2021.06.01.8
Files > Volume 6 > Vol 6 No 1 2021
Phytochemical screening and antioxidant activity of Epidendrum nocturnum
Fernando Mencias1*, Telmo Salazar 2, Marco Cerna3
Available from: http://dx.doi.org/10.21931/RB/2021.06.01.8
ABSTRACT
The objective of this study was to evaluate the antioxidant capacity of Epidendrum nocturnum using the DPPH technique to determine the capacity for scavenging free radicals,as well as to identify secondary metabolites in ethanolic extracts of the previously mentioned species by phytochemical screening, with analysis of alkaloids, flavonoids, saponins,tannins, and triterpenes. The results determined in the phytochemical screening that the secondary metabolites were most present were flavonoids, tannins, and saponins; no alkaloidsor triterpenes were found. In the analysis of antioxidant activity, Epidendrum nocturnun in the three extracts showed that with an average concentration of 3.50 ppm, it could inhibit50% of the free radicals present in the test solution.Keywords. Antioxidant, Epidendrum, DPPH, metabolites, screening.
INTRODUCTION
Orchids belong to the Orchidaceae family, one of the most numerous families with approximately 35,000 species and striking for their beautiful flowers. 1 In Ecuador, Endara and Jost 2 mention that there are 1707 endemic orchid species, and because of this, it is said that this family is considered the most diverse of vascular plants.
We will find several secondary metabolites within the orchids such as alkaloids, flavonoids, phenols, terpenoids, and phenanthrenes; these being complex substances used as active principles in the pharmaceutical industry, they have a pharmacological or physiological action on the body.3
Within the scientific field, interest in verifying the ethnobotanical uses of plants, or obtaining new compounds with pharmacological activity, especially with antioxidant action. Because Ecuador has a great diversity of orchids used in traditional medicine that still do not have a phytochemical screening, the lack of scientific studies that corroborate these applications is evident; with those mentioned above, it is essential since active principles can be obtained to replace synthetic compounds used in the pharmaceutical industry.4
MATERIALS AND METHODS
Location and collection of the sample
The collection was carried out in the "Orquideario de Sarina," located in the province of Pichincha, in the canton of Quito, in the parish of El Quinche.
The plant material selected for this study was 100g of leaves; the leaves were cut with pruning scissors previously sterilized with 96% alcohol, placed in paper bags, and stored in plastic bags with a seam to avoid deterioration the samples.
Obtaining the ethanolic extract
To obtain the ethanolic extracts, a maceration was carried out according to the methodology described by Moreno and Jaramillo,5 the process consisted of: taking 100 g of young leaves, it was crushed in a porcelain mortar of 80 mm in diameter, 70 mL of 96% ethanol and macerated in an amber flask for 8 days in complete darkness. It was then filtered to remove leaf residues and stored in the dark and kept under ambient conditions; all this was repeated three times, obtaining three similar extracts.
Phytochemical screening
Using the Miranda and Cuellar Manual qualitative technique, preliminary colorimetric tests were used, which are fast and straightforward for determining secondary metabolites. The presence of alkaloids (Draggendorf;), flavonoids (Shinoda), saponins (foam), tannins (Gelatin-Salt), and triterpenes (Liebermann-Burchard) were determined in the ethanolic extracts.
If opalescence (+), turbidity (++), or precipitate (+++) is observed, it is considered that the sample contains the secondary metabolite; in all analyses, 96% alcohol was used as a negative control.
Test for alkaloids
Following the methodology described by Carrera et al.,6 using the Draggendorf reagent, 3 mL of sample was placed in a test tube, then 3 drops of the draggendorf reagent were added, vigorously shaken, and waited for 30 minutes. Caffeine was used as a positive control.
Test for flavonoids
Following the methodology described by Ramos et al.,7 using the Shinoda reagent; For which 3 mL of sample was placed in a test tube, then several magnesium filings were added, the test tubes were placed in a water bath at 60 ° C, then 3 drops of concentrated HCl were placed. Apple was used as a positive control.
Test of saponins
Following the methodology described by Moreno and Jaramillo, 5 using the foam test; for which 3 mL of sample was placed in a test tube, then 5 mL of distilled water was added and vigorously stirred for one minute. Quinoa was used as a positive control.
Tannins test
Following the methodology described by Sánchez and Calle, 8 using the Gelatine-Salt reagent, 3mL of the sample was placed in a test tube, then 2 mL of reagent was added. Black tea was used as a positive control.
Triterpenes test
Following the methodology described by Carrera et al., 6 using the Liebermann-Burchard reagent; for which 3 mL of sample was placed in a test tube, then 1 mL of acetic anhydride was slowly added through the wall of the tube, and finally, with caution, 2 drops of concentrated sulfuric acid were added. Calendula was used as a positive control.
Evaluation of antioxidant activity
For this test, the methodology of Noriega et al. 9 was used, for which a solution of 2,2-diphenyl-1-picrylhydracil DPPH was made. For each of the extracts, dilutions were prepared in different concentrations: 10 uL, 50 uL, and 80 uL in amber vials; it was completed with 96% alcohol obtaining a volume of 100 uL; 2.9 mL of DPPH was added to these dilutions until the final volume was 3 mL, it was homogenized and stored in the dark for 30 minutes.
Preparation of the DPPH solution
24 hours before the analysis, 500 mL of DPPH was prepared in 96% ethanol, for which 19.70 mg of DPPH was weighed, it was dissolved in 200 mL of drinking alcohol, then it was added to 500 mL, this The solution was stored in an amber bottle wrapped in aluminum foil refrigerated at 4 ºC, a technique described by Castañeda et al. 10
Vitamin C standard curve
The standard was prepared by dissolving 20 mg of ascorbic acid in 100 mL of ultrapure water as described by Noriega et al. 9
The concentration of vitamin C obtained was 0.2 mg / mL, with which the following dilutions were made:
100 uL of vitamin C + 0 uL of pure water
80 uL of vitamin C + 20 uL of pure water
60 uL of vitamin C + 40 uL of pure water
40 uL of vitamin C + 60 uL of pure water
20 uL of vitamin C + 80 uL of pure water
0 uL of vitamin C + 100 uL of pure water
The prepared solutions were placed in amber vials, and 2.9 mL of previously prepared DPPH was added to these, stirred, and remained in the dark for 30 minutes.
The prepared solutions were placed in 3 mL plastic cells to be analyzed in the spectrophotometer (JASCO V-730) at a wavelength of 517 nm; the samples were analyzed to increase their concentration, each sample was analyzed in triplicate.
The calibration curve obtained in this investigation can be seen in figure 1, which presents an R² = 0.9958, being acceptable because it is close to 1.
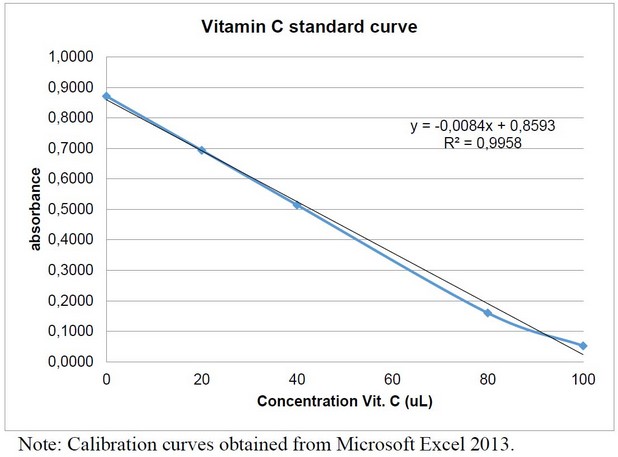
Figure 1: Vitamin C calibration curve
Preparation of samples for analysis in the spectrophotometer
From the extracts of each species under study and the following solutions were made:
10 uL of extract + 90 uL of 96% ethanol
50 uL of extract + 50 uL of 96% ethanol
80 uL of extract + 20 uL of 96% ethanol
100 uL of alcohol 96% + 2.9 mL DPPH
To analyze the samples in the spectrophotometer, the procedure used for vitamin C was replicated.
RESULTS & DISCUSSION
Secondary metabolites identification
The percentage of presence of secondary metabolites in the analyzed extracts of Epidendrum nocturnum were: flavonoids 100% in high concentration, tannins 100% in medium concentration, saponins 66.66% in low concentration, alkaloids and triterpenes 0%, the concerning result the alkaloids contrasts with the study done by Sut et al.,11 who found phenanthrenic alkaloids in species of the genus Epidendrum; This would indicate that the species collected has not had an external threat that induces the production of alkaloids, as explained by Farrán et al.,12 see table 1 and table 2.

Table 1: Phytochemical screening of Epidendrum nocturnum.

Note: Photographs of the tests carried out in the CIVABI UPS.
Table 2: Secondary metabolites analysis.
Evaluation of antioxidant activity
The extracts of E. nocturnum showed antioxidant capacity. The three extracts had similar results that, on average with a concentration of 3.50 ppm they inhibited 50% of the DPPH free radicals. See figure 2.
It was observed that the Epidendrum nocturnum species exceeded the antioxidant capacity of Prosthechea michuacana reported by González et al.,13, which required 13.22 ppm to inhibit the% IC50 of DPPH.

Figure 2: Percentage of inhibition of DPPH of Epidendrum nocturnum.
CONCLUSIONS
The secondary metabolites present in Epidendrum nocturnum in 100% were flavonoids and tannins; in saponins, it was 66.66%.
The antioxidant activity analysis showed in the 3 extracts Epidendrum nocturnum with only 3.5ppm inhibited 50% of DPPH free radicals.
Epidendrum nocturnum presented a significant antioxidant capacity and a high presence of flavonoids in its composition, confirming that this secondary metabolite contributes to these plants' antioxidant capacity.
REFERENCES
1. F. María, Orquídeas, Buenos Aires: ALBATROS, 2011.
2. L. Endara y L. Jost, «Orchidaceae,» de Libro rojo de las plantas endémicas del Ecuador., Quito, Ecuador, Herbario QCA, Pontificia Universidad Católica del Ecuador, 2011.
3. M. Hossain, «Therapeutic orchids: traditional uses and recent advances—An overview,» Fitoterapia, vol. 82, pp. 102-140., 2011.
4. C. Rivas-Morales, M. Oranday-Cárdenas y M. Verde-Star, Investigación en plantas de importancia médica, Nuevo León: OmniaScience, 2016, pp. 105-121.
5. N. Moreno y B. Jaramillo, «Análisis fitoquímico preliminar de Pachira quinata (Jacq.) WS Alverson,» Boletín Semillas Ambientales, vol. 11, nº 1, pp. 30-39, 2017.
6. G. Carrera, E. Benedito, T. Souza-Leal, C. Pedroso-De-Moraes y F. Gaspi, «Testes fitoquímicos em extratos foliares de Oeceoclades maculata Lindl.,» Revista Brasileira de Plantas Medicinais, vol. 16, nº 4, pp. 938-944, 2014.
7. P. Ramos, G. Colareda, M. Rosella, S. Debenedetti, E. Spegazzini y A. Consolini, «Phytochemical profile and anti-inflammatory effect of the orchid Catasetum,» Latin American Journal of Pharmacy, vol. 31, nº 1, pp. 62-67, 2012.
8. A. Sánchez y D. Calle, «Evaluación fitoquímica y determinación de flavonoides en hojas de Ficus benjamina L. Xilema,» Xilema, vol. 28, nº 1, pp. 61-67, 2015.
9. P. Noriega, T. Mosquera, A. Baldisserotto, J. Abad, C. Aillon, D. Cabezas, J. Piedra, I. Coronel y S. Manfredini, «Chemical Composition and in-vitro biological activities of the essential oil from leaves of Peperomia inaequalifolia Ruiz & Pav.,» American journal of essential oils and natural products, vol. 2, nº 4, pp. 29-31, 2015.
10. B. Castañeda, E. Ramos y L. Ibáñez, «Evaluación de la capacidad antioxidante de siete plantas medicinales peruanas,» Horizonte médico, pp. 61-62, 2008.
11. S. Sut, F. Maggi y S. Dall'Acqua, «Bioactive secondary metabolites from orchids (Orchidaceae) 14.11 (2017): e1700172.,» Chemistry & biodiversity, vol. 14, nº 11, pp. 1-30, 2017.
12. A. Farrán, C. López, M. Pérez y M. D. Santa María, Quimica bioorganica y productos naturales, Madrid: UNED, 2017.
13. A. Neira, Aislamiento e identificación de los compuestos con actividad antioxidante del extracto de cloroformo de la orquídea comestible Prosthechea michuacana., México: Escuela Nacional de Ciencias Biológicas. Instituto Politecnico, 2009.
Received: 15 OCtober 2020
Accepted: 14 november 2020
Fernando Mencias1*, Telmo Salazar 2, Marco Cerna3
1 Grupo de investigación Nunkui Wakan, Universidad politécnica Salesiana del Ecuador, Carrera de Biotecnología [email protected] ORCID ID https://orcid.org/0000-0001-7495-2053
Corresponding author:[email protected]
2 Grupo de investigación Nunkui Wakan, Universidad politécnica Salesiana del Ecuador, Carrera de Biotecnología [email protected] ORCID ID https://orcid.org/0000-0002-2109-5243
3 Grupo de investigación Nunkui Wakan, Universidad politécnica Salesiana del Ecuador, Carrera de Biotecnología [email protected] ORCID ID https://orcid.org/0000-0002-0911-9900
