2020.05.04.15
Files > Volume 5 > Vol 5 No 4 2020
INVESTIGATION / RESEARCH
Microbial implications of beef fat and pork fat in the environment
Chinonye Medline Maduka*1, Akuma Oji2, Ugochi Queen Fineboy3 , Gideon Chijioke Okpokwasili2 .
Available from: http://dx.doi.org/10.21931/RB/2020.05.04.15
ABSTRACT
Developing countries are known to dispose of waste indiscriminately into their environment, off which fat is one of them. These fats release awful odor making passersby uncomfortable and also breeds microorganisms. Environmental factors such as rainfall, sunlight, and wind aid the migration of these fats to other sites, thereby leading to contamination. Total heterotrophic plate count of pork fat ranged from 4.0 x 105 cfu/g to 4.2 x 105 cfu/g and its total coliform plate count was from 3.8 x 105 cfu/g to 4.0 x105 cfu/g while the total heterotrophic plate count of beef fat ranged from 3.1 x 105 cfu/g to 3.5 x 105 cfu/g and its total coliform plate count was from 2.4 x 105 cfu/g to 2.8 x105 cfu/g. E.coli and Salmonella sp. were the highest occurring in both fats. Pork fat had more microbial count than beef fat. Fats can be converted to useful products, which will reduce waste in the environment. Statistical analysis showed a significant difference in mean counts of pork and beef fat samples at p≤0.05.
Keywords: Pork fat, beef fat, microorganisms, environment, wastes.
INTRODUCTION
Environmental pollution is an issue that has been a threat to the world for many years and is presently a significant problem because of the rapid population growth in developing countries 1. The burden of increasing waste and its control in urban areas of developing countries is an environmental concern. This situation grew worse due to the lack of technology to help reduce the heap of wastes 2. Another global problem is soil pollution resulting from waste discharges freely dumped into the environment. The richest reservoir of microorganisms in the soil play a significant role in the ecosystem because its continuity depends mainly on it. If the soil becomes polluted, the ecosystem is tilted, and agricultural activities are disrupted 3,4. A major problem of many urban areas in Nigeria is the low sanitary condition best described by Sule 5 as indiscriminate waste disposal and the ability to reduce it to the minimum and the low usage of laws for this waste disposal problem. These abnormal waste dumping methods have created many problems such as sanitation and environmental issues, an imbalance of groundwater, and soil pollution and water resources 6. This pollution problem either on air, water, or land caused by man's day to day activities is rapidly growing to the point that it can no longer be managed and will be seen as a usual way of life. This issue hurts human health and well-being.
One of the by-products of animals is fat 7, and it can be edible or inedible depending on the animal and the animal’s part it is gotten from. Cattle are a source of protein and fats 8. Fat has an essential role in meat quality. Surface fat stops frequent cooling of the underlying muscle tissues, which lowers the tendency of cold-shortening and lowers weight loss due to chilling. The hardness or softness of fat directly impacts its processing efficiency also fat and adds to meat's beneficial properties. Physical properties to look out for in fat are color, hardness, and texture. The better the properties of fat, the higher the market value. It is known that 85% of fat tissue consists of triglycerides located in the fat cells; for every triglyceride, three molecular fatty acids are found in it. The other part of the fat tissue consists of approximately 12% moisture, and the connective tissue is approximately 3%. Different types of collagen and various cross-linking levels are crucial to the structure of fat tissue, and these affect the texture and strength of fat at any temperature, and it depends on the composition of its fatty acid and the molecules that constitute its triglycerides. Palmitic and stearic acids are saturated fatty acids with high melting points of about 65-70°C, and this account for hard fat, unlike palmitoleic and oleic acids, which are unsaturated fatty acids with low melting points of 0-15°C and contribute to softness. The temperature at which fat melts depends on the amount each fatty acid contributes. In Australia, prolonged grain feeding enhances fat color, leads to a uniform product, and a rise in the hardness of fat 9.
Mature Pigs are known to have fat. Feeding habits, the composition of animal feeds, breed diversity, animal age, and other factors are significant contributors to the quality of pork fat 10. Pork's quality may increase or decrease as a result of nutrition, genetics, management, and pork-processing procedures. Pigs' lean genotype fed with diets high in unsaturated fat might possess thinner and soft fat at their bellies, which is of low quality 11. This reduction in fat quality is related to their thinner bellies, which might negatively affect the processing, separation of tissue, and stability during storage. Several factors define Pork's quality, such as color, consistency, and keeping quality, and these are affected by the size of the fat deposits located in the pig and the composition of dietary fat. The fatty acid profile of pork fat represents the role played by each source of dietary fat 12,13,14.
Oleic and palmitic acids are the primary fatty acids in beef fat 15. Many factors, such as the breed of Cattle, sex, diet, weight, age, fatness, and environmental conditions, including climate and season, are known to affect the composition of fat tissue in cattle. Although the factors do not apply to all cattle, the cattle known as Brahman are known for higher unsaturated fat content than a few other breeds. Brahman-cross cattle have softer fat when compared to purebred Hereford and various crossbreeds. Most likely, age plays a significant role in the unsaturation of fat; the older an animal becomes, the more unsaturated fat it is likely to have. Colder climates are known to have Cattles with softer subcutaneous fat. When a healthy cattle grazes green pasture, it utilizes the yellow pigments in these plants known as carotenoids, beta-carotene, which makes up a larger part of carotenoids and gives the fat its color. Most fats are known to have a cream or yellow color.
This research aims to identify the impact of fats in the environment and the microbial load.
MATERIAL AND METHODS
Fat samples were collected from slaughterhouses in Umuahia, Abia State, Nigeria. These samples were collected in sterile polythene bags and transported within 45 minutes for microbiological analysis (Figure 1). All laboratory procedures were done aseptically. One gram of each mashed sample was introduced into 9ml of distilled water, the conical flask was carefully shaken, and from it, ten-fold serial dilution was done 16. The pour plate method was used to isolate microorganisms on MacConkey agar and Nutrient agar at 37oC for 24-48 hours. Pure isolates were identified and characterized. The other part of the fat that was not bought from slaughterhouses was discarded as waste.
Figure 1. Area of Study. Umuahia, Abia State, Nigeria
RESULTS AND DISCUSSION
A total of ten samples were used for this study. Five out of the ten samples were pork fat, while the other five samples were beef fat. Total heterotrophic plate count (THPC) and total coliform plate count (TCPC) of pork and beef fat samples are shown in Figure 2. These samples had higher counts for total heterotrophic plate count than total coliform plate count.
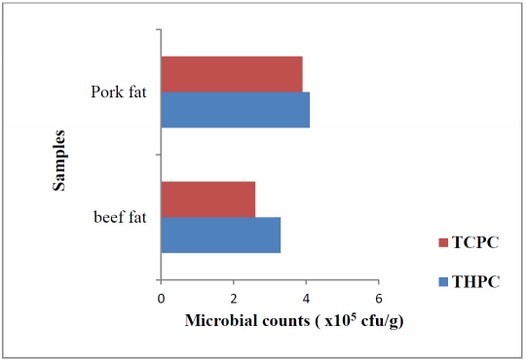
TCPC: Total coliform plate count
Figure 2: Mean bacterial counts of pork fat and beef fat
Figure 2 shows that pork fat has a higher bacterial load than beef fat. This could be as a result of the physical appearance of pork fat. Pork fat appeared wet with more oil content than beef fat. Research by Abdelwhab 17 revealed that beef fat had lesser fat content than mutton fat. This slimy nature of pork fat could be the reason behind its higher number of microbial load. Aymerich et al. 18 identified that meat with aw between 0.94-0.99 promoted microbial growth. Also, pork fat might have higher nutrients than beef fats, which makes microorganisms thrive more in it. Lulietto et al. 19 stated that meat has high protein, lipids, minerals, and vitamin contents but low carbohydrate content, which allows some organisms to survive. Nychas et al. 20 recorded that meat spoilage results from some available substrates such as glucose, lactic acid, nitrogenous compounds, and free amino acids present in the meat.
Four bacterial isolates were identified from this study, and they are Pseudomonas aeruginosa, E. coli, Salmonella sp., and Proteus sp. Odey 16 isolated seven bacterial isolates, and they include Staphylococcus spp., Streptococcus spp., Escherichia coli, Salmonella spp., Bacillus spp., Pseudomonas sp., and Proteus sp. The microbial counts from nutrient agar from their sample was from 1.4 x 105 cfu/g to 3.5 x 105 cfu/g. Lamb 21 identified Samonella sp. in all fat and oil samples, including beef tallow and pig lard. Shaffer 22 stated that the common bacterial contaminants in Pork are E. coli, Salmonella sp., S. aureus, and Yersinia enterocolitica and that the intrinsic properties of meat, such as pH and moisture promotes microbial growth, and also does the extrinsic factor such as temperature.
Results from percentage occurrence showed that E. coli and Samonella sp., highly occurred in both samples, Pseudomonas sp. and Proteus sp., were more in pork fat than beef fat. Yannick et al. 8 recorded 81.8% for Staphyloccocus aureus, 72.7% for Klebsiella pneumonia, 54.4% for Escherichia coli, 45.4% for Salmonella spp., 27% for Proteus vulgaris, and 9% for Shigella spp. Figure 3 is a representation of the percentage occurrence of bacteria in both fats.
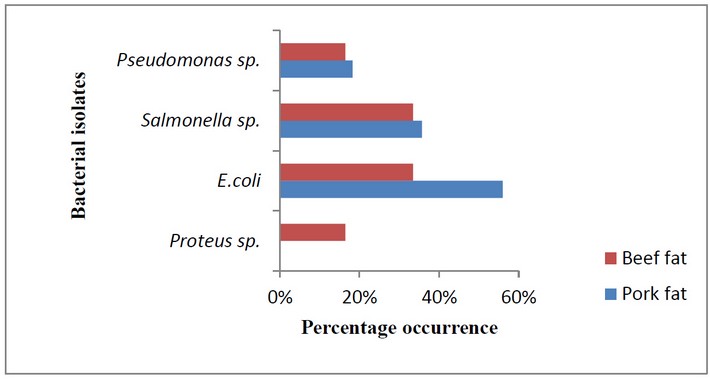
Figure 3: Percentage occurrence of bacterial isolates from pork fat and beef fat
It could be possible that after processing these fats that the bacterial load of these fats will reduce as a result of heating or other techniques used on it. It could also be possible that the microorganisms in these fats are also released into the environment when dumped. When deposited as wastes into the environment, these fats begin to decay and release an offensive odor. The odor released could result from the action of the microorganisms already present in the fat, which most times begin the degradation process or the action of other microorganisms that find the fat as a source of energy. The delicate nature of these fats may have contributed to the easy access of these microorganisms, which, when broken down, will lead to more fat-loving microorganisms inhabiting these fats.
Environmental factors such as sunlight and rainfall further promote the migration of the degraded fats, and this could be beneficial or harmful depending on their quantity in the environment. When these fats are washed in small quantities into the environment, it is tolerated, but when in large quantities, they will either compete or work in synergy with the existing nutrients in such an environment. The oil released by these fats covers the surface (water or soil) they occupy, thereby preventing such environment and the microorganisms in the environment from getting the essential nutrients needed for their growth. This might be the reason for the awful odor given out by these wastes (fats). These decaying fats could trigger the degradation of other materials in the environment. The dumped fats and other wastes are not a sight to behold as it makes the environment look unhealthy. Ifeoluwa 23 stated that solid wastes lead to soil, air, and water contamination, which creates health challenges and is a significant problem to man and its environment, especially those who live closer to areas where these waste release offensive odors a result of decay.
CONCLUSIONS
I also wish to thank Africa Centre of Excellence, University of Port Harcourt for the great knowledge given to me Many thanks to Nweze, N.C.F of LinnC laboratory for his technical support. My sincere appreciation goes to Michael Okpara, the [h1] University of Agriculture Umudike[h2] , for making this research a success.
ACKNOWLEDGEMENTS
Many thanks to Nwoba Chinedu Frank Nweze of the LinnC laboratory for his technical support. My sincere appreciation goes to Michael Okpara, the University of Agriculture, for making this research a success.
COMPETING INTEREST
The authors have no competing interests.
REFERENCES
1. Ali, S.M., Pervaiz, A., Afzal, B., Hamid, N., and Yasmin, A. (2014). Open dumping of municipal solid waste and its hazardous impacts on soil and vegetation diversity at waste dumping sites of Islamabad city. Journal of King Saud University – Science, 26(1): 59 – 65.
2. Baum, B. and Parker, H.C. (1973). Solid waste disposal. Incinerations and landfill, 2nd Edition Ann Arbor Science Publishers Inc. Ann Arbor, Mich. United States. (vol.1) 636 pages.
3. Adedokun, O.M. and Ataga A.E. (2006). Effects of Crude Oil and Oil Products on Growth of Some Edible Mushrooms. Journal of Applied Science and Environmental Management, 10(2): 91 – 93.
4. Igwo-Ezikpe, M.N., Gbenle, O.G., Ilori, M.O., Okpuzor J., and Osuntoki, A.A. (2009). Evaluation of Alcaligenes faecalis Degradation of Chrysene and Diesel Oil with Concomitant Production of Biosurfactant. Research Journal of Environmental Toxicology, 3(4): 159-169.
5. Sule, A. B. (2011). Management of Environments in Africa: A Handbook and References, Lagos: Greenwood Publishing Group Inc, Lagos.
6. Ajibade, F. O., Adewumi, J. R. and Oguntuase, A.M. (2014). Sustainable Approach to Wastewater Management in the Federal University of Technology, Akure, Nigeria. Nigerian Journal of Technological Research, 9(2): 27-36.
7. Tarté, R. (2016). Composition, Functionality and Utilization of Pork Variety Meats. Animal Science Iowa State University. Novel Processing – Pork Variety Meat Workshop Ames. "Last accessed 14/5/2019". http://works.bepress.com/rodrigo_tarte/20/
8. Yannick, N., Rawlings, N. and Emmanuela, A. (2013). Assessment of bacteriological quality of cooked pork meat sold along the commercial streets of Nkwen through Bambili Metropolis, Cameroon. African Journal of Food Science. 7(12): 441-445.
9. Meat Technology Update (2006). Cutting edge technology for the meat processing industry. Food Science Australia. 1-5. "Last accessed 14/5/2019"; https://meatupdate.csiro.au//MeatQualityIndexPage.htm.
10. Pipek, P., Rohlík, B., Potůček, T. and Šimoniová, A. (2012). The composition of pork lard as a raw material in meat production. Journal of Food Science and Technology. 2: 115-119.
11. Gatlin, L.A., See, M.T., Hansen, J.A. and Odle, J. (2003). Hydrogenated dietary fat improves pork quality of pigs from two lean genotypes. Journal of Animal Science. 81(8): 1989 - 1997.
12. Seerley, R.W., Briscoe, J.P. and McCampbell, H.C. (1978). A comparison of poultry and animal fat on performance, body composition and tissue lipids of swine. Journal of Animal Science. 46: 1018–1023.
13. Miller, M. F., Shackelford, S.D., Hayden, K.D. and Regan, J.O. (1990). Determination of the alteration in fatty acid profiles, sensory characteristics and carcass traits of swine fed elevated levels of monounsaturated fats in the diet. Journal of Animal Science. 68: 1624–1631.
14. Madsen, A., Jakobsen, K. and Mortensen, H. (1992). Influence of dietary fat on carcass quality in pigs. A review. Acta Agriculturae Scandinavica Sect A Animal Science. 42(4): 220-225.
15. Nizar, N.N.A., Marikkar, N.M.J. and Hashim, D.M. (2013). Differentiation of Lard, Chicken fat, Beef fat and Mutton fat by GCMS and EA-IRMS Techniques. Journal of Oleo Science. 62 (7): 459-464.
16. Odey, M. O., Mboso, E. O., Ujong, U. P., Johnson, J. T., Gauje, B. and Ategwu, M. A. (2013). Microflora analysis of selected meat and meat products from Calabar, Cross River State-Nigeria. Archives of Applied Science Research. 5(3): 50-56.
17. Abdelwhab, S., Olish, E., Mohammed, S., Khatim, S.B.S., and Mohammed, H. (2017). A Comparative Study of Fat Content in Beef and Sheep Meat. International Journal of Research in Agriculture and Forestry. 4(2): 41-44.
18. Aymerich, M.T., Garriga, M., Costa, S., Monfort, J.M., Hugas, M. (2002). Prevention of ropiness in cooked Pork by bacteriocinogenic cultures. International Dairy Journal, 12(2/3): 239-246.
19. Lulietto, M. F., Sechi, P., Borgogni, E. and Cenci-Goga, B.T. (2015). Meat Spoilage: A critical Review of a Neglected Alteration Due to Ropy Slime Producing Bacteria. Italian Journal of Animal Science. 14(3): 315-326.
20. Nychas, G.J.E., Skandamis, P.N., Tassou, C.C., and Koutsoumanis, K.P. (2008). Meat spoilage during distribution. Meat Science. 78: 77-89.
21. Lamb, K.E. (2017). The survival of various pathogenic organisms in fats. Master of Science Thesis. University of Kentucky. Lexington, Kentucky.
22. Shaffer, C. (2019). Microbes in Raw Meat. 1-5. "Last accessed 14/5/2019". https://www.news-medical.net/lifesciences/Microbes-in-Raw-Meat.aspx
23. Ifeoluwa, O.B. (2019). Harmful Effects and Management of Indiscriminate Solid Waste Disposal on Human and its Environment in Nigeria: A Review. Global Journal of Research and Review, 6(1): 1- 4.
Received: 28 June 2020
Accepted: 26 September 2020
Chinonye Medline Maduka*1, Akuma Oji2, Ugochi Queen Fineboy3 , Gideon Chijioke Okpokwasili2 .
*1World Bank Africa Centre of Excellence, Centre for Oilfield Chemicals Research, University of Port Harcourt, Port Harcourt, Nigeria.
2 Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria; [email protected].
2 Department of Chemical Engineering, University of Port Harcourt, Port Harcourt, Nigeria; [email protected].
3 Department of Microbiology, Michael Okpara University of Agriculture Umudike, Umuahia, Abia State, Nigeria.
ORCID: https://orcid.org/0000-0003-3569-3858.
