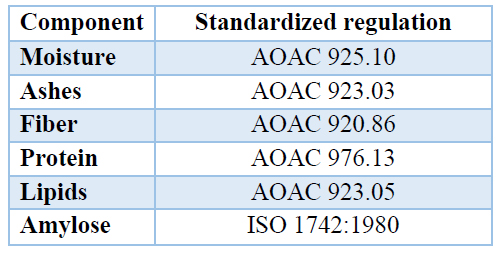
Table 1. Standards used in the proximal analysis.
Cassava bagasse starch was stored at temperatures between 0 - 4 ºC 29, for later use.
Hydrolysis of cassava bagasse starch
A 10 % of the starch/water (w/v) solution was prepared. The solution was heated at 65 ºC for 1 h at 150 rpm in a thermostatic bath 30. Subsequently, a liquefaction process was carried out with α-amylase from pig pancreas A3176-500KU, SIGMA-ALDRICH (USA) in a proportion of 5 μg of enzyme/mg of starch, dissolved in a phosphate buffer solution 400 mM at pH 7.1. Then, it was added NaN3 (0.02 %), CaCl2 (0.25 %), NaCl (0.013%) 31, it was allowed to react in a thermostatic bath for 24 h at 37 ºC and 100 rpm 32. Finally, a saccharification process was carried out with the amyloglucosidase enzyme from Aspergillus niger A7095-50ML, SIGMA-ALDRICH (USA), in a proportion of 0.025 μg of enzyme/mg of starch in 10 mL of a 0.5 M acetate buffer solution and pH 4.8 33. It was heated in a thermostatic bath for 48 h at 65 °C and 150 rpm 34.
After that, the hydrolyzate was stirred at 50 rpm for 12 h and 20 °C. Finally, it was centrifuged at 3000 rpm for 20 min 35.
Determination of reducing sugars in cassava bagasse starch hydrolyzate
The reducing sugars were determined with the colorimetric method by DNS (3.5-Dinitrosalicylic acid) 36. 0.5 mL of the hydrolyzed sample and 0.5 mL of the DNS reagent were placed in a test tube. It was stirred manually and heated in a boiling thermostatic bath for 5 min. Then, it was cooled at 18 °C, and 3.5 mL of distilled water was added. The absorbance was analyzed in a spectrophotometer (Shimadzu, UV-160 A (Brazil)), at a wavelength of 540 nm, and finally, the concentration of reducing sugars was calculated.
Inoculum activation and preparation
A Lactobacillus leichmannii ATCC 7830 strain from Microbiologics Company (USA) was used. A pellet of the microorganism was taken and placed in 10 mL of sterilized MRS agar at 121 ºC for 15 min (Microbiologics, 2018). The agar with the pellet was incubated in a thermostatic bath for 24 h at 37 ºC and 1 mL of this was taken and seeded deeply on MRS agar. The Petri dish with the sowing was incubated for 72 h at 37 ºC 37. To finish, a colony was taken from the agar, placed in 10 mL of MRS agar, vortexed, and incubated similarly at 37 ºC for 18 h 38.
Lactic fermentation
100 mL of cassava bagasse starch hydrolyzate were taken, and enriched with yeast extract 0.5 % (w/v), NH4Cl 0.5 % (w/v) and CaCO3 2.5 % (w/v). The pH was adjusted to 4.5, 5.0 and 5.5 with NaOH 4 % w/v or HCl 5 % w/v. Then, a volume of nitrogen was introduced at a rate of 2 cm3/s for 1 min, and finally, the inoculum at 2 % (v/v) previously prepared was added. The flasks with the substrate to be fermented were placed in an incubator and a thermostatic bath at 37 ºC, with and without shaking, for 120 h 15.
The input variables were: pH (4.5, 5.0, and 5.5) and agitation (150 rpm or absence), while the output variable was the lactic acid content. The performed treatments are detailed in Table 2.
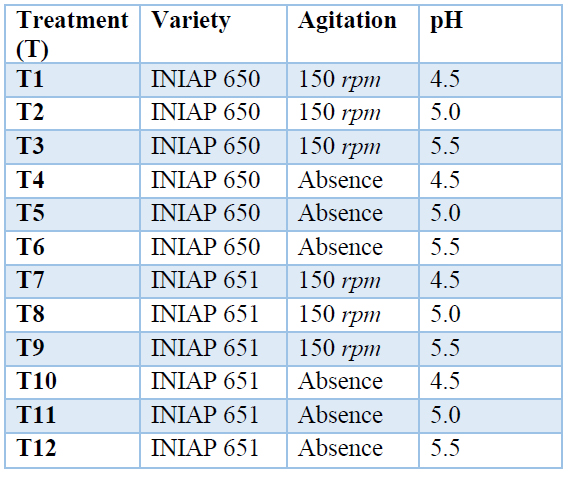
Table 2. Treatments for the production of lactic acid.
Evaluation of the yield and the final composition of lactic acid obtained from fermentation processes
Recovery of lactic acid
The lactic acid recovery was carried out by a hydrolysis and acidification process. All the fermented was filtered with a canvas. For all fermentation, H2SO4 1 M was added until reaching a pH of 2.0, pH at which CaSO4 precipitates and liberates lactic acid. This mixture was stirred for 30 min and centrifuged at 8000 rpm for 15 min. Lactic acid was contained in the supernatant and the CaSO4 precipitated 39. The following section specifies how the lactic acid content was determined
Determination of lactic acid content by acid-base titration
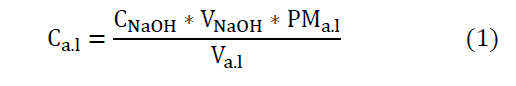
: Lactic acid content (g/L)
: Caustic soda concentration (mol/L): Caustic soda volume (mL)
: Lactic acid molecular weight (g/mol)
: Sample volume used for titration (mL)
Determination of the fermentative efficiency and characterization of lactic acid
The lactic acid solution density was measured with a Mettler Toledo (USA) 40 densimeter and the pH of the final solution containing lactic acid using a Hanna (USA) pH meter. The real fermentative yield was determined by the relationship between the lactic acid obtained and the amount of starch used as a substrate. A conversion of 1 glucose molecule to 2 lactic acid molecules was estimated for the theoretical fermentative yield.
Furthermore, the comparison between the spectrogram obtained by FTIR of the experimentation with a 98 % of lactic acid spectrogram was analyzed. The infrared spectrophotometry equipment used was a Perkin Elmer (USA) brand, Spectrum One 2370 model with a diamond cell. Three repetitions for each simple were performed.
Statistical analysis
The proximal analysis results, lactic acid concentration, optical density, fermentation efficiency, and pH were statistically analyzed with the StatGraphics Centurion XVIII X64 program using a multifactorial ANOVA followed by a Fisher test with a 95 % confidence level to determine statistically significant differences. Three repetitions were made for each treatment.
RESULTS AND DISCUSSION
Evaluation of the effect of pH and agitation in the fermentation process of starch hydrolyzate from cassava bagasse varieties INIAP 650 and INIAP 651
Characterization of cassava bagasse starch
For this study, cassava bagasse starch was used, the by-product of the cassava grating process, of INIAP 650 and INIAP 651 varieties. Table 3. shows the moisture, ash, fiber, protein, lipids, and amylose contents. The carbohydrates content was calculated as the difference in content with these parameters. It is understood that carbohydrates are mainly made up of amylopectin 41.
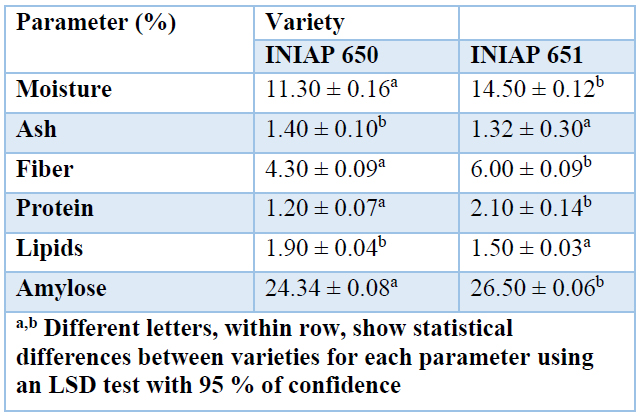
Table 3. Physicochemical characteristics of cassava bagasse starch INIAP 650 and INIAP 651 varieties.
INIAP 650 and INIAP 651 varieties moisture content was 11.30 % (w/w) and 14.50 % (w/w), respectively. A previous study reported the cassava tuber starch had a moisture content in a range of 11 - 13 % 42. The INIAP 650 variety was found within the indicated range, but the INIAP 651 variety surpassed these values, due to higher hygroscopy, at a rate of 3.30 g of water/g of starch.
The ash content in the cassava bagasse starch was 1.40 and 1.32 % for INIAP 650 and INIAP 651 varieties. A study showed the range for ash in cassava bagasse starch from 0.9 to 1.50 % 43. Another work points out values of 0.05 % (w/w) for cassava bagasse starch 42, 28 times less ash content concerning the amount obtained in our study. Conversely, previous work reported that cassava starch had 7 times less ash content than bagasse 44, due to minerals present in its composition 45.
The fiber present in the cassava bagasse starch was 4.30 and 6.00 % for INIAP 650 and INIAP 651 varieties, respectively. No data on the fiber content of bagasse starch was found.
However, the bibliography shows that the amount of fiber present in the cassava tuber starch is 0.30 %. This is due to the high concentration of cellulose present in cassava bagasse, ranging from 15 to 51 %. It is mainly considered a soluble fiber 43,46.
In lipid content, bagasse starch presented values of 1.90 and 1.50 % for INIAP 650 and INIAP 651 varieties, respectively. These values were similar to the range established for cassava starch's lipid material, which is 0.53 - 1.60 % 47. The average value of lipids of the tubers presented ranges from 2 to 5 %, and the lipid material of the cassava starch from the experimentation was lower but not far compared to the average value of cassava lipids. This may be due to different substrates being compared 22.
The amylose content presented 26.50 and 24.34 % values, respectively, for INIAP 651 and INIAP 650 varieties. The range of amylose in bagasse starch varies from 15.9 to 22.4 %, as reported by the literature 48. The varieties of the present study had high values compared to those mentioned in the bibliography. These varieties are species improved by the Center for Agricultural Research (INIAP) for starch production and the tuber's consumption, respectively 26,27.
Hydrolysis of cassava bagasse starch and determination of reducing sugars
Enzymatic hydrolysis using α-amylase and glucoamylase showed conversion levels of cassava bagasse starch to reducing sugars of 71.66 and 85.05 % for INIAP 650 and INIAP 651 varieties, respectively. It should be noted that part of these reducing sugars is the glucose chains that Lactobacillus used. Similar results to this study were reported by the literature 49, with conversion yields from 81 to 109 %, from starch to reducing sugars. Another study stated 95 % conversion from starch to reducing sugars with α-amylase and glucoamylase 8.
Conversions greater than 100 % are standard because the enzymatic hydrolysis process adds one molecule of water for each broken bond and increases the hydrolyzed starch weight. Besides, amylose and amylopectin long chains have n glucose chains. When these chains are hydrolyzed, they break into n glucose, maltose, and dextrins that increase quantification concentration, reducing sugars 24.
Evaluation of the yield and final composition of lactic acid obtained from fermentation processes
Determination of lactic acid content
The multifactorial ANOVA statistical analysis determined that the agitation and the pH within the fermentation of the hydrolyzed cassava bagasse starch of INIAP 650 and INIAP 651 varieties presented significant influence in the response variable (Value-P < 0,05), the lactic acid produced.
Table 4. shows the results of the lactic acid concentration, quantified by titration. Statistically significant differences between the studies treatments were found. Treatment T9 presented higher lactic acid production (33.48 ± 1.87 g/L), which corresponds to the fermentation of the hydrolyzed cassava bagasse starch of INIAP 651 variety pH 5.5 with stirring of 150 rpm.
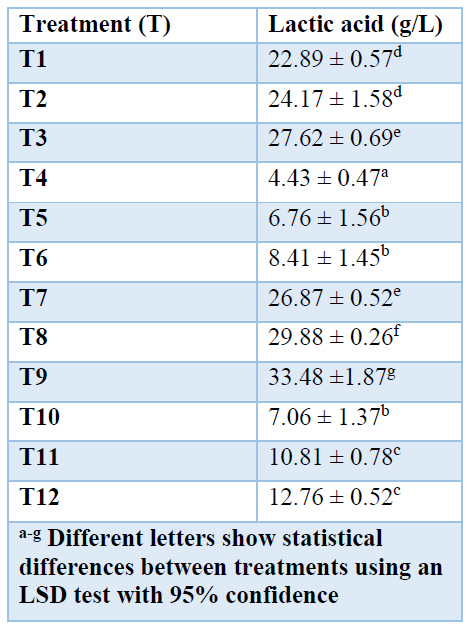
Table 4. Lactic acid production from cassava bagasse starch varieties INIAP 650 and 651 quantified by titration.
In T1, the stoichiometric content of H2SO4 was 6.83 mL, but the real volume used was 5.89 mL until reaching pH 2.0. It is then verified that there is a lack of H2SO4, so it reacted with all the calcium lactate, releasing lactic acid and calcium sulfate 50, so that all the lactic acid produced is quantified. This lactic acid was retained as calcium lactate in the precipitate.
The fermentation treatments, T4 to T6 and T10 to T12, carried out without stirring of the fermentation broth, presented the lowest lactic acid production compared with the other treatments. The lowest lactic acid production was obtained with T4, with a value of 4.43 ± 0.47 g/L at pH 4.5 conditions and without stirring from the fermentation of a hydrolyzed bagasse starch of cassava INIAP 650 variety. The main reason that would explain this is the lack of agitation of the system, which hinders the transfer of nutrients from the medium to the cells 51.
In this study, the lactic acid production rate increased by 80.65 % for the treatments with agitation compared to the treatments without agitation, as seen in T1 and T4, in 120 h of fermentation.
Other studies report an increase in lactic acid production by 42 %, when stirred from 0 to 300 rpm was reached in 36 h, with the formation of alcohol at pH between 4.5 and 5.5 at 37 ºC with a Rhizopus oryzae strain 52,53.
It can be noticed that the production of lactic acid increases as the pH increases in the range of 4.5 to 5.5. In values outside this range, there is an inhibition of the microorganisms that act in transforming glucose to lactic acid 51. Besides, there are statistically significant differences between the treatments T3 and T9, the latter with the highest production of lactic acid.
The Lactobacillus delbrueckii strain develops in the pH range of 4.0 to 6.0, but its growth occurs at pH 5.0 for optimal lactic acid production 54. However, in this work, the Lactobacillus leichmannii developed better and obtained higher lactic acid production at pH 5.5 (33.48 ± 1.87 g/L) than at pH 5.0 (27.62 ± 0.69 g/L). This is due to the accumulation of lactic acid within the fermentation process that creates an environment not suitable for developing microorganisms, an acidic medium that reduces microbial growth, and therefore the low transformation of glucose to lactic acid. Furthermore, when the initial pH of the solution approaches the pKa of lactic acid (3.8), the undissociated form of this acid has a high inhibitory effect compared to the dissociated lactate form 7,52.
Studies performed with Lactobacillus strains indicate high lactic acid productions for pH ranges from 5.0 to 6.5. The literature reports lactic acid productions of 74.01 g/L from a hydrolyzed cassava starch with a starch concentration of 120 g/L at pH 5.0 with a 61.67 % degree of conversion 24. This is a higher production than the major content of lactic acid obtained in our study (33.48 %), because cassava bagasse starch has non-hydrolyzed lignocellulosic matter that generates interference in fermentation.
A previous study showed lactic acid production from cassava bagasse starch with a Lactobacillus delbrueckii strain reaching 136.8 and 196.4 mg/g of lactic acid at pH 4.0 and 5.0, respectively 50. In our study, a yield of 334.80 and 276.20 mg of lactic acid was obtained for each gram of cassava bagasse starch at pH 5.5 and pH 5.0, respectively, so higher contents of lactic acid than those mentioned in the literature. This is because, in the experimentation, cassava bagasse starch was used and not directly the bagasse.
The substrate plays a significant role in fermentation. Cassava bagasse starch presented a lignocellulosic fiber content that was not hydrolyzed by lactic acid bacteria. On the other hand, cassava starch, in general, does not contain this lignocellulosic material. Thus, cassava bagasse presented lower yields than starch because it presented a high degree of lignocellulosic matter without hydrolyzing; therefore, lactic acid production was lower.
Evaluation of the fermentation efficiency of Lactobacillus leichmannii of a starch hydrolyzate from cassava bagasse starch
The stoichiometric relationship was used under the microorganism's ideal conditions to perform the calculations for the theoretical production of lactic acid from glucose (pH 5.0 at 37 ºC) 51. However, it should be considered the conditions of pH, temperature, and agitation are the essential variables in fermentation. As seen in Figure 1., there is low production of natural lactic acid compared to the theoretical one that should reach 71.66 g/L and 85.95 g/L of lactic acid for INIAP 650 INIAP 651 varieties, respectively. Thus, maximum values of 27.62 ± 0.69 g/L and 33.48 ± 1.87 g/L were obtained at pH 5.5 with agitation at 150 rpm for INIAP 650 and INIAP 651 varieties, respectively. This may be because the theoretical production will always be greater than the production that was reached experimentally. Due to the type of microorganisms for fermentation, fermentation times, pH, temperature, and agitation, the medium and substrate are not considered.
Furthermore, the theoretical yield was determined, assuming that reducing sugars correspond entirely to glucose. The determining factors were pH and substrate. Moreover, the microorganism used in the fermentation specifically consumes glucose for the production of lactic acid, and in the hydrolysis of starch, reducing sugars were obtained that include maltose, dextrins, and glucose. Therefore, the conversion of reducing sugars to lactic acid will not reach the total.
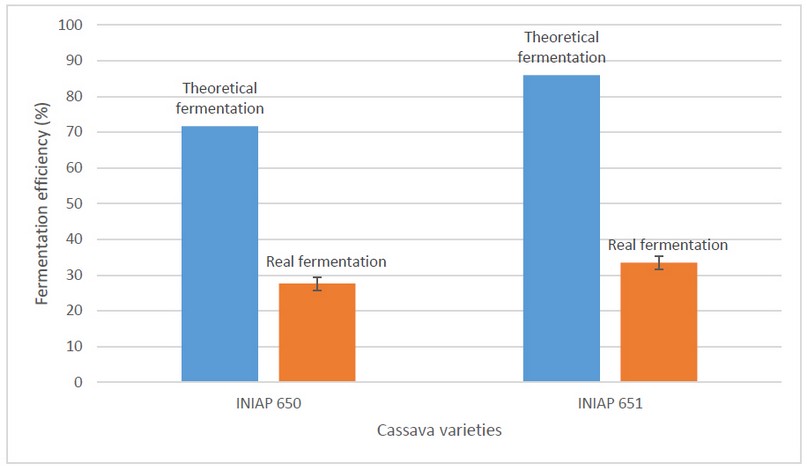
Figure 1. Theoretical (blue bars) and real (orange bars) fermentation efficiency at pH 5.5 with cassava varieties' agitation.
Characterization of the final lactic acid solution
The characterization of the purified lactic acid solution in the different treatments is presented below. The parameters to be evaluated were pH and density. Furthermore, the samples were characterized by FTIR with the representative lactic acid spectrograms.
Physicochemical characterization of lactic acid solutions.
The physical properties, density, and pH were evaluated in lactic acid solution. In Table 5. the density and final pH of the lactic acid solutions are presented. The density in treatments T7, T8, and T9 reached the highest values because they present the highest lactic acid concentration. The highest lactic acid production was at pH 5.5, stirring the hydrolyzed cassava bagasse starch of INIAP 651 variety. Therefore, the density directly relates to lactic acid concentration with a correlation coefficient of R2 = 0.961. The higher the concentration, the higher the density because the water concentration decreases, and the density takes the value of pure lactic acid.
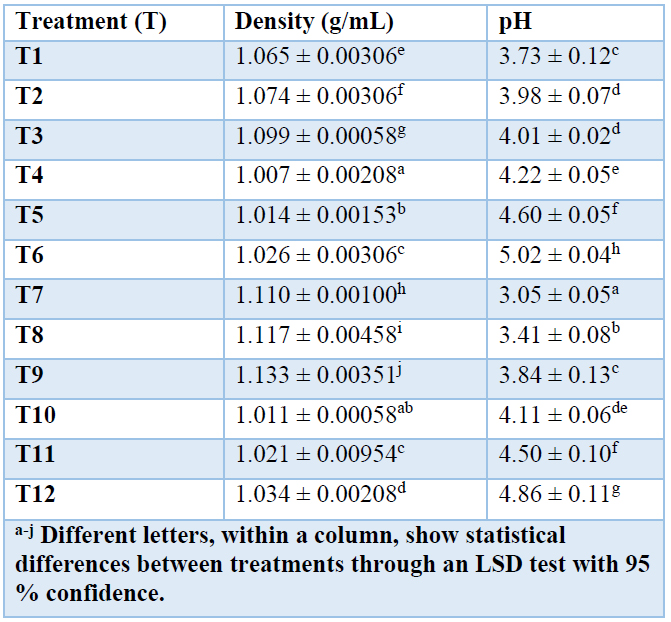
Table 5. Physico-chemical characteristics of the final lactic acid solution.
Within this work, density values were obtained in the range of 1.007 g/mL to 1.133 g/mL. The literature reports density in 98 % lactic acid of 1.215 g/mL 55. The value is not within the range obtained in our work because the final lactic acid concentration reached a maximum of 33.48 g/L. The pH of all the treatments decreased due to the presence of lactic acid in the solution. This varies according to the initial pH for fermentation 51.
Evaluation of the organic compounds present in the final lactic acid samples
FTIR analysis was carried out to verify the presence of lactic acid in the samples obtained after fermentation. Figure 2. showed the FTIR spectrogram of the lactic acid sample that presented the best production, T9 of INIAP 651 variety, pH 5.5, and stirring, in which the characteristic bands of this organic compound appear, and the spectrogram of a commercial lactic acid. All the treatments presented similar characteristics of lactic acid spectrograms.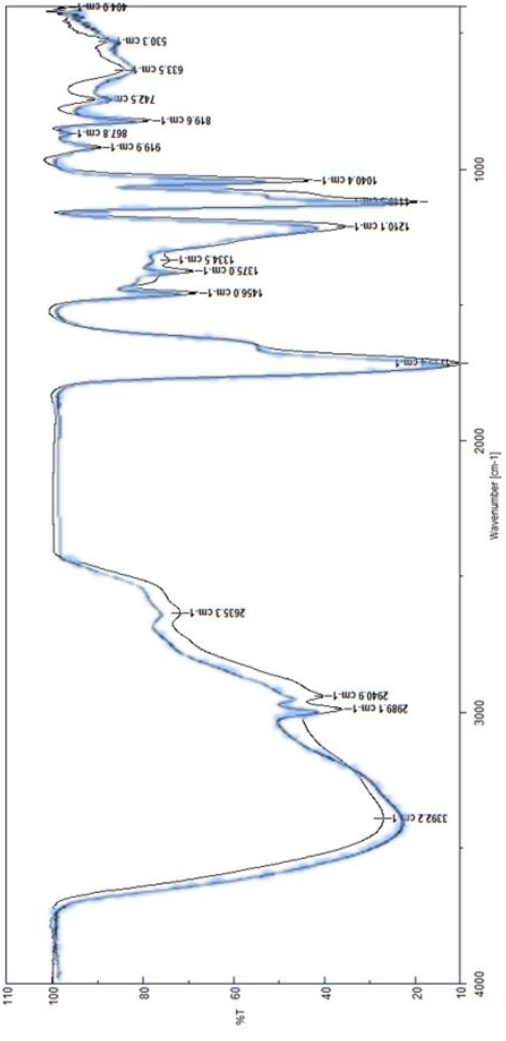
Figure 2. FTIR spectrogram of lactic acid obtained from T9 of INIAP 651 variety, pH = 5.5 with stirring (blue line) and FTIR spectrogram of commercial lactic acid (black line) (Carlo Erba brand, Pharmacopeia grade) 15.
The experimental spectrogram shows the OH stretch bands at 3392.2 𝑐𝑚−1, alcohol and carboxyl groups at 2940.9 and 2989.1 𝑐𝑚−1. For the COOH group, the bond presented a tension at 1715.4 𝑐𝑚−1 , typical of the acid group. Groups C𝐻3 and 𝐶𝐻 showed symmetric flexures at 1375 and 1456 𝑐𝑚−1. The C-O stretches of the alcohol and acid groups at 1210.1, 1119.3, and 1040.4 𝑐𝑚−1. The characteristics of the commercial lactic acid spectrogram are linked to OH stretches at 3338 𝑐𝑚−1, alcohol and carboxyl groups at 2944 and 2995 𝑐𝑚−1. For the COOH group, the bond presented a tension at 1716 𝑐𝑚−1, typical of the acid group. Groups showed symmetric flexures at 1376 and 1432 𝑐𝑚−1. The C-O stretches of the alcohol and acid groups at 1220, 1120, and 1070 𝑐𝑚−1 56. Thus, the bands of the spectra resulting from the lactic acid analysis of this work compared to those of the commercial one were similar. The resulting spectra showed the characteristic bands of this compound. Therefore, lactic acid was confirmed in all the final samples, with approximately 99% accuracy, compared to the characteristics bands' values.
In our research, the characteristic spectrogram presented a band at the level of 2635 𝑐𝑚−1, because of the formation of amino compounds, due to protein in the initial substrate. Besides, the spectrograms of this work did not present peaks own of other substances. It is worth mentioning that the samples did not present the characteristic band of water that ranges from 3600 to 4000 𝑐𝑚−1, because the samples were pre-treated by evaporation at 65 ºC and thus, there was no interference in the sample scanning with the equipment.CONCLUSIONS
Lactic acid production from INIAP 650 and INIAP 651 varieties of cassava made of bagasse starch hydrolyzate reached relevant yields. The highest production of lactic acid was obtained at pH 5.5 with stirring. Thereby, it could be thought that at higher pH, the microorganism works better in the production of lactic acid. Agitation maximizes the production of lactic acid in the fermentation processes of hydrolyzed cassava bagasse starch. So, the pH-agitation system significantly affects lactic acid production in the fermentation processes of hydrolyzed cassava bagasse starch. The infrared spectrogram showed the characteristic of lactic acid bands for all the treatments analyzed. Finally, the quality of lactic acid from cassava by-products was verified and could have significant market value for Ecuador's sustainable development.
Further research to improve the quality and yield of lactic acid from a cassava bagasse starch is essential. Thus, to analyze the pH in ranges from 6 to 7, evaluate if there is a greater production of lactic acid and determine the optimal pH condition. The influence of enzymatic hydrolysis with cellulases to degrade all the cellulosic matter present in the cassava bagasse starch should be studied. The quantification of the initial amount of glucose and its consumption in the fermentation process is relevant for subsequent investigations. Evaluating the incidence of agitation of the fermentation broth at different levels until reaching 300 rpm is also in perspective. The work with cassava bagasse directly for the enzymatic hydrolysis process and thus evaluate lactic acid production with cellulase enzymes in the hydrolysis process of the lignocellulosic material present in the residue is relevant.
ACKNOWLEDGMENTS
This research was supported economically by EPN through the project PREDU-2016-015. The authors thank H. Caballero (UTM) for their collaboration in collecting samples, starch extraction and information on the provenance of study cassava varieties. And I. Chango (CIAP - EPN) for assistance in analysis, recording, and interpreting spectra.
REFERENCES
1. Alves de Oliveira R, Komesu A, Vaz Rossell CE, Maciel Filho R. Challenges and opportunities in lactic acid bioprocess design-From economic to production aspects. Biochem Eng J. 2018;133:219–39.
2. Alves de Oliveira R, Komesu A, Vaz Rossell CE, Wolf Maciel MR, Maciel Filho R. A study of the residual fermentation sugars influence on an alternative downstream process for first and second-generation lactic acid. Sustain Chem Pharm. 2020;15(December 2019):100206.
3. Ge L, Wang P, Mou H. Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew Energy. 2011;36(1):84–9.
4. Kwon EE, Yi H, Jeon YJ. Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresour Technol. 2013;136(March):475–80.
5. Bertoft E. Understanding starch structure: Recent progress. Agronomy. 2017;7(3).
6. Omoregie H. Chemical Properties of Starch and Its Application in the Food Industry. In: Chemical properties of starch. Intechopen; 2019. p. 13.
7. Ghaffar T, Irshad M, Anwar Z, Aqil T, Zulifqar Z, Tariq A, et al. Recent trends in lactic acid biotechnology: A brief review on production to purification. J Radiat Res Appl Sci. 2014;7(2):222–9.
8. Chen H, Chen B, Su Z, Wang K, Wang B, Wang Y, et al. Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and Lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind Crops Prod. 2020;146(15):112175.
9. Kundrat L. October 2010 Dear Microbiologics Customer, The name of Microbiologics product 0235 (ATCC Licensed Derivative® ATCC® 7830TM*) is [Internet]. 2010. Available from: https://www.microbiologics.com/core/media/ media.nl?id=900&c=915960&h=c860a1caa7f78e5ebf50&_xt=.pdf
10. Abdel-Rahman MA, Tashiro Y, Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Vol. 31, Biotechnology Advances. Elsevier; 2013. p. 877–902.
11. Tejada R. Obtención de ácido láctico por fermentación de almidón de ñame espino mediante el Lactobacillus delbrueckii ssp. bulgaricus y el Streptococcus thermophilus para su uso en la producción de ácido poliláctico (Proyecto de titulación previo a la obtención del [Internet]. Universidad de Cartagena –Universidad Nacional de Colombia sede Medellín; 2015. Available from: https://repositorio.unal.edu.co/handle/unal/55489
12. Delgado Troya J, Realpe Ortega P. Producción de ácido láctico por fermentación de la bacteria (Lactobacillus casei) utilizando cáscara de cacao (Theobroma cacao). Tesis [Internet]. Universidad de Guayaquil; 2019. Available from: http://repositorio.ug.edu.ec/handle/redug/40005
13. Torres MG, Gómez SL. Lactic Acid : a Review on Determination and Purification. Biociencias. 2019;14(2):149–75.
14. Yang X, Zhu M, Huang X, Lin CSK, Wang J, Li S. Valorisation of mixed bakery waste in non-sterilized fermentation for l-lactic acid production by an evolved Thermoanaerobacterium sp. strain. Bioresour Technol. 2015;198:47–54.
15. Rojas AM, Bastidas MJ. Producción de ácido láctico a partir del lactosuero utilizando Lactobacillus delbrueckii subsp. bulgaricus y Streptococcus thermophilus. Rev Colomb Química. 2015;44(3):5–10.
16. Wischral D, Jr NP. Statistical optimization of lactic acid production by Lactobacillus pentosus using hemicellulose hydrolysate from sugarcane bagasse. Rev Ing. 2018;29(1):41–51.
17. Caballero Salinas JC, Moreno Reséndez A, Reyes Carrillo JL, García Valdez JS, López Báez W, Jiménez Trujillo JA. Competencia del uso del rastrojo de maíz en sistemas agropecuarios mixtos en Chiapas. Rev Mex Ciencias Agrícolas. 2017;8(1):91.
18. Arias Zabala M, Henao Navarrete L, Castrillón Gutiérrez Y. Lactic acid production by fermentation of coffee mucilage with Lactobacillus bulgaricus NRRL-B548. DYNA. 2009;76(158):147–53.
19. INEC. Superficie según producción y ventas de yuca (Raíz), por región y provincia. https://www.ecuadorencifras.gob.ec › web-inec › espac › espac-2018,. 2018;
20. Zambrano JL, Ph D, D VBP, Sc IMHM, Domínguez JM, Ph D. Plan Estratégico De Investigación Y Desarrollo Tecnológico Del Iniap 2018 - 2022 [Internet]. Guayaquill: INIAP; 2018. 76 p. Available from: https://www.iniap.gob.ec/pruebav3/wp-content/uploads/2018/03/281-iniap-OK-baja.pdf
21. Tang Y, Li X, Chen PX, Zhang B, Hernandez M, Zhang H, et al. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015;174(5):502–8.
22. Román Y, Techeira N, Yamarte J, Ibarra Y, Fasendo M. Caracterización físico-química y funcional de los subproductos obtenidos durante la extracción del almidón de musáceas, raíces y tubérculos. Interciencia. 2015;40(5):350–6.
23. Sivamani S, Baskar R. Process design and optimization of bioethanol production from cassava bagasse using statistical design and genetic algorithm. Prep Biochem Biotechnol [Internet]. 2018 Oct 21;48(9):834–41. Available from: https://doi.org/10.1080/10826068.2018.1514512
24. Ortíz C, Ospina L, Sánchez S. Producción de ácido Láctico a partir de almidón de yuca por bacterias acidolácticas del género Lactobacillus. UJTL. 2014;10.
25. Comité de Reconstrucción y Reactivación Productiva. Plan de Reconstrucción y Reactivación Productiva post terremoto [Internet]. 2017. p. 76. Available from: https://www.reconstruyoecuador.gob.ec/wp-content/uploads/2018/02/Plan-de-Reconstrucción-y-Reactivación-Productiva-post-terremoto.pdf
26. INIAP. INIAP 650- Estación Experimental Portoviejo [Internet]. Ecuador: SENECYT; 2012. p. 1–2. Available from: https://repositorio.iniap.gob.ec/bitstream/41000/1199/1/INIAP PORTOVIEJO-650.pdf
27. INIAP. INIAP 651- Estación Experimental Portoviejo [Internet]. Ecuador; 2012. p. 1–2. Available from: https://repositorio.iniap.gob.ec/bitstream/41000/1115/1/INIAP PORTOVIEJO-651.pdf
28. Vargas Aguilar P. Obtención de almidón fermentado a partir de yuca (Manihot esculenta crantz) variedad valencia, factibilidad de uso en productos de panadería. Tecnol en Marcha. 2010;23(3):15–23.
29. INEN 1529-2. Control Microbiológico de los Alimentos. Toma, envío y preparación de muestras para el análisis microbiológico [Internet]. Vol. Primera ed. 2013. p. 7–12. Available from: http://normaspdf.inen.gob.ec/pdf/ntel1529-2-1R.pdf
30. Chookietwattana K. Lactic Acid Production from Simultaneous Saccharification and Fermentation of Cassava Starch by Lactobacillus Plantarum MSUL 903. APCBEE Procedia [Internet]. 2014;8:156–60. Available from: http://www.sciencedirect.com/science/article/pii/S2212670814000992
31. Hofvendahl K, Hahn–Hägerdal B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb Technol [Internet]. 2000 Feb [cited 2015 Mar 27];26(2–4):87–107. Available from: http://www.sciencedirect.com/science/article/pii/S0141022999001556
32. Gómez MB, Cárdenas ON, Santa C, Carpio C, Román G. Purificación Parcial y Caracterización de Alfa Amilasa de granos germinados de Chenopodium quinoa (Quinua). Rev ECIPeru. 2018;52–8.
33. Maldonado-Alvarado P. Facteurs déterminants du pouvoir de panification de l’amidon de manioc modifié par fermentation et irradiation UV. Université Montpellier 2. PhD thesis. 190 pag.; 2014.
34. Mera I, Cataño JC. Obtención de glucosa a partir de almidón de yuca (Manihot Sculenta). Fac Ciencias Agropecu [Internet]. 2005;3:54–63. Available from: file:///C:/Users/PEDROM~1.1-1/AppData/Local/Temp/Dialnet-ObtencionDeGlucosaAPartirDeAlmidonDeYucaManihotScu-6117970.pdf
35. Bomrungnok W, Sonomoto K, Pinitglang S, Wongwicharn A. Single Step Lactic Acid Production from Cassava Starch by Lactobacillus plantarum SW14 in Conventional Continuous and Continuous with High Cell Density. APCBEE Procedia. 2012;2:97–103.
36. Bello Gil D, Carrera B. E, Diaz M. Y. Determinación de azúcares reductores totales en jugos mezclados de caña de azúcar utilizando el método del ácido 3,5 dinitrosalicílico. ICIDCA obre los Deriv la Caña Azúcar. 2006;40:45–50.
37. Alonso L, Poveda A. Estudio comparativo en técnicas de recuento rápido en el mercado y placas petrifilm. Proyecto de titulación previo a la obtención del título de Microbiólogo Industrial). Pontificia Universidad Javeriana, Bogotá, Colombia. Comercial Experimental. 2008.
38. Cardona M, Larrosa-Fuentes J. Manual para la observación de medios [Internet]. 1st ed. ITESO; 2014. Available from: http://www.jstor.org/stable/j.ctvdmwzct
39. Miller C, Fosmer A, Rush B, McMullin T, Beacom D, Suominen P. Industrial Production of Lactic Acid. 2nd Ed. Vol. 3, Comprehensive Biotechnology. Elsevier B.V.; 2011. 179–188 p.
40. ASTM -D7777. Standard Test Method for Density, Relative Density, or API Gravity of Liquid Petroleum by Portable Digital Density Meter. 2016;1–8.
41. Vargas Aguilar P, Hernández Villalobos D. Harinas y almidones de yuca, ñame, camote y ñampí: propiedades funcionales y posibles aplicaciones en la industria alimentaria. Rev Tecnol en Marcha. 2013;26(1):37.
42. López JA, Rodríguez E, Sepúlveda JU. Evaluación de características fisicas y texturales de pandebono. Acta Agron. 2012;61(3):273–81.
43. Pandey A, Soccol CR, Nigam P, Soccol VT, Vandenberghe LPS, Mohan R. Biotechnological potential of agro-industrial residues. II: cassava bagasse. Bioresour Technol. 2000;74(1):81–7.
44. Montilla J, Arcila J, Aristizábal M, Montoya EC, Puerta GI, Oliveros CE, et al. Propriedades Físicas y Factores de Conversión del Café en el Proceso de Beneficio. Av Técnicos Cenicafé. 2008;370.
45. Vargas y Vargas M de L, Figueroa Brito H, Tamayo Cortez JA, Toledo López VM, Moo Huchin VM. Aprovechamiento de cáscaras de frutas: análisis nutricional y compuestos bioactivos. Cienc ergo sum. 2019;26(2):1–11.
46. Arroyo P, Mazquiaran L, Rodriguez P, Valero T, Ruiz E, Ávila JM, et al. Frutas y hortalizas: Nutrición y Salud en la España del SXXI. Fund Española la Nutr. 2018;198.
47. Woiciechowski AL, Nitsche S, Pandey A, Soccol CR. Acid and enzymatic hydrolysis to recover reducing sugars from cassava bagasse: An economic study. Brazilian Arch Biol Technol. 2002;45(3):393–400.
48. Hernández-Medina M, Torruco-Uco JG, Chel-Guerrero L, Betancur-Ancona D. Caracterización fisicoquímica de almidones de tubércules cultivados en Yucatán, México. Cienc e Tecnol Aliment. 2008;28(3):718–26.
49. Souto LRF, Caliari M, Soares Júnior MS, Fiorda FA, Garcia MC. Utilization of residue from cassava starch processing for production of fermentable sugar by enzymatic hydrolysis. Food Sci Technol. 2017;37(1):19–24.
50. John RP, Nampoothiri KM, Pandey A. Solid-state fermentation for L-lactic acid production from agro wastes using Lactobacillus delbrueckii. Process Biochem. 2006;41(4):759–63.
51. Eiteman MA, Ramalingam S. Microbial production of lactic acid. Biotechnol Lett. 2015;37(5):955–72.
52. Bahry H, Abdalla R, Pons A, Taha S, Vial C. Optimization of lactic acid production using immobilized Lactobacillus Rhamnosus and carob pod waste from the Lebanese food industry. J Biotechnol. 2019;306(September):81–8.
53. Bai DM, Jia MZ, Zhao XM, Ban R, Shen F, Li XG, et al. L(+)-lactic acid production by pellet-form Rhizopus oryzae R1021 in a stirred tank fermentor. Chem Eng Sci. 2003;58(3–6):785–91.
54. Ai Z, Lv X, Huang S, Liu G, Sun X, Chen H, et al. The effect of controlled and uncontrolled pH cultures on the growth of Lactobacillus delbrueckii subsp. bulgaricus. LWT - Food Sci Technol. 2017;77:269–75.
55. Farmaquímica. Ficha técnica de Ácido Láctico. [Internet]. 2013. Available from: http://archivosdemedicinadeldeporte.com/articulos/upload/Revision_acido_lactico_270_120.pdf
56. Rojas AM, Montaño L, Bastidas MJ. Producción de ácido láctico a partir del lactosuero utilizando Lactobacillus delbrueckii subsp. bulgaricus y Streptococcus thermophilus. Rev Colomb Química. 2015;44(3):5–10.
Received: 23 November 2020
Accepted: 16 February 2021Shirley Inguillay1, Felipe Jadán2 and Pedro Maldonado-Alvarado1*,
1Department of Food Science and Biotechnology, Escuela Politécnica Nacional (EPN), Quito, Ecuador
Departament of chemical processes, Universidad Técnica de Manabí (UTM). Portoviejo, Ecuador
ORCID
Shirley Inguillay https://orcid.org/0000-0003-1422-4374
Felipe Jadán https://orcid.org/0000-0002-5640-2207
Pedro Maldonado-Alvarado https://orcid.org/0000-0001-7096-249X