2023.08.02.59
Files > Volume 8 > Vol 8 No 2 2023
Optimum conditions of the treatment of organic matters in gray water by Ozone
Esraa Hashim Ali1,*, Amal A. Hussein2,*, and Natheer Jamal Imran3,*
¹ Department of Applied Sciences, Division of Biotechnology, University of Technology- Iraq;
2 Department of Applied Sciences, Division of Biotechnology, University of Technology- Iraq;
3 Environment and Water Directorate, Ministry of Science and Technology, Baghdad, Iraq;
* Correspondence: [email protected]. [email protected]. [email protected].
Available from: http://dx.doi.org/10.21931/RB/2023.08.02.59
ABSTRACT
Wastewater treatment processes are critical and economical to improve the quality, remove the more significant part of the contaminants from wastewater and solve the water crisis, which may provide new water sources. To conserve water, the gray water (GW) must be recycled. The use of Ozone in water treatment is a chemical treatment based on the infusion of Ozone into water or wastewater. In this study, the best conditions for treating gray water with Ozone were investigated to achieve the best removal of organic matters represented by chemical oxygen demand (COD), Biochemical oxygen demand (BOD₅), Total organic carbon (TOC), Oils and Greases. It was experimentally concluded that the ozone treatment is positively affected by the increase in pH, the ozone concentration and the time of exposure to Ozone. The ideal conditions for ozone treatment were determined according to the quality of the resulting water and not according to the removal values of organic materials. These conditions are pH 8, ozone concentration of 40mg/l and ozone exposure time of 30 min.
Keywords: Gray water; Ozonation; Water Treatment; COD.
INTRODUCTION
Water scarcity is one of the significant challenges faced by human beings. Drought, conflicting demand for water in most parts of the world and increasing pollution rates in natural water sources¹ have caused intense pressure on water sources. As a result, it has become necessary to rely on wastewater as a source of reusable water. Wastewater treatment processes are critical and economical to improve the quality, remove the greater part of the contaminants from wastewater and solve the water crisis, which may provide new sources of water²’³. On the local scale, one method of conserving water is by recycling gray water (GW). Gray water eliminates toilet wastewater and includes water from baths, hand basins, showers, kitchen sinks and washing machines in households, schools, office buildings and others⁴. The total percentage of GW constitutes about 75% of the volume of residential wastewater. GW treatment for reuse is an essential strategy for reducing global water shortage, especially in places where water is scarce, and converting it to a better quality. The treated water doesn't have to be drinkable but can at least be used for various other purposes, such as gardening and agriculture, industrial purposes, steam generation, others⁵’⁶’⁷’⁸. Among the main components of gray water are organic materials, including proteins, carbohydrates and fats, at rates of approximately 50%, 40% and 10%, respectively. These substances may be biodegradable, nonbiodegradable or toxic carbohydrates and proteins are readily biodegradable. The fragmentation of fats by bacteria is more complicated. Many phenols, surfactants, and recalcitrant organic compounds resist decomposition by bacteria in biological treatment. Chemical oxygen demand (COD) measures organic substances and represents the total amount of oxygen wanted to oxidize the organic matter by strong oxidizing chemicals (potassium dichromate) in acidic condition⁹.
Presently used technologies in wastewater treatment are no longer efficient in getting rid of certain types of pollutants that can stay in the treated wastewater; the advanced treatment technologies that are effective in wastewater treatment plants have been investigated in recent years. In the current study, we will treat the water with Ozone and see how it affects the gray water, specifically, its effect on organic compounds in gray water.
Ozonation (also referred to as ozonization) is the use of Ozone in water treatment as a chemical treatment based on the infusion of Ozone into water or wastewater. Ozone (O₃) is a strong oxidant with almost 10 times higher solubility in water than pure oxygen, reacting with organic and inorganic compounds directly via a hydroxyl radical mechanism and by Advanced oxidation processes (AOPs), which are considered a highly efficient technology regarding water treatments for the reduction of organic pollutants classified as bio-recalcitrant and for the inhibition of pathogen microorganisms resistance to conventional techniques⁹’¹⁰’¹¹.
Ozone applications can be divided into destruction and synthesis, whereas its ability to oxidize substances can be used to break them down or synthesize new chemical compounds. Ozone has an influential and comprehensive role in water purification and treatment applications in various types of water: raw, fresh or salt water or groundwater for use as drinking water; it is also used in the treatment of domestic and industrial wastewater, whether for reuse or disposal to natural water sources, in addition to the possibility of reusing this treated water in swimming pools, cooling tower systems, and even for irrigation purposes¹².
Due to the nature of its reactive molecule and its high ability to oxidize substances, Ozone can achieve specific objectives if it is used alone in treatment or enhanced by other technology or catalysts. These objectives may include disinfection, color removal, the degradation of organic micropollutants and the reduction of chemical oxygen demand (COD)¹³. This study aims to install a wastewater treatment system consisting of an ozone treatment unit, then investigate the ability of this system to treat and minimize organic substances in gray water.
MATERIALS AND METHODS
Study Site and Sampling
Gray water samples (hand washing and kitchen sink) were manually collected from Baghdad, Iraq. The sample type is composite; the greywater samples were taken in three stages from November to February 2021-2022. Each stage includes collecting a 100-liter sample of gray water and storing it at 4°C to reduce biodegradation until treatment.
Ozone Generator
The Ozone used in this study was produced by an ozone generator (The POG-5G Portable Ozone Generator is equipped with a built-in internal air compressor and heavy-duty cooling fan) based on the corona discharge method, Where the electricity passes through a narrow cavity filled with air or oxygen, the oxygen molecules will cleavage into oxygen atoms (Oˉ) and these atoms combine with other oxygen molecules (O₂) to give Ozone (O₃). Figure (1) shows the ozone generator used in this study.

Figure 1. The POG-5G Portable Ozone Generator.
Chemicals
All solvents and chemicals were at least of analytical purity (> 95%) and bought from several suppliers.
Experimental steps
Initially, GW samples were brought to the laboratory, stored at 4°C, and then analyzed to determine the characteristics of GW in terms of its organic compounds before treatment. GW characterization was done according to steps and working methods described in APHA (the Standard Methods for the Examination of Water and Wastewater)¹⁴. pH measured by pH meter (WTW, InoLab), Chemical Oxygen Demand (COD) measured by Open Reflux Method 5220 B.¹⁴, Biochemical oxygen demand (BOD₅) values were determined by measuring oxygen consumption using an oxygen meter (WTW, InoLab) after 5 days of incubation of samples, Total Organic Carbon (TOC) measured by the direct method, Oil and Grease measured by Partition-Gravimetric Method 5520 B. ¹⁴, and the Turbidity was measured by the turbid meter (Lovibond). pH of the samples was adjusted using NaOH and H₂SO₄; all experiments were performed at room temperature.
Treatment design
Experiments were conducted to determine the effect of the ozone concentration used in the treatment, the pH and duration of exposure to Ozone (Time) on organic content degradation, and thus determine the optimum conditions for degradation. Ozonation was carried out on 5 liters of gray water for each variable, operated in semi-batch mode, for various pH values between 2 and 10, and ozone exposure times between 30 and 150 min. And the applied ozone concentrations changed from about 20 to 40 mg O3/L; for selecting these variables, previous works were considered. In all experiences, the ozone doses refer to the ozone concentrations in the gas phase. At the end of the treatment under specified conditions, samples of the treated gray water were withdrawn from the reactor to perform the analyses required to determine values of COD, BOD₅, TOC and Oil and Grease after treatment for the estimation of the treatment efficiency.
Starting and before performing each treatment, the gray water is examined, and the values of each of the following parameters are determined: pH, COD (mg/l), BOD₅ (mg/l), TOC (mg/l) and Oil and grease (mg/l), as shown in Table 1.
In the first stage, the treatment was carried out using five concentrations of Ozone, a fixed exposure time of 30 minutes, and pH=8. These conditions degraded the organic matters represented by COD, BOD₅, TOC and Oil values, as shown in Table 2.
In the second stage, the treatment was done by changing the pH value and fixing the exposure time and the ozone concentration. These conditions degraded the organic matters represented by COD, BOD₅, TOC and Oil values, as shown in Table 3.
In the third stage, the treatment was done by changing the exposure time to Ozone and stabilizing the ozone concentration and pH value. These conditions degraded the organic matters represented by COD, Oil and Grease and TOC values, as shown in Table 4.
Experimentally, the gray water retreated at the variables that produced the highest removal of organic matter, pH 10, Ozone concentration 40 mg/l and ozone exposure time 150min., which produced water with a pH of 10 and alkaline water of limited use. Therefore, the second optimal condition was chosen with high degradation, but less than the first condition, and it produces water with a pH of 8 within the values of natural water¹⁵.
Data Processing
The Removal percentage was calculated according to the formula¹⁴:
Removal % = (Co-C)/Co ˟ 100
Where Co = concentration before treatment.
C = concentration after treatment.
RESULTS
Gray water characteristics
Gray water characteristics are presented in Table 1 without treatment. All water samples were considered slightly alkaline since the initial pH (before treatment) was above seven. No pH changes were observed over time during our experiments. The water sample taken was characterized by its yellowish-white color; it has a thicker texture than regular water and is soapy and misty because it contains suspended solids, oils, washing liquids, soaps and kitchen wastes.
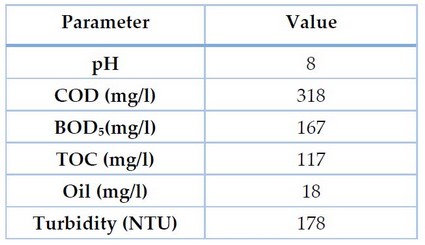
Table 1. Parameters values of gray water before treatment
pH, COD, BOD₅, TOC, Oil and Grease and Turbidity values are normal as values of gray wastewater of mixed origin (bathroom, hand wash and kitchen sink)⁵. This information is essential to evaluate the efficiency of the treatment or the possibility of reuse.
Ozonation
Impact of ozone concentration
All parameters responded to the ozone treatment and were degraded below the initial value. In the first stage, the treatment was carried out using five concentrations of ozone 20,25,30,35 and 40 mg/l and a fixed exposure time of 30 minutes, pH=8. These conditions degraded the organic matters represented by COD, BOD₅, TOC and Oil and Grease values, as shown in Table 2. Note: Decimals are neglected in the result.
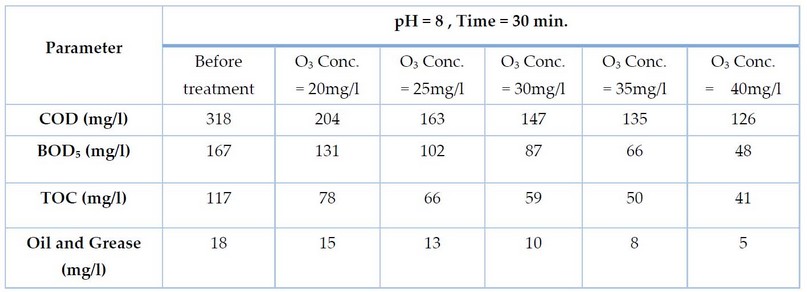
Table 2. Parameters values of gray water after ozonation at different ozone concentrations.
Exposing gray water to ozone concentrations of 20, 25, 30, 35 and 40 mg/l at pH 8 and a time of 30 minutes led to obtaining a removal percentage of COD of 35, 48, 53, 57 and 60%, respectively, and BOD removals of 21, 38, 47, 60 and 71%, respectively, and removal of TOC 33, 43, 49, 57 and 64%, respectively, and oil and grease removal 16, 27, 44, 55 and 72 percent, respectively, as shown in Figure 2:
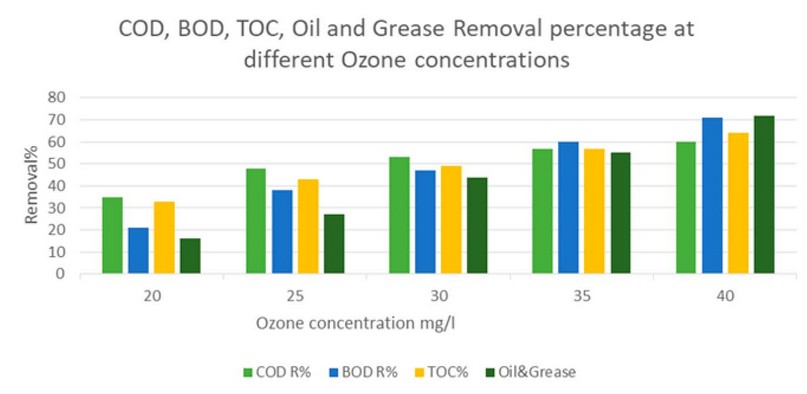
Figure 2. COD, BOD₅, TOC and Oil and Grease Removal at different ozone concentrations.
Impact of pH
In the second stage, the treatment was done by changing the pH values and fixing the exposure time and the ozone concentration. These conditions degraded the organic matters represented by COD, BOD₅, TOC and Oil and Grease values, as shown in Table 3.
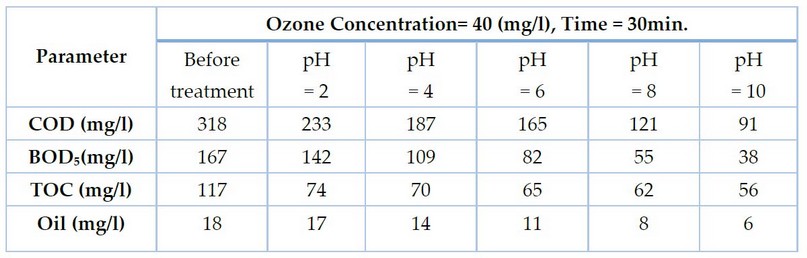
Table 3. Parameters values of gray water after ozonation at different pH values.
Treatment at pH values 2, 4, 6, 8, and 10 produced a removal of COD of 26, 41, 48, 61, and 71%, respectively, removal of BOD of 14, 34, 50, 67, and 77%, and of TOC 36, 40, 44, 47, and 52%, respectively, and oil and grease removals of 5, 22, 38, 55 and 66%, respectively, as shown in Figure 4:
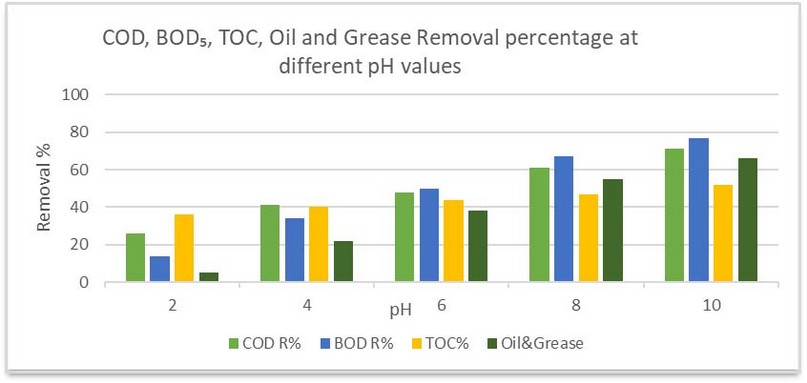
Figure 3. COD, BOD₅, TOC and Oil and Grease Removal at different pH values.
Impact of ozone exposure time
In the third stage, the treatment was done by changing the exposure time to Ozone and stabilizing the ozone concentration and pH value. These conditions gave a degradation of the organic matters represented by COD, Oil and Grease and TOC values, as shown in Table 4:
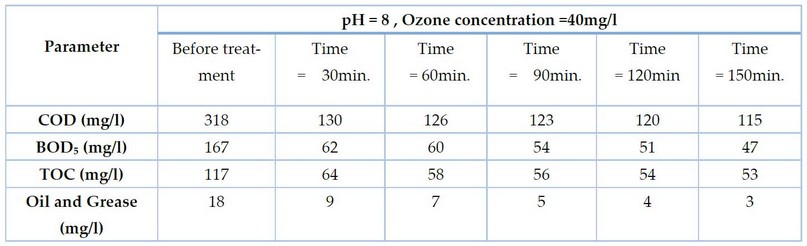
Table 4. Parameters values of gray water after ozonation at different ozone exposure times.
These exposure times (30, 60, 90, 120 and 150) produced a removal of COD of 59, 60, 61, 62 and 63%, respectively, and a removal of BOD 62, 64, 67, 69, and 71%, and a removal of TOC 45,50,52,53, and 56% and removal of oils and greases, amounting to 50,61,72,77 and 83%, respectively, as shown in Figure 4:
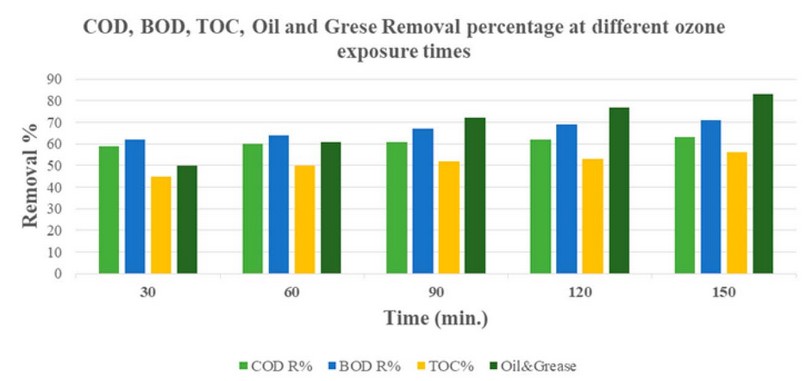
Figure 4. COD, BOD₅, TOC and Oil and Grease Removal at different ozone exposure times.
During all three stages of treatment, the foam was formed continuously throughout the treatment period, and the pH value changed slightly and did not affect the treatment.
Optimum conditions
Although the most degradation of organic matters represented by COD, BOD₅, TOC and Oil and Grease occurred at PH 10, exposure time of 150 minutes and Ozone concentration of 40mg/l, they cannot be considered optimum conditions, as they are conditions in which the results of the degradation of organic materials are the best possible compared to the other conditions within this study. Still, they produce water of limited use due to its alkalinity and the length of the treatment period.
Therefore, it is possible to determine less optimum conditions in terms of organic matter degradation. Still, it produces water of higher quality, especially as it achieves a very good removal of organic matter, and a shorter treating time, which makes treatment more practical. These conditions are PH 8, Ozone concentration of 40mg/l and exposure time of 30 min.
The water retreated under both determined conditions, as shown in Table 5:
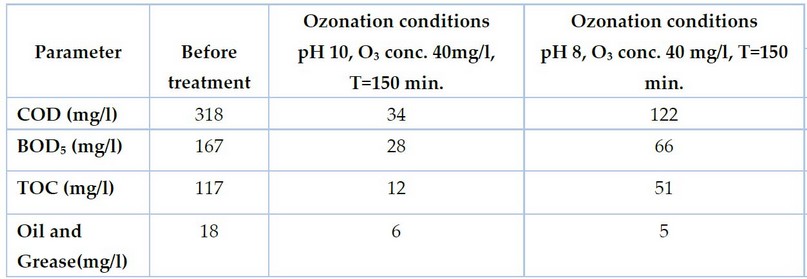
Table 5. Result of gray water treatment under two sets of optimum conditions
The Ozonation conditions of pH 10, O₃ conc. 40mg/l, T=150 min. produced a removal of COD of 89%, a removal of BOD₅ 83%, a removal of TOC of 89% and a removal of oils and greases of 66%,
While the Ozonation conditions of pH 8, O₃ conc. 40 mg/l, T=150 min. produced a removal of COD of 61%, a removal of BOD₅ 60%, a removal of TOC of 56% and a removal of oils and greases of 72%; these conditions produce water with pH = 8, which is The same pH value as natural water⁵ and within the permissible pH value of irrigation water, which makes its use more widely. Ozone also showed high efficiency in removing color at these conditions, as shown in Figure 5, knowing that Ozone removed the color with good efficiency under all the treatment conditions used in this study.

Figure 5. gray water (A) before treatment, (B) after treatment
DISCUSSION
The Impact of ozone concentration, the above results showed that as the concentration of ozone increases, the concentrations of organic substances decrease, and this corresponds to several studies¹⁷’¹⁸’¹⁹. The total COD removal was between 35 and 60 %, increasing with the ozone concentration and depending on the sample's initial COD content. Among ozone dosages, after 30 minutes, COD removal at 40 mg/l became significantly higher than at the other four concentrations. The explanation is that the increase in ozone concentration means more Ozone available per molecule of organic substances in water. In addition to the concentration of Ozone reflecting on the production of hydroxyl radicals, increasing the concentration of Ozone directly leads to the activation of the generation of hydroxyl radicals, which in turn increases the degradation of organic compounds. Although another study was conducted, it proved that increasing the ozone concentration to more than 40mg/l does not lead to an essential difference in the COD and TOC removal²⁰ rate. These results accord well with previous studies²¹’²²’²³. Another study concluded that higher ozone dosage might adversely affect hydroxyl radicals generation through Eq.1. In this case, Ozone will react with hydroxyl radicals, producing hydroperoxyl radicals, which are less reactive than hydroxyl radicals²³. Then, this reaction might suppress the removals of COD and TOC in ozone treatment.
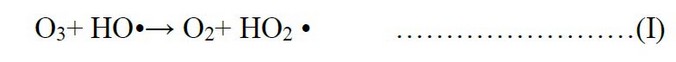
This study concluded that the best treatment of organic materials at an ozone concentration of 40 mg/l represents the highest concentration used in this study. The decrease in BOD₅ has been referred to as the formation of smaller, oxygenated types of organic compounds, which is easier for microbes to consume and more suitable for microbial attack. It may be to the reduction of bactericidal components, for example, phenolics²⁴. The Impact of pH, the organic substances removal by Ozone is influenced mainly by pH²⁵. Direct mechanical prevailed by molecular Ozone at the pH within the acidic range (less than 7); at pH values within the basal range, more significant amounts of hydroxyl radicals are formed. For pH 10, the decomposition of O₃ into hydroxyl radicals is prompt, which means that the indirect mechanism will prevail (via OH radicals)¹¹. The results of the second stage proved that the pH significantly influenced the treatment efficiency. At pH 10, the organic matter removal achieved the highest values pointing that a larger quantity of OH radicals was formed, and the indirect reaction prevailed because hydroxyl radicals are less selective and more reactive to degrade organic compounds leading to the increase in organic substances removal. This is not true for some complex chemical substances that disintegrate more and faster at an acidic pH²⁶. From the preceding, it can be considered that pH 8-10 is ideal for the degradation of organic materials in grey water; despite the highest degradation at pH 10, other studies have found the same result²⁵’²⁷. During the Impact of ozone exposure time, increases in ozone exposure time can lead to the extensive breakdown of organic compounds (for instance, aliphatic and aromatic structures and other electron-rich targets) into lesser molecular weight compounds, thus increasing the amount of biodegradable organic compounds.
Moreover, increasing the time makes the water more saturated with Ozone, thus allowing a higher rate of oxidation and fragmentation of organic matter. It was noted that the highest fat removal was done at the most prolonged time due to the increase in the oxidation period of oils and greases. In contrast, the double bonds in oils and greases can undergo oxidation. This can also be attributed to the hydrogen peroxide H₂O₂ produced by two hydroxyl radicals (OH) are produced from two O₃ molecules which further oxidize and decompose the Oils and Greases²⁸.
CONCLUSION
Grey water treatment with Ozone exhibited high efficiency in organic substance removal. At pH 10, ozone concentration of 40 mg/L, and ozone exposure time of 150 minutes, organic matter was removed most. Still, it is not considered optimum conditions because it produces alkaline water unsuitable for use in most fields. Therefore, the optimum conditions were pH 8, ozone concentration 40 and treatment time 30. The reactivity of Ozone with organic substances is great and promises a significant development in treating and reusing wastewater, especially gray ones. Ozone breaks down organic materials at acidic and basic conditions, although the efficiency of the treatment increases with increasing pH. This may enable us to produce potable water if a second treatment with Ozone eliminates salts and inorganic materials. Ozone treats the wastewater without forming any sludge or other solid waste. Thus, no residue will be left after the treatment, reducing waste disposal costs. Enacting a law to separate gray water from black water in sewage networks is recommended to facilitate its treatment and utilization.
Funding: This research received no external funding.
Acknowledgments: I would like to thank the Environment and Water Department staff at the Ministry of Science and Technology - Iraq for providing technical and administrative assistance to complete my research.
REFERENCES
1. Hussein, A. A. Monthly changes of some physicochemical parameters for Tigris river- Baghdad between2002-2003. Engineering and Technology Journal, 2009, 27(2), 64-70.
2. He, Z.; Lyu, Z.; Gu, Q.; Zhang, L. and Wang, J. Ceramic-based Membranes for Water and Wastewater Treatment. Journal of Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 578, 123513. https://doi.org/10.1016/j.colsurfa.2019.05.074.
3. Ait-Mouheb, N.; Bahri, A.; Thayer, B.B.; Benyahia, B.; Bourrié, G.; Cherki, B.; Condom, N.; Declercq, R.; Gunes, A.; Héran, M.; Kitir, N.; Molle, B.; Patureau, D.; Pollice, A.; Rapaport, A.; Renault, P.; Riahi, K.; Romagny, B.; Sari, T.; Sinfort, C.; Steyer, J.P.; Talozi, S.; Topcuoglu, B.; Turan, M.; Wéry, N.; Yıldırım, E. and Harmand, J. The reuse of reclaimed water for irrigation around the Mediterranean Rim: a step towards a more virtuous cycle? Journal of Regional Environmental Change, 2018, 18, 693–705. https://doi.org/10.1007/s10113-018-1292-z.
4. Ali A. A. The Reuse of Greywater for irrigation in suburban area, Thesis, Building and Construction Engineering Department, University of Technology-Iraq, 2013.
5. Eriksson, E.; Auffarth, K.; Henze, M. and Ledin, A. Characteristics of grey wastewater. Journal of Urban Water, 2002, 4, 85–104. http://dx.doi.org/10.1016/S1462-0758(01)00064-4.
6. Etchepare, R.; Peter, J. and Der Hoek, V. Health risk assessment of organic micropollutants in graywater for potable reuse. Journal of Water Researches, 2014, 72, 186–198. https://doi.org/10.1016/j.watres.2014.10.048.
7. Khalil, M. and Liu, Y. Greywater biodegradability and biological treatment technologies: A critical review. International Biodeterioration & Biodegradation, 2021, 161. https://doi.org/10.1016/j.ibiod.2021.105211.
8. Shaikh, I. and Ahammed, M. Quantity and quality characteristics of greywater: A review. Journal of Environmental Management, 2020, 261, 110266. https://doi.org/10.1016/j.jenvman.2020.110266.
9. Qasim, S. R. and Zhu, G. Wastewater Treatment and Reuse Theory and Design Examples Volume 1: Principles and Basic Treatment. Taylor & Francis Group.Taylor & Francis Group, London, UK, 2018; pp.34-66.
10. Najim, Y. S. .; Mohammed, T. T. .; Hussain, F. M. . The Impact Of Varying Azolla Dosages On Male Broilers Diets In Terms Of Economic Feasibility And Physiologic Performance. JLSAR 2022, 3, 42-45.
11. Cardenas, J.; García, B.; Agüera, A.; Pérez, J. and Agugliaro, F. Wastewater Treatment by Advanced Oxidation Process and Their Worldwide Research Trends. International Journal of Environmental Research and Public Health, 2019, 17(1), 170. https://doi:10.3390/ijerph17010170.
12. Gottschalk, C., Ann Libra, J. and Saupe, A. Ozonation of Water and Waste Water 2nd Ed., Wiley-VCH, Germany(2010); pp. 27-56.
13. Paraskeva, P. and Graham, N. Ozonation of Municipal Wastewater Effluents. Journal of the Water Environment Federation, 2002, 74, 569-581. https://doi.org/10.2175/106143002X140387.
14. Standard Methods for the Examination of Water and Wastewater, 20th ed. APHA, AWWA, WPCF: Washington DC, 1998.
15. Tolgyessy, J. Chemistry and biology of water, air and soil environmental aspects, Elsevier, 1993; pp. 24-28.
16. Chu, W.; Gao, N.; Yin, D.; Deng, Y. and Templeton, M. R. Ozone–biological activated carbon integrated treatment for removal of precursors of halogenated nitrogenous disinfection by-products. Journal of Chemosphere, 2012, 86(11), 1087-1091. https://doi.org/10.1016/j.chemosphere.2011.11.070.
17. Gerrity, D.; Pisarenko, A. N.; Marti, E.; Trenholm, R. A.; Gerringer, F.; Reungoat, J. and .Dickenson, E. Nitrosamines in pilot-scale and full-scale wastewater treatment plants with ozonation. Journal of Water Research, 2015, 72, 251-261. https://doi.org/10.1016/j.watres.2014.06.025.
18. Reaume, M. J.; Seth, R.; McPhedran, K. N.; da Silva, E. F.; and Porter, L. A. Effect of media on biofilter performance following ozonation of secondary treated municipal wastewater effluent: Sand vs. GAC. Journal of Ozone-Science & Engineering, 2015, 37(2), 143-153. https://doi.org/10.1080/01919512.2014.939741.
19. Barzegar, J.; Wu, J. and Ghanbari, F. Enhanced treatment of greywater using electrocoagulation/ozonation: Investigation of process parameters. Journal of Process Safety and Environment Protection, 2018, 121, 125-132. https://doi.org/10.1016/j.psep.2018.10.013.
20. Ekblad, M.; Falas, P.; El-taliawy, H.; Nilsson, F.; Bester, K.; Hagman, M. and Cimbritz, M., Is dissolved COD a suitable design parameter for ozone oxidation of organic micropollutants in wastewater? Journal of Science of the Total Environment, 2019, 658, 449–456. https://doi.org/10.1016/j.scitotenv.2018.12.085.
21. Altmann, J.; Ruhl, A.S.; Zietzschmann, F. and Jekel, M., Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Journal of Water Research, 2014, 55(15), 185–193. https://doi.org/10.1016/j.watres.2014.02.025.
22. Song, S.; He, Z.; Qiu, J.; Xu, L. and Chen, J. Ozone assisted electrocoagulation for decolorization of CI Reactive Black 5 in aqueous solution: An investigation of the effect of operational parameters. Journal of Separation and Purification Technology, 2007, 55(2), 238-245. https://doi.org/10.1016/j.seppur.2006.12.013.
23. Rivas, J.; Beltran, F.; Acedo, B. and Gimeno, O. Two-step wastewater treatment: sequential ozonation – aerobic biodegradation. Journal of Ozone Science and Engineering, 2000, 22, 617-636. https://doi.org/10.1080/01919510009408803.
24. Pereira, M.; Borges, A.; Heleno, F.; Faroni, L. and Silva, J. Experimental Design Optimization of Dairy Wastewater Ozonation Treatment. Journal of Water, Air, & Soil Pollution, 2018, 229, 1-11. https://doi.org/10.1007/s11270-018-3737-x.
25. Urbano, V.; Maniero, M.; Moya, M. and Guimarães, J. Influence of pH and ozone dose on sulfaquinoxaline ozonation. Journal of Environmental Management, 2017, 195(2), 224-231. http://dx.doi.org/10.1016/j.jenvman.2016.08.019.
26. Malik, S.; Ghosh, P.; Vaidya, A. and Mudliar, S. Hybrid ozonation process for industrial wastewater treatment: Principles and applications: A review. Journal of Water Process Engineering, 2020, 35, 101193. https://doi.org/10.1016/j.jwpe.2020.101193.
27. Kishimoto, N.; Hatanaka, M. and Kinoshita, Y. Applicability of ozonized water treatment for controlling fat, Oil, and grease deposition onto a drainpipe. Journal of Applied Research in Water and Wastewater, 2018, 10, 417-420. https://dx.doi.org/10.22126/arww.2018.974.
Received: May 15, 2023/ Accepted: June 10, 2023 / Published: June 15, 2023
Citation: . Ali , E.H.; Hussein , A.A.; Imran ,N.J. Optimum conditions of the treatment of organic matters in gray water by Ozone.Revis Bionatura 2023;8 (2) 59. http://dx.doi.org/10.21931/RB/2023.08.02.59
