2023.08.04.80
Files > Volume 8 > Vol 8 no 4 2023
Production and characterization of flavored goat milk gels using zinc and calcium salts Producing functional foods
Qausar ALKaisy 1,2*, Ali Alrikabi1, Jasim Al-Saadi2
1 Department of Food
Science/ College of Agriculture/ University of Basrah/ Iraq; ORCID.
2 Department of Dairy
Science and Technology/ College of Food Science/ AL-Qasim Green University/
Iraq. ORCID 0000-0002-0344-3566.
*Correspondence: [email protected]. edu.iq; Tel.:
009647715967596.
Available from. http://dx.doi.org/10.21931/RB/2023.08.04.80
ABSTRACT
Goat milk gels were prepared using calcium and zinc salts.
The viscosity of gel prepared by adding zinc chloride and flavored with banana
and orange was higher than that of gel prepared using calcium chloride. WHC of
gels prepared using zinc chloride and calcium chloride was high on the first
day and then gradually decreased during storage time at 7 °C. The hardness of
the sweetened, flavored goat milk gel prepared with calcium chloride was lower
than that of the gel prepared with zinc chloride. The Sensory evaluation study
showed that, in general, flavoring gels prepared from goat milk using zinc and
calcium salts had a high degree of acceptability.
Keywords: flavored gel; Zinc chloride; Goat milk; Rheological
properties
INTRODUCTION
Goat's milk is
similar to cow's milk in its elemental composition 1. Goat milk contains 12.2%
total solids, consisting of 3.8% fat, 3.5% protein, 4.1% lactose, and 0.8% ash.
It has more fat, protein, ash, and less lactose than cow's milk 2. Goat milk
contains less total casein but higher non-protein nitrogen than cow's milk 3.
Goat milk has less αs-CN fraction and more κ-CN and β-CN fractions than cow
milk 4.
Milk protein gel formation is an essential and fundamental process in
the dairy industry. This phenomenon occurs when protein-protein interactions
form a three-dimensional network capable of trapping water mole-cules. Several
types of protein gels are produced from milk and its components. The formation
of acid gels is the most important because of its role in the formation of
yogurt and similar products 5. Whey proteins form a gel substance due to
exposure to a temperature higher than 70°C 6. While gels containing both whey proteins
and casein can form during high-temperature storage of UHT milk 7. Heating milk
after adding calcium chloride at concentrations ranging from 7 to 20 mM results
in a gel similar to yogurt's but with a pH close to natural milk's pH of 8.
Despite extensive studies on the effect of added calcium salts on casein
particles, pure caseins, or pure whey protein systems, information about the
effect of heat treatments on the interactions of added calcium with casein and
whey proteins present in milk is limited. The effects of calcium ion activity,
pH, temperature, and ionic strength on the physicochemical and rheological
properties of the gel produced by adding calcium to skim milk were previously
reported 9,10,11. The rheological properties of this type of gel were also
indicated by some researchers 11. This study aimed to prepare flavor gels from
goat milk using calcium and zinc salts and identify their textural and sensory
properties.
MATERIALS AND METHODS
Milk samples
Samples of mixed local goat milk
were collected from several farms in Al-Hilla-Babil - Iraq, and fat was
separated from milk using a milk separator to obtain skim milk.
Preparation of goat milk gels using
calcium and zinc salts
Several preliminary experiments
were established to determine the best conditions to form goat milk protein
gel. Skimmed goat milk samples (100 ml) were heated at 85 °C for 20 minutes,
sugar (7.5%) and flavoring materials (banana, orange, and cocoa flavors used
according to manufacturing companies (made in China) were added to milk
samples, mixed, and cooled to 22 °sC. Calcium chloride and zinc chloride (13.5
mM) were added separately within the normal pH of milk. After that, the milk
samples were heated to 85°C for 20 minutes and left without stirring to produce
a gel. Gels were stored at 7 °C for 28 days.
Gel hardness
Textural properties were evaluated
using a texture analyzer (CT3(4500), Brookfield engineering lab). The hardness
of the samples was measured. The operation conditions were an artificial
plastic cylinder (20 mm in diameter) inserted into each product to a depth
of 20 mm with a 10.0 g trigger and
speed of 1 mm/s 12.
Water Holding Capacity (WHC)
Water-holding capacity (WHC) of
sweetened flavored gel samples was determined as described by 13.
Briefly, 10 g of gel sample was centrifuged at 5000xg for 10 min at 5oC. The
resulting supernatant was carefully weighted to determine the amount of
excluded water, WHC % = [1-(w2 / w1)] ×100
WHC% = [1-(w2 / w1)] ×100
whereas:
W1: Weight of the gel used
W2: Whole weight after
centrifugation
Spontaneous Whey separation (SWS)
Spontaneous whey separation was
determined according to the procedure described by 14. A cup of
flavored gel was removed from the refrigerator and placed at a 45º angle. A
needle connected to a syringe was used to withdraw the liquid whey from the
sample's surface, and the whey's volume was measured. The process lasted for
less than 10s to avoid further leakage of whey from the gel. The spontaneous
whey separation was expressed as the percentage volume of whey over the initial
weight of the gel sample.
Viscosity
The gel was broken by stirring with
a glass rod (10 times clockwise; 10 times anticlockwise). Rotational viscosity
measurements were done utilizing a Brookfield viscometer (model DV- E;
Brookfield Engineering laboratories) utilizing spindle No 3. Separately,
measurement was caused at room temperature at 50 rpm for 1 minute, as described
by 15.
Sensory evaluation
Eight panelists did a sensory
evaluation among the College of Food Science/ Al-Qasim Green University staff
for flavored goat milk gels to estimate the acceptability of gel samples. Gel
specimens were assessed for flavor, body, texture, bitterness, and appearance/color
on a 100-point scale according to the ensuing: flavor 40, body 15, texture 15,
bitterness 20, and appearance/color 10. Mid scores from the eight panelists
were documented16.
RESULTS
Viscosity
of flavored goat milk gels
Figure (1) shows the storage
effect on the viscosity of flavored goat milk protein gel. The viscosity of gel
prepared by adding zinc chloride and flavored with banana, orange, and cocoa
were 2110, 1395, and 1154 cp, respectively, while the viscosity for gel
prepared using calcium chloride were 303, 296, and 301 cp, respectively, at day
1 of storage. The viscosity values gradually changed after 28 days of storage
at 7 °C, and they were 1630, 1882, and 786 cp for gel prepared with zinc
chloride and 223, 190, and 200 cp for gel prepared with calcium chloride,
respectively.
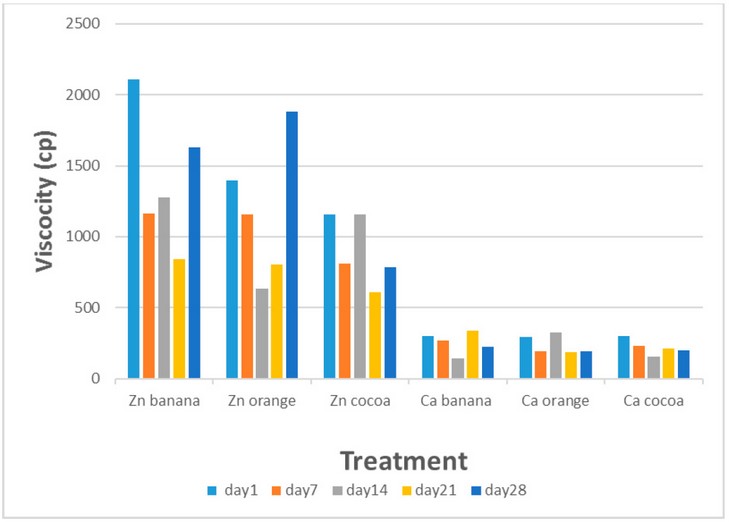
Figure 1. Changes in viscosity of
flavored goat milk gel prepared using 13.5 mM of zinc and calcium chloride
during storage at 7 ℃ for 28 days.
Water holding capacity of
flavored goat milk gels
Water
holding capacity is the ability of the gel to hold water within its structure.
Figure (2) shows that the WHC of gels prepared using zinc chloride and calcium
chloride was high on the first day and then gradually decreased during the
progress of storage time at 7 °C. WHC on the first day for goat milk gels
prepared using zinc chloride and flavored with banana, orange, and cocoa
flavors were 31, 29, and 28%, respectively, while WHC for gels prepared with
calcium chloride was 25, 28, and 29 % respectively. These values gradually
decreased to 16, 19.5, 21.4%, 21.4, 22.1, and 24.6%, respectively, after 28
days of storage at 7 °C.
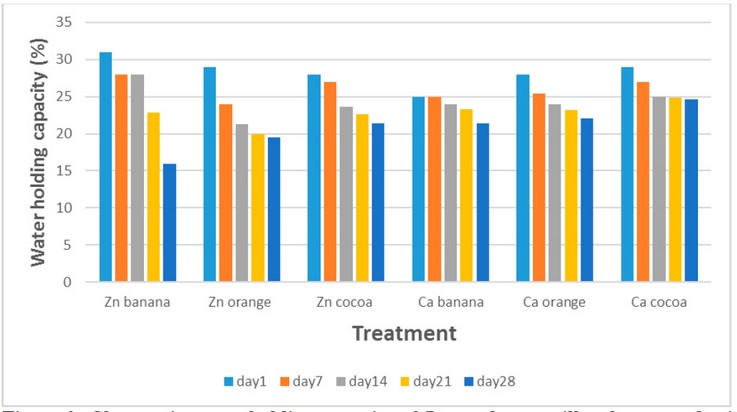
Figure 2. Changes
in water holding capacity of flavored goat milk gel prepared using 13.5 mM of
zinc and calcium chloride during storage at 7 ℃ for 28 days.
Spontaneous whey separation of flavored
goat milk gels.
Spontaneous whey separation means liquid exudation at the
top of the gel, which is one of the common defects of yogurt and sweetened gel.
Figure (3) showed that SWS in goat milk gels prepared with zinc chloride and
flavored with banana, orange, and cocoa were 0.9, 1, and 1.1 % in the first-day
storage, while these values in the gel prepared with calcium chloride were 0.5,
0.2, and 0 %, respectively. These values changed after 28 days of storage to
reach 1.4, 1.2, and 0.2 % for the gel prepared with zinc chloride and 1.5, 1,
and 1.2 % for the gel prepared with calcium chloride, respectively.
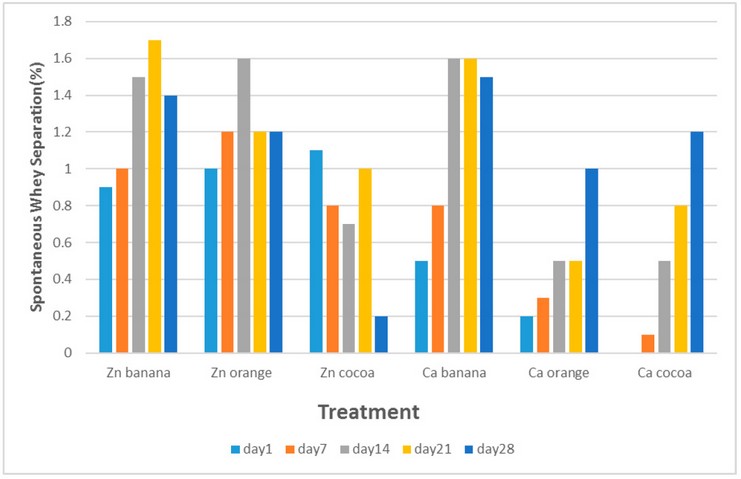
Figure 3. Changes in spontaneous
whey separation of flavored goat milk gel prepared using 13.5 mM of zinc and
calcium chloride during storage at 7 ℃ for 28 days.
Hardness of flavored goat milk
gels
Hardness is the force needed to induce deformation in a gel,
and it is a standard measure to indicate the strength of the gel network being
analyzed. Figure No. (4) showed that the hardness of the sweetened flavored
goat milk gel prepared with calcium chloride was lower than the hardness of the
gel prepared with zinc chloride, where the hardness values for calcium gels
flavored with banana, orange, and cocoa were 64.3, 57.9, 47.6 g, while the
hardness in zinc gels where 207.3, 178.6, 158.4 g, respectively in the 1 day of
storage, and these worth's altered after warehouse for 28 days at 7 ° C to
63.7, 54.4, 30 g for calcium gel and 237.2, 209.4, 147.3 g for zinc gels,
respectively. The difference in the hardness of the gel prepared using
different ions is due to the different ability of these minerals to interfere
with milk proteins and the difference in their binding sites with proteins,
which causes a difference in the three-dimensional structure of the gel network
17.
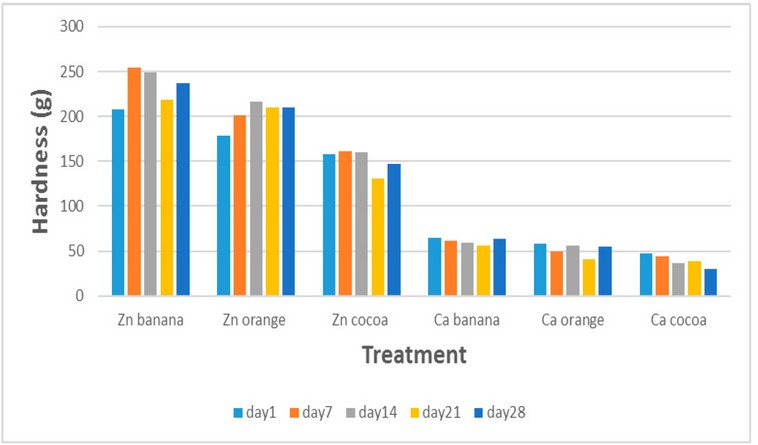
Figure 4. Changes in flavored
goat milk gel hardness were prepared using 13.5 mM of zinc and calcium chloride
during storage at 7 ℃ for 28 days.
Sensory evaluation of flavored
goat milk gels
In general, flavoring gels prepared from goat milk using
zinc and calcium salts were highly accepted (Figure 5). The total sensory
evaluation scores for the goat milk proteins gel prepared with zinc chloride
and flavored with banana, orange and cocoa on the first day were 90.6, 88.1,
and 92.75, while the scores for gel prepared with calcium chloride were 86,
85.75, 89.25, respectively. These values changed during storage to reach after
28 days to 94.25, 89.5, and 94.75 for gel prepared with zinc chloride and
94.25, 90.25, and 90 for gel prepared with calcium chloride. The gels produced
in this study were distinguished by their delicate texture and acceptable
hardness.
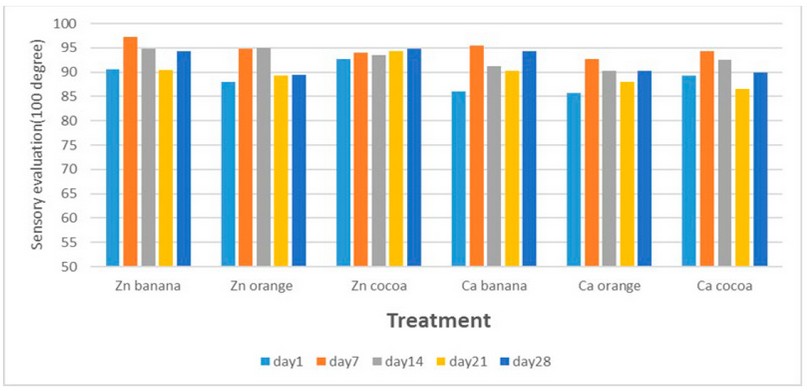
Figure 5.
Sensory evaluation score of flavored goat milk gel crafted utilizing 13.5 mM of
zinc and calcium chloride during storage at 7 ℃ for 28 days.
DISCUSSION
In general, the viscosity values for the sweetened
flavored gel prepared with zinc chloride were higher than the gel prepared with
calcium chloride, and this was due to the extensive loss of water from the gel
prepared by adding zinc chloride. Besides, the change in viscosity values is
due to the change in the properties of the sweetened gel during storage
resulting from increasing interactions between milk proteins and salts. These
results are consistent with what was indicated by 17,18, who
indicated that the hardness of the cow milk gel increased during the storage
period, accompanied by an increase in viscosity. Concerning WHC values
gradually decreased respectively after 28 days of storage at 7°C, and this is
due to the increase in the interaction between salts, caseins, and whey
proteins, which causes caseins to lose their net charges and increase their
hydrophobicity, which leads to a decrease the ability of the gel to hold water.
These results are consistent with the results found by 5,18, and
overall, we notice an increase in SWS during the storage period. It has an
inverse relationship with the water retention capacity of the protein network21,
and this can be due to the increase in the bonds between proteins and minerals
in the gel network, which increases its hydrophobicity and allows the exudation
of whey 18. as well as the difference in the hardness of the gel
prepared using different ions is due to the different ability of these minerals
to interfere with milk proteins, as well as the difference in their binding
sites with proteins, which causes a difference in the three-dimensional
structure of the gel network 18. The differences between the
hardness of milk protein gel produced with different flavors during the storage
period are related to the reactions between these flavors and milk proteins and
ionic salts that affect the interaction between milk proteins, which affects
the hardness6. This may be due to the addition of sugar during the preparation
of the gel, which causes a reduction in the interactions between milk proteins 19.
Panelists did not notice any undesirable taste in the resulting gels, and
although the gels were crafted from skim goat milk, multiple panelists related
them to be creamy. Hence, it may be achievable to utilize this technique to
make quiet-fat dairy outputs with identical textures to those with more
increased fat content.
.
CONCLUSIONS
the
study investigated the effects of different salts on the properties of
sweetened goat milk gels. The results showed that the viscosity of the gels was
higher when zinc chloride was used instead of calcium chloride. This was due to
the more extensive water loss from the gels prepared with zinc chloride. The
WHC of the gels gradually decreased over time, and the SWS increased. This was
due to the increase in the interactions between the salts, caseins, and whey
proteins, which caused the caseins to lose their net charges and increase
hydrophobicity. The panelists did not notice any undesirable taste in the
resulting gels and found them creamy. Therefore, this technique could produce
low-fat dairy products with the same texture as those with higher fat content.
Goat
milk protein gels were constructed from skim milk utilizing heat therapy and
13.5 mM calcium chloride and zinc chloride. Flavoring gels prepared from goat
milk using zinc and calcium salts were highly accepted.
Author
Contributions: Qausar ALKaisy and Jasim Al-Saadi: Methodology,
Resources, Writing-Original Draft. Ali Alrikabi: Conceptualization
Writing-Review & Editing.Jasim Al-Saadi: Writing-Review & Editing. All
authors have reviewed the final manuscript.
Funding: This research received no external funding.
Acknowledgments: We highly thank her (Ashwaq Kadhim-Rahia) for
their valuable help and support through this work. In addition, the financial
support for the in vitro part of this work by Dr. Qaisar Hamad Ghayeb is highly
acknowledged.
Conflicts of
Interest: The authors
declare no conflict of interest.
REFERENCES
1. Rai, D. C., Rathaur, A.,
Yadav, A. K. (2022). Nutritional and nutraceutical properties of goat milk for
human health: A review. Indian Journal of Dairy Science, 75(1). https://epubs.icar.org.in/index.php/IJDS/article/view/121647
2. Park, Y. W. (2010). Goat
milk products: quality, composition, processing, and marketing. Encyclopedia of
animal science. 2nd edition. Taylor and Francis. CRC Press. Boca Raton, FL. e
DOI: 10.1081/E-EAS-120045703
3. Delger, M. (2021). Effect
of seasonality and processing on physicochemical characteristics of goat and
sheep milk: a thesis presented in partial fulfilment of the requirements for
the degree of Master of Food Technology at Massey University, Palmerston North,
New Zealand. Massey University. http://hdl.handle.net/10179/16989
4. Khudai M Y, Abdulateef S
M, Mouhammed T Th, Alamili H S. Use of modern geometric design of fish ponds to
increase welfare and blood parameters. Revis Bionatura 2023;8 (2) 82. http://dx.doi.org/10.21931/RB/2023.08.02.82
5. Lucey,
J., Singh, H. (1998). Formation and physical properties of acid milk gels: a
review. Food research international, 30(7), 529-542. https://doi.org/10.1016/S0963-9969(98)00015-5
6. Zain,
H.; Tatar , A.; Alabi, O. M. .; Samiei Zafarghandi, M. . The Effect Of Using Different Levels Of Vitamin E On The
Antioxidants Status Of Broiler Chickens. JLSAR 2023, 4, 37-44.
7. Torrance L, Cowan GH,
McLean K, MacFarlane S, Al-Abedy AN, Armstrong M, Lim TY, Hein I, Bryan GJ.
Natural resistance to Potato virus Y in Solanum tuberosum Group Phureja.
Theoretical and Applied Genetics. 2020 Mar;133:967-80. https://doi.org/10.1007/s00122-019-03521-y
8. Datta, N., Deeth, H.
(2001). Age gelation of UHT milk—a review. Food and Bioproducts Processing,
79(4), 197-210. https://doi.org/10.1205/096030801753252261
9. Sievanen, K., Huppertz,
T., Kelly, A. L., Fox, P. F. (2008). Influence of added calcium chloride on the
heat stability of unconcentrated and concentrated bovine milk. International
journal of dairy technology, 61(2), 151-155. https://doi.org/10.1111/j.1471-0307.2008.00391.x
10. Koutina, G., Christensen,
M., Bakman, M., Andersen, U., Skibsted, L. H. (2016). Calcium induced skim-milk
gelation during heating as affected by ph. Dairy Science & Technology,
96(1), 79-93.
11. Lin, L., Wong, M., Deeth,
H., Oh, H. (2018). Calcium-induced skim milk gels using different calcium
salts. Food chemistry, 245, 97-103. https://doi.org/10.1016/j.foodchem.2017.10.081
12. Ramasubramanian, L.,
D’Arcy, B. R., Deeth, H. C., Oh, H. E. (2014). The rheological properties of
calcium-induced milk gels. Journal of Food Engineering, 130, 45-51. https://doi.org/10.1016/j.jfoodeng.2014.01.020
13. Ibraheem M W, Muhaimeed A
R, Mohammed Th. T. Leg cuts from Awaasi lambs fed a diet with varying levels of
Rhus coriaria L., Physical dissection and chemical composition. Revis Bionatura
2022;7(4) 4. http://dx.doi.org/10.21931/RB/2022.07.04.4
14. Harte,
F., Luedecke, L., Swanson, B., Barbosa-Canovas, G. (2003). Low-fat set yogurt made from milk subjected to combinations
of high hydrostatic pressure and thermal processing. Journal of Dairy Science,
86(4), 1074-1082. https://doi.org/10.3168/jds.S0022-0302(03)73690-X
15. Amatayakul, T., Sherkat,
F., Shah, N. P. (2006). Syneresis in set yogurt is affected by EPS starter
cultures and levels of solids. International journal of dairy technology,
59(3), 216-221. https://doi.org/10.1111/j.1471-0307.2006.00264.x
16. Donkor, O. N., Nilmini,
S., Stolic, P., Vasiljevic, T., Shah, N. (2007). Survival and activity of
selected probiotic organisms in set-type yoghurt during cold storage.
International Dairy Journal, 17(6), 657-665.
17. Bodyfelt, F. W., Tobias,
J., Trout, G. M. (1988). The sensory evaluation of dairy products. Van Nostrand
Reinhold.
18. N. Khorshed, A., S.
Ahmed, A. Cultivation Of Reishi Mushroom (Ganoderma Lucidum) On Different Local
Substates In Kurdistan Region, Iraq. Anbar Journal Of Agricultural Sciences,
2023; 21(1): 158-173. doi: 10.32649/ajas.2023.179727
19. Ali,
Z. K., Al-Saadi, J. M. (2019). Textural
and sensory properties of milk proteins gels made by ferrous salts. Euphrates
Journal of Agriculture Science, 11(4).
20. Belyakova,
L. E., Antipova, A. S., Semenova, M. G., Dickinson, E., Merino, L. M.,
Tsapkina, E. N. (2003). Effect of
sucrose on molecular and interaction parameters of sodium caseinate in aqueous
solution: relationship to protein gelation. Colloids and Surfaces B:
Biointerfaces, 31(1-4), 31-46. https://doi.org/10.1016/S0927-7765(03)00041-9
21. Niamah,
A. K., Al-Sahlany, S. T. G., Al-Manhel, A. J. (2016). Gum Arabic uses as prebiotic in yogurt production and study
effects on physical, chemical properties and survivability of probiotic
bacteria during cold storage. World applied sciences journal, 34(9),
1190-1196. DOI: 10.5829/idosi.wasj.2016.34.9.184
Received: 26 September 2023 / Accepted: 15 April
2023 / Published: 15 December 2023
Citation: Qausar, A.; Ali A.; Jasim A..Production and characterization
of flavored goat milk gels using zinc and calcium
salts Producing functional foods. Revis Bionatura 2023;8
(4) 80. http://dx.doi.org/10.21931/RB/2023.08.04.80
Publisher's Note: Bionatura stays neutral concerning jurisdictional
claims in published maps and institutional affiliations.
Copyright: © 2023 by the authors. Submitted for possible
open-access publication under the terms and conditions of the Creative Commons
Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
