2018.03.01.5
Files > Volume 3 > Vol 3 No 1 2018
INVESTIGATION / RESEARCH
Detection of pathogenic microorganisms in urine samples with BluBAC system
Detección de microorganismos patógenos en muestras de orina con sistema BluBAC
Nardo Ramírez-Frómeta1, Carlos Abel Lamothe-Nuviola1, Elier
Riverón-Rodríguez1, Carmen Yamilet Moreno-Barrios2, Angel Regueiro-Gómez3.
Available from: http://dx.doi.org/10.21931/RB/2018.03.01.5
ABSTRACT
The main purpose of this research was to evaluate the performance of BluBAC- an innovative system for the detection of pathogenic microorganisms in urine samples (uroculture). BluBAC system is based on the combination of photostimulation and turbidimetry. The urine samples were analyzed by the following three methods: direct count in Petri dish (reference method), DIRAMIC system and BluBAC system. Based on the conventional protocol for the obtention of samples for the analysis of uroculture, 350 samples from inpatients and outpatients. Results show a 100 % coincidence with the reference method and a detection time lower than 20 minutes in the 90 samples classified as positive. Although the obtained results must be amplified and corroborated with other studies, the sensitivity shown by BluBAC system in the detection of various enterobacteria is a new alternative to the diagnosis of urinary tract infections if consideration is given to the high costs of the systems for the rapid microbiological diagnosis available in the market and their negative impact on the economy of small and medium-sized laboratories, clinics and hospitals.
Keywords: Microbiological diagnostics, urinary tract infections, enterobacteria, blue light, photostimulation, BluBAC.
RESUMEN
El propósito principal de esta investigación fue realizar la evaluación del desempeño del nuevo sistema BluBAC en la detección de microorganismos patógenos en muestras de orina (urocultivo). El sistema BluBAC se basa en la combinación de las técnicas de fotoestimulación y turbidimetría. Las muestras de orina fueron analizadas mediante tres métodos: conteo directo en la placa de Petri (método de referencia), el sistema DIRAMIC y el sistema BluBAC. Teniendo en cuenta el protocolo convencional de obtención de las muestras para el análisis de urocultivo fueron analizadas un total de 350 muestras provenientes de pacientes hospitalizados y ambulatorios. Los resultados alcanzados muestran un 100 % de coincidencia con el método de referencia y un tiempo de detección inferior a los 20 minutos de las 90 muestras clasificadas como positivas. Aunque los resultados obtenidos deben ser ampliados y corroborados con otros estudios la sensibilidad mostrada por el sistema BluBAC en la detección de diferentes enterobacterias constituye una nueva alternativa para el diagnóstico de infecciones del tracto urinario si se tienen en cuenta los altos costos de los sistemas para el diagnóstico rápido microbiológico disponibles en el mercado y su impacto negativo en la economía de los pequeños y medianos laboratorios, clínicas y hospitales.
Palabras clave: Diagnóstico microbiológico, infecciones del tracto urinario, enterobacterias, luz azul, fotoestimulación, BluBAC.
INTRODUCTION
Infectious diseases are the world’s second cause of death, and among these, those which are urinary tract-related have a significant impact (nearly an estimated 80 percent) on hospitalized patients 1. During pregnancy, this pathology is one of the most frequent complications, accounting for 17 to 20 percent of pregnant women due to the fact that the pregnancy-associated physiological changes predispose the occurrence of complications that can significantly damage the mother and the fetus 2. Despite the development of new antibiotics, urinary tract infection remains associated with an increased morbidity and mortality of both the mother and her unborn fetus accounting for around 27 percent of the premature deliveries worldwide 3.
Throughout time a large number of methods have been developed to quickly detect the presence of bacteria in urine samples. An example of these is the development of various chemical, physical and immunological methods, and the latest ones based on flow cytometry among others 4. Chemical methods are based on chemical reactions produced by the microorganism in front of the urine’s own substrates or by those added, which change their color due to the bacteria’s chemical action (example nitrate reduction, catalase production, etc.) Physical methods, many of which are already automated, mainly detect the presence of bacteria when their growth reaches a certain threshold (e.g. turbidity measurement, bioimpedance).
The development of quick and automated methods for microbiological diagnosis is growing worldwide; Sysmex system is an example. Based on the complex and expensive technique of flow cytometry, Sysmex system is useful to conduct large-scale screenings and discriminate the negative samples 5. At present, the reference method for the analysis of urine culture is still the direct counting of microorganisms in a Petri dish. This is a manual, painstaking routine work which requires the samples to be cultivated for a period of 18 to 24 hours with controlled temperature and humidity.
As we looked at the detection times and the high costs of the systems for the rapid microbiological diagnosis available in the market- especially those systems intended for the diagnosis of urinary-tract infections, we were encouraged to conduct the evaluation of the performance of the new system –BluBAC- for the detection of pathogenic microorganisms in urine samples (uroculture) 6. BluBAC is an economic system with appropriate benefits, integrating photostimulation, λ=470 nm (blue light) and acquisition of data with turbidimetric method.
During the research conducted at the Microbiology Laboratory at the Medical-Surgical Research Center (CIMEQ Hospital, Havana-Cuba), the conventional protocol for the obtention of samples for the analysis of uroculture was taken into account and three methods were used: direct count in Petri Dish (reference method), DIRAMIC system (installed at the working laboratory) and BluBAC system. Both systems were developed at CNIC (Cuba’s National Center for Scientific Research).
MATERIALS AND METHODS
For the realization of the evaluative phase we used the DIRAMIC system (CNIC, Cuba), BluBAC system (CNIC, Cuba), and the Colony counter system on Petri Dish (RETOMED cc 2007, Cuba). Also used a Memmert INE 700 incubator and 5-1000 µl adjustable Eppendorf Pippette.
Other reagents:
Clinical strains of Enterobacteria (Laboratory of Microbiology at CIMEQ hospital Havana-Cuba).
Culture medium DETID-Ec, culture medium for the identification of E. coli (CNIC, Cuba).
Culture medium DKD, consists of modified Mueller-Hinton broth (OXOID), a PH 7.4 ± 0.2 sterile solution which additionally includes a polymer. (CNIC, Cuba).
Research Protocol for the detection of urinary infections
In the study of urine samples, three measurement alternatives were used. The first one uses the reference conventional method (viable count in Petri dish), using Petri dish available for their clinical use with 30 ml volume which includes tryptic soy Agar medium and neutralizers produced by German company MERCK. In order to perform the UFC/ml count in the samples, 5 μl urine was cultivated based on the reference method in the indicated dishes. These were incubated for 24 h to 37oC and were afterwards examined. The result of the culture was reported positive if the count exceeds 105 CFU/ml of one or two potentially pathogenic microorganisms.
For the second alternative for the diagnosis of urinary infections, the DIRAMIC 7 system was used. In it 250 μlof each urine simple was inoculated in 2.25 ml of DETID-Ec culture medium and an initial reading was made, which was considered as reference reading. The inoculated flasks were incubated for four hours at 37oC in the working incubator. After this time, a final reading is made. In those samples where the classification criterion was doubtful the incubation period was extended for one hour and the final reading was repeated. The obtained difference of readings is used for the diagnosis of the sample.
The third alternative uses a new developed system: BluBAC, in which 500 μl of each urine sample is inoculated in 4.5 ml of DKD culture medium. The programmable readings are made every 5 minutes in order to analyze the kinetics of the culture growth. In this case, the samples were kept in the incubator and were also optically stimulated with a λ=470 nm.
Results were classified according to the reference method (direct count in Petri dish):
(i) Positives samples: ≥ 105 CFU/ml.
(ii) Doubtful samples: < 105 CFU/ml.
(iii) Contaminated samples (Polymicrobial): more of 3 different colonies of microorganisms.
(iv) Negative samples: without growth.
Samples
During this research developed in the laboratory of microbiology of CIMEQ hospital (Havana-Cuba), a number of 350 samples were analyzed; out of which eleven were classified as contaminated (more than 3 colonies of different microorganisms) therefore they were excluded from the statistical analysis. Thus a total of 339 samples of voluntary people were considered valid for our research.
Data Validation
The data validation was conducted as the results of the measurements stored in the databases of the DIRAMIC and BluBAC systems were reexamined. In addition, such results were compared with the results in the book of control of uroculture at the microbiology laboratory. Results in the book of control of uroculture were classified as positive, contaminated o negative samples, respectively.
Evaluation of the performance of the BluBAC system
The performance evaluation of BluBAC system was made with four indicators: E, percentage of coincidence versus direct count in Petri dish; sensitivity (S, related with the capacity of equipment to detect the sick people), and positive and negative predictive values (VPP, VPN: related with the probability of result to indicate the existence or absence of clinical pathology, respectively):
RESULTS AND DISCUSSION
Conventional standard method (direct count in Petri dish) and DIRAMIC system were used to evaluate the research results with BluBAC system.
In its working procedure, the DIRAMIC system uses two culture media: DKD and DETIC-Ec. Culture medium DKD is used for both analyzing the urine culture and determining the antibiogram (a scheme of susceptibility to antibiotics). Culture medium DKD consists of modified Mueller-Hinton broth (OXOID), a PH 7.4 ± 0.2 sterile solution which additionally includes a polymer. The inclusion of such polymer to the culture medium allows for the elimination of the inhibiting effect of catabolic products accompanying the inoculum of the samples to be tested.
On the other hand, culture medium DETIC-Ec can be used when E coli wants to be identified in the tested sample in addition to analyzing the urine culture. This medium, in addition to the conventional nutritious bases that the culture medium Mueller Hinton (OXOID) contains has the subtracts MU-ß-D-glucuronide (0,1), L-Tryptophan (1 g), as well as the polymer used as depressor agent (1 g). These components are solubilized in 50 mM Potassium Phosphate to the pH of such medium between 7-7,5. These already used subtracts are useful to detect the activities of the ß-D-glucuronidase and Tryptophanase enzymes produced by E. coli, through the formation of Indole. That is why after the bacterial growth, an auxiliary reagent is added to such medium (modified Kovac’s reagent) for the revealing process. The product of the interaction of enzymes with its subtracts is detected in a first step, by exposing the samples where an increase in the turbidity (positives) is detected to an UV light source.
For the DIRAMIC system, no matter the medium used, each analysis (urine culture and/or antibiogram) lasts 4 hours. In order to make preliminary evaluation of the BluBAC system, medium DKD was used as it is more economical. However, medium DETIC-Ec was used in the work with DIRAMIC system as the objective of the hospital was to identify the presence of E. coli in the tested samples.
With the purpose of minimizing the impact of the false positives, every patient who took part in the test was given a sterilized flask in autoclave. In addition, they were instructed to follow the protocol to take the urine sample, which included the hygiene of the genitals, and that such sample should be the first urine sample taken in the morning. Such sample should be refrigerated and should be handed in the lab no later than two hours from the time it was taken. Also, in order to avoid the false negatives, a necessary condition was established: participation in the test should only be allowed if the patients should not be taking antibiotics, or at least seven days should have passed after the conclusion of any therapy with antibiotics. However, in a later evaluation phase, a study of the residual microbial activity is being considered.
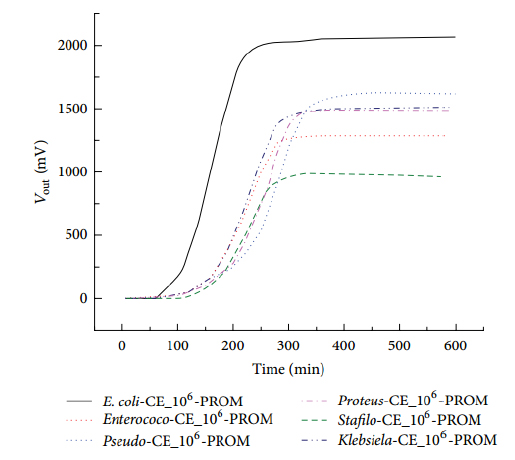
Figure 1. Shows typical registers related with kinetics of growth in different microorganisms.
The percentage of positive samples found was 26,54%. One-hundred percent of results (339 samples) coincided with both methods. No false positive or negative results were registered (Table 1), which means that the coincidence percentage for the statistical analyzed sample is considered satisfactory. With these results, a 100 % sensitivity and specificity was achieved. The predictive value of the positive samples and the negative ones was 100%.
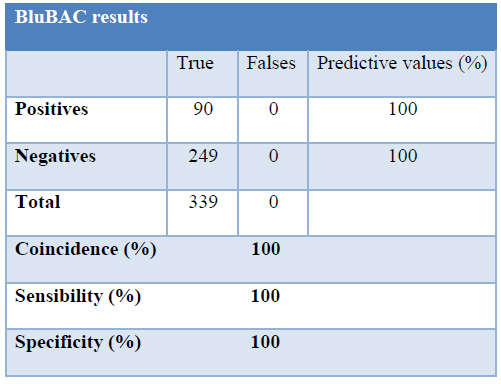
Table 1. Performance evaluation of BluBAC system
The higher percentage of infections is related to the presence of E. coli as the main pathogenic microorganism. These values coincide with the studies conducted by Alvarez and col. 8 with the DIRAMIC system. They report that in Cuba the incidence of Urinary Tract Infection due to Escherichia coli reaches up to 82% (Fig. 2). Ninety-seven percent of infections were caused by gram-negative enterobacteria. This fact coincides with what Kang and his collaborators reported 1.
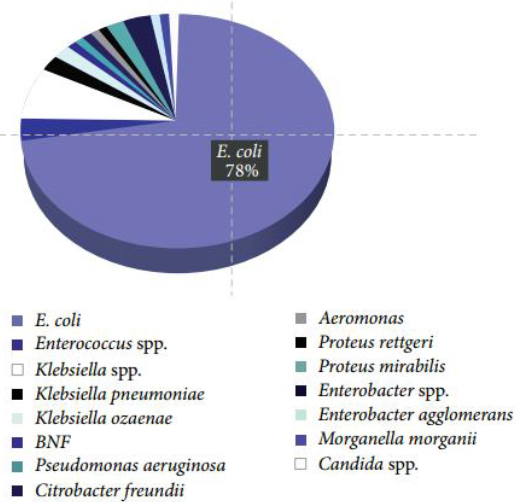
Figure 2. Mains microorganisms founded during the conduct of the research (Escherichia coli, Enterococcus spp, Klebsiella spp, Klebsiella neumoniae, Klebsiella ozaenae, Pseudomonas aeruginosa, Citrobacter freundii, Aeromonas, Proteus rettgeri and Proteus mirabilis).
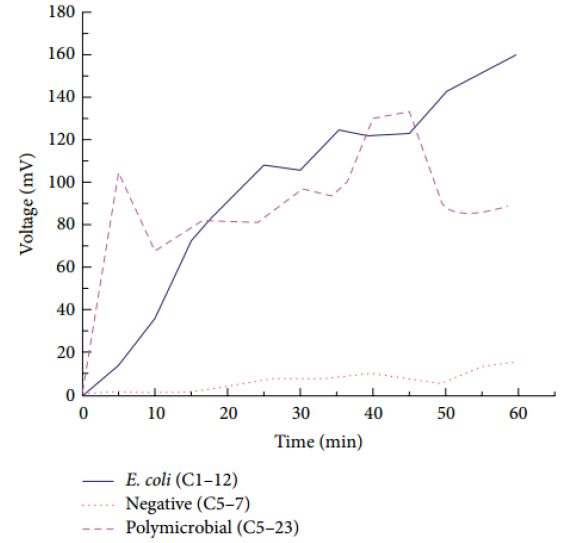
Figure 3. Examples of curves in urocultive samples when these samples were stimulated with blue light.
The employment of photostimulation with λ= 470 nm significantly influenced the growth of enterobacteria studied and its detection time (< 20 minutes). Photostimulation effect on direct urine samples practically caused the absence of the lag phase and a longer exponential phase, which results in a higher level of microorganism’s growth. The 2016 work table, by Nardo and collaborators 6 shows a brief characterization (costs, detection time and operation method) of the main commercial systems available for the rapid diagnosis of microbial infections, where it can be seen that the obtained results are way below the detection time by these systems at a lower cost. These results have been legally protected through the Cuban Office of Intellectual Property 9.
The constant photostimulation of samples with blue light drives a growth expedited of the present microorganisms in the samples. We believe that the effect of photo-stimulation on doubling time of E. coli (often 20 to 30 minutes for most wild-type strains in a rich medium), can arise from the presence of factors in the urine or components of the infection site relating to the photoreceptors, quantitatively or qualitatively 10, 11. This result coincides with the point made by Tiphlova and Karu that photo-stimulation of growth depends on the conditions of cellular metabolism at the time of photostimulation 12. However, investigations are required specially designed to elucidate the factors involved in such behavior.
CONCLUSION
The results obtained in the evaluation of the performance of the new BluBAC system for the detection of pathogenic microorganisms in urine samples (uroculture) can be considered satisfactory. BluBAC system- based on the combination photostimulation and turbidimetry - showed 100 % coincidence with the reference method (direct count in Petri dish). The detection time of 90 samples classified as positive was four (4) hours by DIRAMIC system while by BluBAC system it was lower than twenty (20) minutes.
Although the results obtained must be amplified and corroborated with other studies, the sensitivity shown by BluBAC system in the rapid detection of various enterobacteria is a promising alternative to the diagnosis of urinary tract infections if consideration is given to the high costs of the systems for the rapid microbiological diagnosis available in the market and their negative impact in the economy of small and medium-sized laboratories, clinics and hospitals.
ACKNOWLEDGMENTS
This project was funded in part by the collaboration between Department of Bioengineering (Universidad Tecnológica de La Habana), Department of Microbiologic Diagnostics (CNIC: National Center for Scientific Research) and Laboratory of Microbiology - CIMEQ hospital.
REFERENCES
[1] Cheol-In Kang, Doo Ryeon Chung, Jun Seong Son, Kwan Soo Ko, Kyong Ran Peck and Jae-Hoon Song. Clinical significance of nosocomial acquisition in urinary tract-related bacteremia caused by gram-negative bacilli. American Journal of Infection Control 2011, 39(2): 135-140. doi: 10.1016/j.ajic.2010.03.022.
[2] Vazquez JC, Abalos E.Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database Syst Rev. 2011 Jan 19;(1)1-6:CD002256. doi: 10.1002/14651858.CD002256.pub2.
[3] Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3): 2-16. CD001209.
[4] Dalet, F and del Rio-G (1997). Infecciones urinarias. Ed. Panamericana. Madrid.
[5] de Frutos-Serna M, Asensio-Calle ML, Haro-Pérez AM, Blázquez-de Castro AM, Gutiérrez-Zufiaurre MN, Iglesias-García J. Evaluation of the Sysmex UF-1000i flow cytometer for screening of urinary tract infection. Enferm Infecc Microbiol Clin. 2014, Mar; 32(3):147-51. doi: 10.1016/j.eimc.2013.02.015. Epub 2013 Apr 30.
[6] Ramírez-Frómeta N, Lamothe-Nuviola CA, Riverón-Rodríguez E et. al. BluBAC system for determining microbial growth in clinical microbiological diagnosis samples by combining photostimulation and turbidimetry. Biotecnología Aplicada 2016; Vol. 33, No. 2. http://elfosscientiae.cigb.edu.cu/PDFs/Biotecnol%20Apl/2016/33/2/BA003302EN2401-2405.pdf. Accessed on July 31, 2017.
[7] Zayas-Tamayo AM, Barreras-García G, Álvarez-Varela E. Detección mediante el Sistema DIRAMIC de Staphylococcus aureus resistente a meticilina (SARM) y comparación con otros métodos utilizado en la práctica clínica Revista CENIC. Ciencias Biológicas 2013, 44 (2).
http://revista.cnic.edu.cu/revistaCB/articulos/detecci%C3%B3n-mediante-el-sistema-diramic-de-staphylococcus-aureus-resistente-meticilina-sarm. Accessed on July 31, 2017.
[8] Alvarez-Varela E, Contreras-Alarcón R y Alvarez-Pineda A. Resistencia microbiana en la red nacional cubana de laboratorios con equipos Diramic durante los años 2002 al 2004. Revista CENIC Ciencias Biológicas, Vol. 36, No. Especial, 2005.
http://revista.cnic.edu.cu/revistaCB/sites/default/files/articulos/CB-2005-4-CB-015.pdf. Accessed on July 31, 2017.
[9] Ramírez-Frómeta N, Lamothe-Nuviola CA, Riverón-Rodríguez E, Moreno-Barrios CY, Regueiro-Gómez A, Contreras OR. Sistema para la detección de microorganismos fotosintéticos y no fotosintéticos en muestras biológicas por fotoestimulación controlada. Patent pending, CU 2016-0070; 2016.
[10] Masuda, S. Light Detection and Signal Transduction in the BLUF Photoreceptors. Plant Cell Physiol. 2013 Feb;54(2):171-9. doi: 10.1093/pcp/pcs173. Epub 2012 Dec 14.
[11] Jung A, Domratcheva T, Tarutina M, Wu Q, Ko WH, Shoeman RL, Gomelsky M, Gardner KH, Schlichting I. Structure of a bacterial BLUF photoreceptor: Insights into blue light-mediated signal transduction. Proc Natl Acad Sci USA.2005,102 (35): 12350–12355. doi: 10.1073/pnas.0500722102.
[12] Tiphlova, O and Karu;T (1991). Action of low-intensity laser radiation on Escherichia coli. Crit Rev Biomed Eng. 18(6): 387-412.
https://www.isan.troitsk.ru/dls/publ/126.pdf Accessed on July 31, 2017.
Received: 11 october 2017
Approved: 29 december 2017
Nardo Ramírez-Frómeta1, Carlos Abel Lamothe-Nuviola1, Elier Riverón-Rodríguez1, Carmen Yamilet Moreno-Barrios2, Angel Regueiro-Gómez3.
1 Centro Nacional de Investigaciones Científicas (CNIC) Ave. 25 y Calle 158, Playa, Apartado Postal 6412, La Habana, Cuba.
2 Centro de Inmunología Molecular (CIM) Calle 216 esq. 15. Atabey, Playa, La Habana. Cuba
3 Centro de Bioingeniería (CEBIO), Universidad Tecnológica de la Habana
Corresponding Author: Nardo Ramírez-Frómeta.
E-mail: [email protected]
