2023.08.01.50
Files > Volume 8 > Vol 8 No 1 2023
Role of Vitamin D in the diagnosis of acute Myeloid Leukemia
1National Diabetes Center-Mustansiriyah University/Baghdad Iraq
2 Medical Technical College, Al-Farahidi University, Al-Jadiriyah Bridge, Baghdad, Iraq
3 Iraqi Center for Cancer and Medical Genetics Research -Mustansiriyah University Baghdad, Iraq
*Corresponding author is: [email protected], [email protected]
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.50
ABSTRACT
A range of hematological and biochemical markers have been investigated in Acute Myeloid Leukemia (AML) patients to determine the relationship between cancer growth and metabolic problems. This study aimed to determine the effects of vitamin D deficiency in Iraqi patients with acute myeloid leukemia who had recently been diagnosed. There was a significant inverse correlation between the total serum cholesterol (TC) level of acute myeloid leukemia (AML) patients group [(148.77±12.2) for males, (165.29±9.64) for females] and the control group [(164.50±7.26) for males, (180.05±7.31) for females], also an inverse correlation between high-density lipoprotein (HDL) level of acute myeloid leukemia (AML) patients group [(46.00±2.04) for males, (46.18±1.08) for females] and control group [(54.25±1.86) for males,(51.94±1.37) for females]. A significant difference was between the serum triglyceride (TG) level of acute myeloid leukemia (AML) patients group [(128.71±13.07) for males, (152.48±10.6) for females] and control group [85.12±11.30) for male, (90.50±10.90) for females], also between vitamin D level of acute myeloid leukemia (AML) patients group [(17.23±1.18) for males, (12.96±0.74) for females] and control group [(42.62±1.43) for males, (40.76±0.82) for females]. A statistically significant difference was between the serum calcium levels of individuals with acute myeloid leukemia [(8.99±0.32) for males, (8.91±0.23) for females] and the control group [(13.13±1.16) for males, (10.73±0.28) for females]. AML patients can benefit from vitamin D treatment, according to a pairwise analysis of receiver operating characteristic (ROC) curves. The above results are related to concluding that Vitamin D can be utilized as a diagnostic test for AML patients.
Keywords; acute myeloid leukemia (AML), Hypereosinophilia, ROC curve, hypocholesterolemia, vitamin D.
INTRODUCTION
Leukemia is a type of cancer that affects the blood and blood-forming tissues, such as the bone marrow and the lymphatic system. Several risk factors for leukemia have been identified, including smoking, a genetic disorder such as Down syndrome, exposure to a large amount of radiation or exposure to specific chemical compounds such as benzene, certain chemotherapy used to treat previous cancer, and having a family history of leukemia 1 . Chronic leukemia progresses more slowly than acute leukemia. Patients have a higher proportion of mature cells. In acute leukemia, the immature cells are rapidly advancing, and these cells cannot perform their normal functions 2. Malignancy myeloid cells lead to myeloid leukemia, whereas that including T and B lymphocytes leads to lymphocytic leukemia 3. Acute myeloid leukemia (AML) is a cancer that develops when long-lived myeloid blasts in the bone marrow and peripheral blood undergo unchecked transformation and proliferation. Normal cells are replaced by malignant ones, lowering the number of healthy cells 4.
Lipid metabolism disorders are characterized by anomalies of lipids and lipid metabolites occurring mostly in plasma and other tissues, which cause genetic or acquired factors 5. Several related genes, hormones and enzymes organize the level of lipids. Abnormalities of these factors lead to lipids metabolism disorders, which cause cardiovascular disorders, metabolic diseases and cancers6. Different studies have indicated that abnormal levels of lipids are closely related to carcinogenesis and cancer metastasis. Malignant cancer cell proliferation requires high energy to transform and accelerate, leading to lipid metabolism alterations 7. Some studies showed a decrease in plasma lipid levels in patients with cancer. This may result in increased utilization of blood lipids by malignant cells as a competing factor 8. Despite these positive correlations between hypercholesterolemia and carcinogenesis, some epidemiologic observations suggest no association exists between cholesterol and cancer progression 9.
Different studies have reported a relationship between Vitamin D deficiency and AML 10. An inverse relationship exists between circulating vitamin D levels and malignancy for colorectal 11 and breast cancer 12. This research sought to determine the levels of lipids, high-density lipoprotein (HDL), calcium, vitamin D, and various aspects of the hematological picture in newly diagnosed Acute Myeloid Leukemia (AML) patients in Iraq, with the ultimate goal of improving treatment outcomes.
MATERIALS AND METHODS
This study proceeded at the National Center for Hematology of Al-Mustansiriyah University in Baghdad from April 2019 until November 2019. This research enrolled 55 newly diagnosed AML patients before any specific medication was administered. Their ages ranged from (33-80) years and were compared to 26 healthy people who served as a control group. Body mass index was determined as weight in kilograms divided by the square of height (kg/m2). Before starting therapy for AML, blood samples were taken from patients at diagnosis and before they were treated for AML. The separated serum was used for total cholesterol, triglycerides and HDL measurements by Auto Analyzer Kenza 240 TX ( Biolabo, France). Vitamin D3 was determined by a mini vidas analyzer (biomerieux, France). Serum Ca was measured by the easy kit analyzer. (Beckman Coulter, Ireland) were used to analyze the Complete Blood Count (CBC).
Statistical Analysis
SAS was used to analyze the data statistically (Statistical Analysis System - version 9.1). To determine if there were any statistically significant differences between means, a one-way ANOVA and the Least Significant Differences (LSD) post hoc test were used. The p-value (P < 0.05) is regarded as statistically significant when it is less than one. The receiver operation characteristic (ROC) curve was used to determine the validity of markers as indicators of illness in a particular population. The area under the curve (AUC) of the features was calculated and compared (AUC). The analysis was completed with the help of the Med Calc software 13, 14.
RESULTS
This study reported different parameters for patients with AML compared with the control. In table (1), some of the baseline characteristics showed no change in an age when compared between patients and controls; there were increases in the weight of male patients and base mass index. Moreover, some of the hematological studies showed no change in values of Red blood cells (RBC), Basophils but increased Eosinophil values when compared between patients and controls.
Table 2 determined total serum cholesterol, triglyceride, high-density lipoprotein lipids, serum calcium and vitamin D for AML compared with the control. There was a decrease in TC, HDL,D 3 and calcium, while there was an increase in TG.
Figures 1 (A and B) illustrate the receiver operating characteristic curve (ROC) for calcium (A) and vitamin D (B).
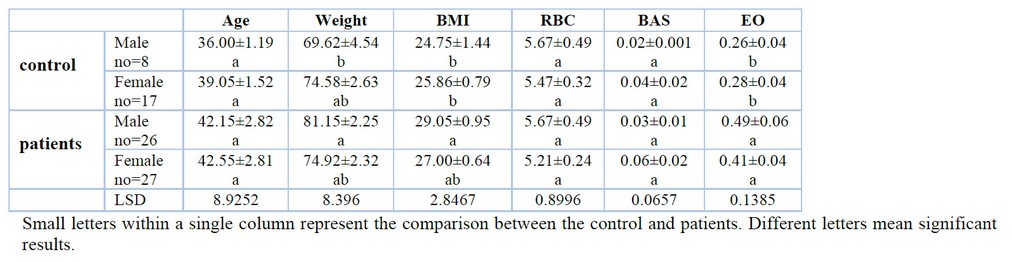
Table 1. Baseline characteristics and some hematological parameters in AML patients and controls.
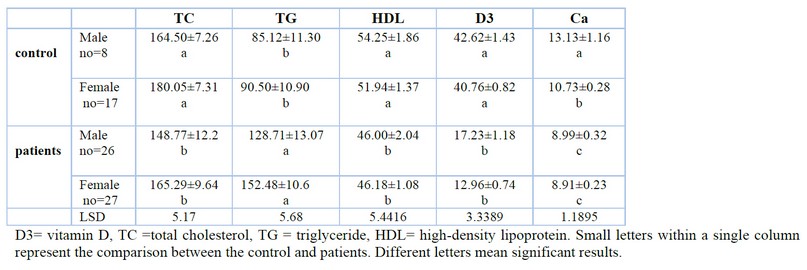
Table 2 Some biochemical studies for AML and control.
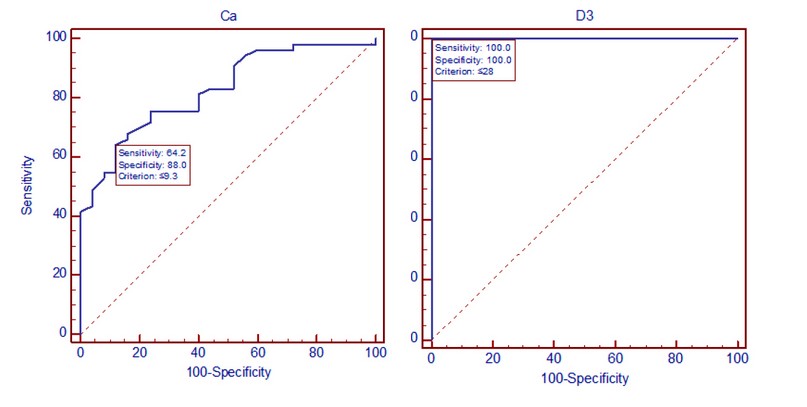
Figure 1. ROC curve for identifying optimal calcium (left) and vitamin D (right) cutoff points
Figure hows the area under the curve (AUC) for vitamin D and calcium. While in table 3 shows the AUC, standard error (SE) and confidence interval (CI) for vitamin D and calcium.
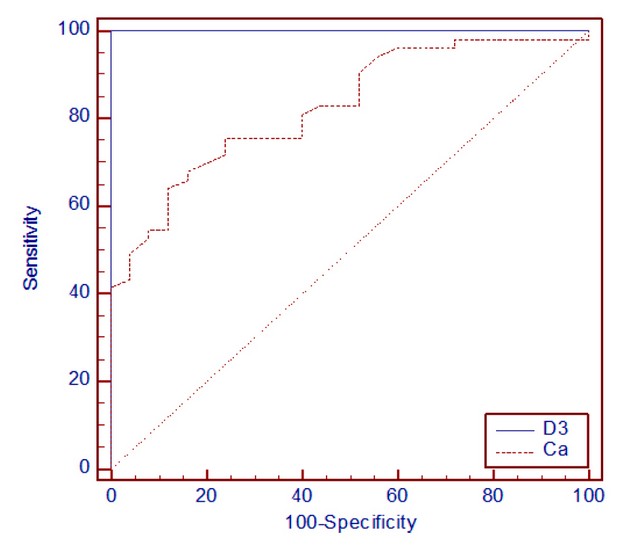
Figure 2. The ROC curves with different areas under the curve
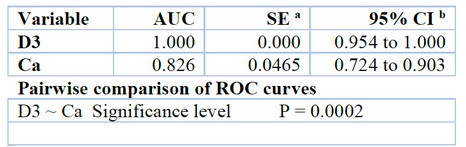
Table 3. AUC, SE and CI for vitamin D and calcium
DISCUSSION
There was no statistically significant difference in age between the patients and the control group. However, there was a statistically significant difference in male weight between the control and the patients (Table 1) and a significant difference in BMI among males and females. Being overweight was related to raised risk of AML. Adipose tissue is one of the active and complex endocrine organs. Recent studies highlighted the bidirectional interactions, based on adipokines and lipids, between white adipose tissue and tumors and their role in cancer progression (later, "adipose tissue" will refer to white adipose tissue). In addition, adipocyte-secreted factors have been shown to regulate the expression of genes associated with cancer progression (adhesion, invasion, angiogenesis, signal transduction and apoptosis) in non-cancerous mammary cells suggesting a role in cancer initiation 15; they may also participate to the leukemogenesis of AML16.
Hypereosinophilia is classified to Primary (Chronic eosinophilic leukemia, Familial eosinophilia, Clonal hypereosinophilia, and Idiopathic hypereosinophilia) and secondary (Infections, autoimmune diseases, allergic diseases, drugs and Malignancies) 17. Through search in articles, we found similar results were matched with our results 18, 19. Many surveys have been performed considering the serum lipids abnormalities 20; in table 2, no statistically significant variations in plasma total cholesterol, triglycerides, or high-density lipoprotein were detected between men and females, neither for AML patients nor controls. There was a considerable decrease(p˂0.05) in cholesterol and HDL, whereas triglycerides value a significantly elevated(P˂0.05) when compared with males between( patients and controls) and females between (patients and power). This results in agreement with the other findings 21. Different researchers explained the relation between hypocholesterolemia and the stage of maturation of leukemic blast cells in acute myeloid leukemia 22. The elevated LDL receptor in AML patients was caused by hypocholesterolemia 23. Several research has been conducted to characterize HDL's potential to boost proliferation, immigration, and survival in cancer cell cultures 24. According to many studies, HDL-C is related to an increased risk of cancer because it continues to provide extra cholesterol to and fuel the development of the tumor 25.
In this study, we found a low level of Vitamin D in newly diagnosed AML patients before the beginning of their treatment. In the Middle East, different factors cause Vitamin D deficiency, such as Low exposure to sunlight, lack of Vitamin D in food, the nature of clothes, type of skin, and lack of complements 26. But, recently, the use of Vitamin D supplements has obtained great interest among older adults; they essentially have sufficient levels of Vitamin D 27. Different epidemiological and experimental studies support that Vitamin D deficiency has increased the risk of developing several cancers 28. Various studies have indicated that Vitamin D can usually modify several critical cellular methods, involving suppression of carcinogenesis by creating cellular differentiation, suppression of proliferation and promoting apoptosis 29,30. Vitamin D has other significant effects, such as inhibiting tumor angiogenesis, invasion and metastasis 31.
On the other hand, a previous study showed a positive correlation between low vitamin D levels and higher BMI or obesity 32. Hypocalcemia is unusual in hematoma malignancy. Various factors, including low albumin, inadequate nutrition, low vitamin D, low magnesium, or persistent respiratory alkalosis, may cause it. In tumor lysis, increased serum phosphorous may cause calcium-phosphate deposition, decreasing serum calcium 33. Figures 1 (A and B) illustrate the receiver operating characteristic (ROC) curves. Generally, the ROC curve measures a test's effectiveness by establishing appropriate cutoff points. The graph depicts calcium sensitivity, and specificity (A) was 64.2. % and 88.0%, then sensitivity and specificity for vitamin D (B) were 100.0% and 100.0%, respectively.
Table 3 shows that AUC for vitamin D was 1.000 (95% CI = 0.954–1.000), and AUC for calcium was 0.826 (95% CI = 0.724 –0.903). The AUC is a standard measure of the accuracy of a diagnostic test. Tests are often classed based on the area under the ROC curve (figure 2). The bigger the AUC, the higher the test's total score. The positive and false favorable rates for the vitamin D test ( figure 1) are greater than those for calcium test A at all cutoffs, making it the better choice. The AUC for vitamin D is larger than the area under the curve for the calcium test. The pairwise comparison of the ROC curves revealed a statistically significant difference (P= 0.0002) for vitamin D and calcium parameters. In a paired ROC analysis, the vitamin D measure was shown to be more discriminatory than the calcium measure. A perfect test has an AUC of 1.0. So that vitamin D is an ideal test for diagnostic AML patients than calcium. Recently, cancer disease can be diagnosed by PCR, such as CML 34 and Adenocarcinoma 35,36. This molecular technique was also employed to diagnosis different microorganisms that caused pathogenicity in human such as Clostridium perfringens37, Brucella melitensis 38, Proteus vulgaris 39,40, Staphylococcus aureus 41, Pseudomonas aeruginosa 42,43 , and Toxoplasma spp 44,45
CONCLUSIONS
The pairwise comparison among the ROC curves for vitamin D and calcium showed that the vitamin D measure was more discriminative than calcium. So vitamin D is a perfect test for early diagnosis of AML patients.
Acknowledgment: Thanks going to all who support us.
Conflict between authors: No conflict
Funds: self by authors
REFERENCES
1. Lupatsch JE, Kreis C, Konstantinoudis G, Ansari M, Kuehni CE, Spycher BD. Birth characteristics and childhood leukemia in Switzerland: a register-based case–control study. Cancer Causes & Control. 2021; 32(7):713-23.
2. Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. American journal of hematology. 2020;95(6):691-709..
3. Juárez-Avendaño G, Méndez-Ramírez N, Luna-Silva NC, Gómez-Almaguer D, Pelayo R, Balandrán JC. Molecular and cellular markers for measurable residual disease in acute lymphoblastic leukemia. Boletín médico del Hospital Infantil de México. 2021;78(3):159-70.
4. Aitken MJ, Ravandi F, Patel KP, Short NJ. Prognostic and therapeutic implications of measurable residual disease in acute myeloid leukemia. Journal of Hematology & Oncology. 2021;14(1):1-5.
5. Tian L, Yu X. Lipid metabolism disorders and bone dysfunction-interrelated and mutually regulated. Molecular Medicine Reports. 2015;12(1):783-94.
6. Kansal R. Acute myeloid leukemia in the era of precision medicine: recent advances in diagnostic classification and risk stratification. Cancer biology & medicine. 2016;13(1):41-54.
7. Agrawal AG, Nagarajappa AK, Bandela V, Agrawal G, Chaturvedi SS, Patil SR. Alteration in Serum Lipid Profile Pattern in Oral Squamous Cell Carcinoma and Potentially Malignant Disorders. Pesquisa Brasileira em Odontopediatria e Clínica Integrada. 2021;14;21.
8. Saha SP, Kalathiya RJ, Davenport DL, Ferraris VA, Mullett TW, Zwischenberger JB. Survival after pneumonectomy for stage III non-small cell lung cancer. Oman Medical Journal. 2014;29(1):24.
9. Cholesterol Treatment Trialists'(CTT) Collaboration. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PloS one. 2012 ;7(1):e29849.
10. Swords R, Sznol J, Elias R, Watts J, Zelent A, Martin E, Vargas F, Bethel-Ellison S, Kobetz E. Acute leukemia in adult Hispanic Americans: a large-population study. Blood cancer journal. 2016;6(10):e484-
11. Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH, Calvo MS, Cashman KD, Combs G, De‐Regil LM, Jefferds ME. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low‐and middle‐income countries. Ann. N.Y. Acad. Sci., 2018;1430:44–79.
12. Zhang L, Zou H, Zhao Y, Hu C, Atanda A, Qin X, Jia P, Jiang Y, Qi Z. Association between blood circulating vitamin D and colorectal cancer risk in Asian countries: a systematic review and dose-response meta-analysis. BMJ open. 2019;9(12):e030513.
13. MedCalc Statistical Software version 16.4.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2016.
14. SAS.2010.SAS/STAT Users Guide for Personal Computer. Release 9.13.SAS Institute, Inc., Cary, N.C., USA.
15. Tahergorabi Z, Khazaei M, Moodi M, Chamani E. From obesity to cancer: a review on proposed mechanisms. Cell biochemistry and function. 2016 ;34(8):533-45.
16. Li S, Chen L, Jin W, Ma X, Ma Y, Dong F, Zhu H, Li J, Wang K. Influence of body mass index on incidence and prognosis of acute myeloid leukemia and acute promyelocytic leukemia: A meta-analysis. Scientific reports. 2017 ;7(1):1-10.
17. Kolobovnikova YV, Dmitrieva AI, Yankovich KI, Vasil’eva OA, Purlik IL, Poletika VS, Novitskii VV, Urazova OI. Expression of galectins-1 and galectin-3 in stomach and colorectal cancer with tissue eosinophilia. Bulletin of Experimental Biology and Medicine. 2018;165(2):256-258.
18. Reiter A, Gotlib J. Myeloid neoplasms with eosinophilia. Blood, The Journal of the American Society of Hematology. 2017;129(6):704-714.
19. Zhang X, Wang B, Zhang R, Chai X, Chao H. Hypereosinophilia (HE) in acute myeloid leukemia (AML) with normal karyotype: A report of two cases. Nigerian Journal of Clinical Practice. 2020;23(1):116-.117.
20. Zhao L, Zhan H, Jiang X, Li Y, Zeng H. The role of cholesterol metabolism in leukemia. Blood Science. 2019;1(1):44-49.
21. Ozturk E. The Relationship Between Hematological Malignancy and Lipid Profile. Medeniyet Medical Journal. 2021;36(2):146.
22. Parsa N, Taravatmanesh S, Trevisan M. Is low cholesterol a risk factor for cancer mortality?. European Journal of Cancer Prevention. 2018;27(6):570-576.
23. Oztas Y. Hypocholesterolemia: a neglected laboratory finding. Acta Medica. 2016;47(1):19-22.
24. Panchoo M, Lacko A. Scavenger receptor class B type 1 regulates neuroblastoma cell proliferation, migration and invasion. Biochemical and biophysical research communications. 2018 ;495(1):614-20.
25. Morin EE, Li XA, Schwendeman A. HDL in Endocrine carcinomas: biomarker, drug carrier, and potential therapeutic. Frontiers in endocrinology. 2018;9:715.
26. Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, Fuleihan GE, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. European journal of endocrinology. 2019;180(4): 23-54.
27. Alizadeh N, Khalili H, Mohammadi M, Abdollahi A. Serum vitamin D levels at admission predict the length of intensive care unit stay but not in-hospital mortality of critically ill surgical patients. Journal of research in pharmacy practice. 2015;4(4):193.
28. Ma Y, Johnson CS, Trump DL. Mechanistic insights of vitamin D anticancer effects. Vitamins & Hormones. 2016;100:395-431.
29. Thompson T, Andreeff M, Studzinski GP, Vassilev LT. 1, 25-Dihydroxyvitamin D3 Enhances the Apoptotic Activity of MDM2 Antagonist Nutlin-3a in Acute Myeloid Leukemia Cells Expressing Wild-type p531, 25D Accelerates Apoptosis in AML Cells. Molecular cancer therapeutics. 2010;9(5):1158-68.
30. Fernández-Barral A, Bustamante-Madrid P, Ferrer-Mayorga G, Barbáchano A, Larriba MJ, Muñoz A. Vitamin D effects on cell differentiation and stemness in cancer. Cancers. 2020;12(9):2413.
31. Skrajnowska D, Bobrowska-Korczak B. Potential molecular mechanisms of the anti-cancer activity of vitamin D. Anticancer research. 2019;39(7):3353-3363.
32. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. The American journal of clinical nutrition. 2000 ;72(3):690-693.
33. Luciano RL, Brewster UC. Kidney involvement in leukemia and lymphoma. Advances in Chronic Kidney Disease. 2014;21(1):27-35.
34. Ahmed AA, Khaleel KJ, Fadhel AA, Al-Rubaii BA. Chronic Myeloid Leukemia: A retrospective study of clinical and pathological features. Bionatura. 7(3):41. DOI. 10.21931/RB/2022.07.03.41.
35. Ali SM, Lafta BA, Al-Shammary AM, Salih HS. In vivo oncolytic activity of non-virulent newcastle disease virus Iraqi strain against mouse mammary adenocarcinoma. InAIP Conference Proceedings 2021; 2372(1):030010. AIP Publishing LLC.
36. Ali SM, Laftah BA, Al-Shammary AM, Salih HS. Study the role of bacterial neuraminidase against adenocarcinoma cells in vivo. InAIP Conference Proceedings 2021; 2372: 030009). AIP Publishing LLC.
37. Hashim ST, Fakhry SS, Rasoul LM, Saleh TH, ALRubaii BA. Tropical Journal of Natural Product Research. 2021; 5(4):613-616.
38. abdulkaliq Awadh H, Hammed ZN, Hamzah SS, Saleh TH, AL-Rubaii BA. Molecular identification of intracellular survival related Brucella melitensis virulence factors. Biomedicine. 2022;42(4):761-765.
39. Abdul-Gani MN, Laftaah BA. Purification and characterization of chondroitinase ABC from Proteus vulgaris, an Iraqi clinically isolate. Current Science. 2017;113(11):2134-2140.
40. kadhim AL-Imam MJ, AL-Rubaii BA. The influence of some amino acids, vitamins and anti-inflammatory drugs on activity of chondroitinase produced by Proteus vulgaris caused urinary tract infection. Iraqi Journal of Science. 2016; 57(4A):2412-2421.
41. Sabah Fakhry S, Noori Hammed Z, Abdul-elah Bakir W, Abdullah Laftaah ALRubaii B. Identification of methicillin-resistant strains of Staphylococcus aureus isolated from humans and food sources by Use of mecA 1 and mecA 2 genes in Pulsed-field gel electrophoresis (PFGE (technique. Revis Bionatura 2022; 7 (2) 44. Doi: 10.21931/RB/2022.07.02.44.
42. Shehab ZH, AL-Rubaii BA. Effect of D-mannose on gene expression of neuraminidase produced from different clinical isolates of Pseudomonas aeruginosa. Baghdad Science Journal. 2019;16(2):291-298.
43. Shehab ZH, Laftah BA. Correlation of nan1 (Neuraminidase) and production of some type III secretion system in clinical isolates of Pseudomonas aeruginosa. BIOSCIENCE RESEARCH. 2018; 15(3):1729-1738.
44. Abdulla L, Ismael MK, Salih TA, Malik SN, Al-Rubaii BA. Genotyping and evaluation of interleukin-10 and soluble HLA-G in abortion due to toxoplasmosis and HSV-2 infections. Annals of parasitology. 2022;68(2):385-390.
45. Jiad¹ AL, Ismael MK, Muhsin SS, Al-Rubaii BA. ND2 Gene Sequencing of Subfertile Patients Recovered from COVID-19 in Association with Toxoplasmosis. Bionatura, 7(3): 45. http://dx.doi.org/10.21931/RB/2022.07.03.45.
Received: December 23, 2022 / Accepted: January 30, 2023 / Published:15 February 2023
Citation: Thair Tahir N, Thamer N. A, Mahmood N A. Role of Vitamin D in the diagnosis of acute Myeloid Leukemia.
Revis Bionatura 2023;8 (1) 50. http://dx.doi.org/10.21931/RB/2023.08.01.50
