2019.04.04.10
Files > Volume 4 > Vol 4 No 4 2019
REVISION/REVIEW
Pyrazoline as a medicinal scaffold
Gurinderdeep Singh1*, Anju Goyal2, R S Bhatti3 & Sandeep Arora2
Available from: http://dx.doi.org/10.21931/RB/2019.04.04.10
ABSTRACT
Heterocyclic Chemistry is the backbone of medicinal compounds that exhibits numerous biological activities. Pyrazole and its derivatives possess nitrogen atom along with carbon atom as a substitution and show a diversity of biological activities such as antibacterial, antimicrobial, anti-inflammatory, antioxidant, antidiabetic, anticancer, antifungal, antidepressant, anticonvulsant, analgesic, and monoamine oxidases (MAOs)as shown by pyrazoline prepared from chalcone(Intermediate). The synthesized compounds are checked by the TLC and further analyzed by the IR, NMR, and UV spectroscopy.
Keywords: Anti-microbial, heterocyclic, and pyrazoles, biological activity.
INTRODUCTION
Pyrazole, well known heterocyclic compound with two adjacent nitrogen atoms within the ring and having a five-membered ring carries one endocyclic double bonds and essential in nature1, represented by the molecular formula C3H4N22.Pyrazole melts at 700C, in spite of its low molecular weight. Clinically the substitution derivatives at 3 and five positions are indistinguishable from one another whereas the properties disappear the hydrogen atom on the nitrogen atom immediately is replaced by an alkyl group 3
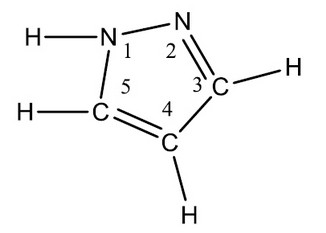
Figure 1. Pyrazole representation with two adjacent nitrogen atoms within the ring
In 1883, Ludwig Knorr coined the term pyrazole, which is a weak base, acquires pKb 11.5(pKa value of the conjugated acid 2.49 at 250C). Pyrazole having unique pharmacological effects on human beings and from watermelon seeds, 1-pyrazolyl-alanine was isolated and then classified as alkaloids on composition, first natural pyrazole in 1959.4
Pyrazole exhibits various biological activities and also has a potent medicinal scaffold.5 Pyrazoline and its derivatives are having an antibacterial6, antimicrobial7, anti-inflammatory8, antioxidant9, antidiabetic10, anticancer11, antifungal12, antidepressant13, anticonvulsant14, analgesic15, and monoamine oxidases (MAOs) 16.
Chemistry and Synthetic approaches
In medicinal chemistry, heterocyclic rings such as Pyrazole containing active pharmacophore agents play an essential role in refined and efficient ways to make these heterocyclic heads. Pyrazole includes two nitrogen atoms also carry a π-excessive heterocycle, as seen in pyrrole at position1 and pyridine at positions 2. Pyrazole subsists in three partially reduced forms.
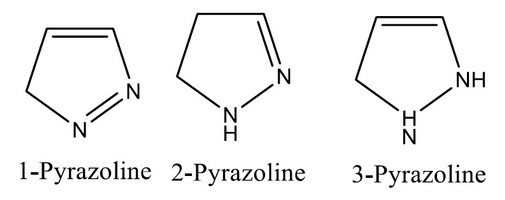
Figure 2. Pyrazole contains two nitrogen atoms also carry a π-excessive heterocycle, as seen in pyrrole at position1 and pyridine at positions 2.
Pyrazole carries a boiling point of (186-188°C) due to its hydrogen bonding and exhibits two identical and non-separable tautomers owing to rapid interconversion of tautomers. In other words, In pyridine, nitrogen is prone to electrophilic attack, and the hydrogen atom bonded to the nitrogen at position 1 is more acidic than pyrrolic N-H, thus easily removed by nucleophiles. It can be synthesized via Claisen-Schmidt condensation followed by cyclizations with hydroxylamine HCl and hydrazine hydrate under the appropriate conditions. Pyrazole lower basicity with weak base (pKa= 2.52) because of extra destabilization of π-bonding after protonation and increases acidity with very weak acid (pKa=14.21) by the introduction of the electron-withdrawing group (-I & -M effect).17
Synthetic approach to the pyrazole
1-(4,5-disubstitutedpyrazol-1-yl)-ethanone Synthesis: On the contrary, pyrazoles have been carried out by the reaction of β-formyl enamides with hydroxylamine hydrochloride catalyzed by potassium dihydrogen phosphate in acidic medium and become novel synthesis. 18
3,5-substituted-1H-pyrazole Synthesis: From tosylhydrazones, α, β-unsaturated carbonyl compounds synthesis of pyrazole derivatives possessing a β-hydrogen is proposed and exploiting microwave activation coupled with solvent-free reaction conditions and appears as novel approach .19
Tri- and tetra-substituted pyrazoles Synthesis; For the facile synthesis of tri- and tetra-substituted pyrazoles using Dioxygen gas as the oxidant undergoing intramolecular oxidative CN coupling method catalyzed by A ruthenium (II) carried out the transformation, and the reaction demonstrates excellent reactivity, functional group tolerance, and high yields.20
For the Regio, selective synthesis; By the reaction of diarylhydrazones and vicinal diols via the route of 1,3- and 1,3,5-substituted pyrazoles undergoes iron-catalyzed synthesis of 1, 3-substituted pyrazoles.21
Synthesis of 1,3,5-trisubstituted-1H-pyrazole: An easily accessible reaction 1, 3-bisaryl-monothio-1,3-diketone or 3-(methylthio)-1,3-bisaryl-2-proponents gives 1-aryl-3,5-bisarylpyrazoles with arylhydrazines and with complementary Regioselectivity at position 3 and 5.22
General procedure for synthesizing pyrazoline
A substituted Chalcone (0.01 mole) mixed with hydrazine hydrate (0.012 moles) and acetic acid(10ml) in methanol for five hours. The reaction mixture was poured in chilled water, and solid separated was filtered and recrystallized from ethanol. Again, the reaction completion was confirmed by the TLC and monitored.
Pharmacological activity
The usefulness and great therapeutic value of pyrazole nucleus have been recognized, and the most comprehensive range of activities of this nucleus evaluated for a long time. However, as the first synthetic organic compound carries pyrazoline-5-one nucleus to find use as an essential drug. Derivatives of the pyrazolopyrimidine ring system are known to possess potent biological properties 23 Out of these many natural and synthetic products having heterocyclic rings as Derivatives of the pyrazolopyrimidine ring system are known to possess potent biological properties24.Also condensed pyrazoles are biologically active and their chemistry has received considerable attention as pyrano[2,3-c]pyrazoles are reported to have useful biological effects, such as analgesic and anti-inflammatory activities .25In recent years significant progress has been made relating to oxidation in biological cells resulted from reactive oxygen species (ROS) and initiates lipid peroxidation in healthy cells leading to Alzheimer’s disease, atherosclerosis, diabetes, Parkinson’s disease, etc. Among plant kingdom and animal kingdom coumarins, Xanthones pyrazoles and acrylonitriles showed widespread use in medicine26.Synthetically, several methods have been published for the synthesis of 2-pyrazolines for the treatment of tumor, fungal and viral infection, Tuberculosis and depression, etc.27
Antimicrobial activity
Sahu SK et al., synthesized a series of 4-(5-substituted aryl-4, 5-dihydropyrazole-3-yl-amino) phenols 2a-f have been reported novelty by treating substituted Aryl-N-chalconyl amino phenols 1a-f with hydrazine hydrate from p-aminoacetophenone By the substitution of the p-nitro and p-hydroxy group in aryl moiety of the pyrazoleanalogs 2c(-4-NO2-C6H4) and 2e(-2-OH-C6H4) produce compounds with potent analgesic, anti-inflammatory but also in a few cases, antimicrobial properties. IR, 1H NMR spectral data.28 confirmed the structures of synthesized compounds
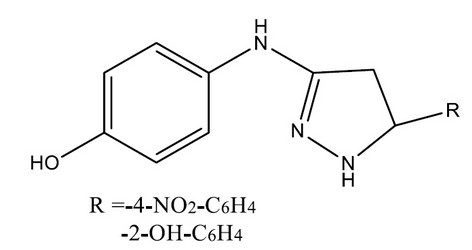
Figure 3. Pyrazoleanalogs 2c(-4-NO2-C6H4) and 2e(-2-OH-C6H4) produce compounds with potent analgesic, anti-inflammatory but also in a few cases, antimicrobial properties
Kendre M.M. and BASEER M.A. synthesized efficiently biologically active Pyrazoline derivatives with excellent yields via cyclization reaction of chalcones and hydrazine hydrate. The compounds 2a, 2b, and 2h showed significant activity in comparison with the standard drug. The presence of pyrazoline moiety, substituents, particularly having Bromo, Chloro, Hydroxyl, Iodo and Methyl groups in the ring may be responsible for antimicrobial activity of this class of compounds and screened that also reflects moderate to good activity against different strains of bacteria and fungi employed. All the synthesized compounds were confirmed by IR, 1HNMR and Mass spectral data.29
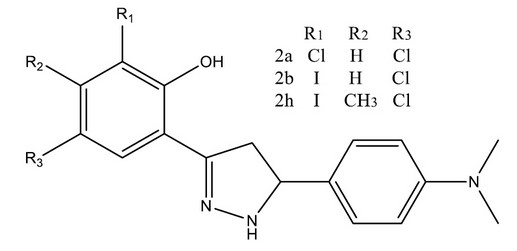
Figure 4. Substituted Pyrazoline moiety
Ahmet O¨ zdemir et al., synthesized 1-(p-Methyl phenyl)-3,5-diaryl-2-pyrazoline derivatives (2a–f) via the treatment of 1-(1H-indol-3-yl)-3-aryl-2-propen-1-ones (1a–f) with p-methylphenylhydrazine hydrochloride in hot acetic acid. Pyrazoline derivatives, compound 2e exhibits 2,5-dichlorophenyl moiety identified the most promising agent against Klebsiella pneumoniae (ATCC 13883) and Candida glabrata (ATCC 36583) due to its inhibitory effects on K. pneumoniae and C. glabrata with a MIC value of 100 mg/mL as a nontoxic agent (LC50 > 1000 mg/mL). Structural elucidation of these compounds by IR, 1H NMR, and mass spectral data and elemental analysis and investigated toxicity by Brine-Shrimp lethality assay for their antimicrobial activity 30
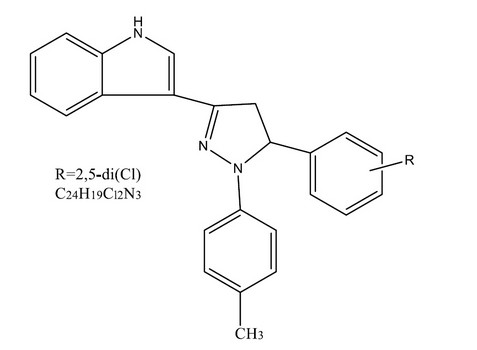
Figure 5. 1-(p-Methyl phenyl)-3,5-diaryl-2-pyrazoline derivatives
Dipankar et al. studied the reports of twelve 2-pyrazoline derivatives against S. aureus and A. niger thoroughly (Table1). Two varieties of acetophenones were condensed with three varieties of substituted benzimidazole derivatives to get six chalcone derivatives, which undergo condensation followed by cyclization with isoniazid and 1-(2-napthyloxy acetate) hydrazine two get the final 2-pyrazoline derivatives. Compound D7 exhibited the highest antibacterial activity, and compound D12 exhibited highest anti-fungal activity as well as comparable to the antibacterial activity and antifungal activity of the standard drugs at 200 μg/ml. These compounds were characterized by IR, 1H-NMR and Mass spectral studies. The synthesized compounds were found to have good antimicrobial activity in the range of 20-70 μg/ml.31
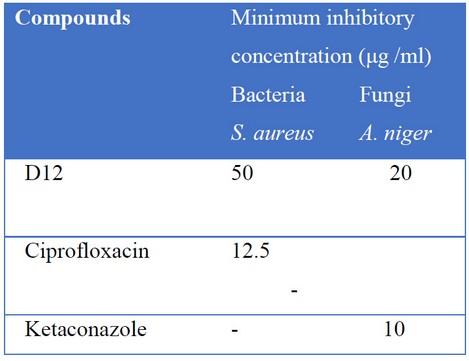
Table 1. reports of twelve 2-pyrazoline derivatives against S. aureus and A. niger
N.C. Desai. et al synthesized a series of compounds 2-(5-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-3-(aryl)-4,5-dihydro-1Hpyrazol- 1-yl) thiazol-4(5H)-ones (4a–q) and screened invitro synthesized against the representative panel of Gram-positive (Staphylococcus aureus, Streptococcus pyogenes) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa) bacteria. These compounds were also tested for their inhibitory action against strains of fungi (Candida albicans, Aspergillus niger, Aspergillus clavatus). The synthesized compounds showed potent inhibitory action against the test organisms by the use of an electron-withdrawing group on the benzene ring in basic structures was worthy. Compounds bearing 2-Cl, 4-Cl, 2-F, 3-F,4-F, 2-NO2, and 4-NO2 exhibited more pronounced activity 32.
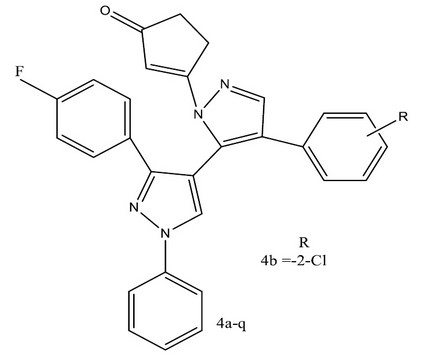
Figure 6. Series of 2-(5-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-3-(aryl)-4,5-dihydro-1Hpyrazol- 1-yl) thiazol-4(5H)-ones for antimicrobial activity
Antidepressant activity
B. K. Kaymakcıoğlu, S. Gumru, N. Beyhan, F. Aricioglu investigated a series of new 2-pyrazoline derivatives and activity was evaluated by using tail suspension test, Compounds 3d and 3e were effective and a significant reduction in immobility time was observed as compared to results of imipramine, as the reference standard drug. Compounds 3d and 3e remarked the potential for the treatment of depression.33.
Figure 7. -pyrazoline derivatives for Antidepressant activity
Anti-convulsant activity
Ahsan synthesized 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide and assayed the anticonvulsant activity and neuroprotection according to Antiepileptic Drug Development Programme protocol. Compound 4b showed neuroprotection activity with 26.2 ± 1.9% of total propidium iodide uptake at 100 µM, and inhibitory concentration 50 (IC50) of the compound was found to be 159.20 ± 1.21 µM 34
Sudhakarararao G et al., Synthesized compounds and then evaluated to suppress seizures and provide neuroprotection by minimizing the effects of the seizure attacks. To attain this some chalcone and chalcone based pyrazolines were Evaluated for their anticonvulsant activity and then structural elucidation was taken on the basis of the elemental analysis and spectroscopic studies (NMR, IR and Mass Spectroscopy) Among all compounds only compounds Ph1, Ph2, Py 3and Py 4shown to be good anticonvulsant activity with dose level of 4mg/kg b.w.35
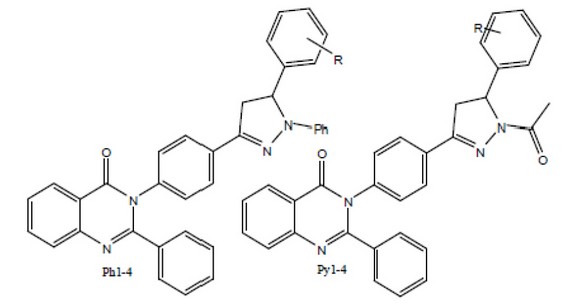
Figure 8. Substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c] pyrazole-2-carboxamide for Anticonvulsant activity
Abdel-Aziz et al., have described two synthetic paths for the formation of diacylhydrazines, 5amino-1-substiuted pyrazole-3,3,4-tricarbonitriles and oxadiazole, pyrazoline derivatives, Compounds 4a and 4b showed good activity in comparison to imipramine at a dose of 10 mg/kg dose level and showing antidepressant activity using tail suspension behavioural despair test and anticonvulsant activity against pentylenetetrazol induced seizures in mice. 35
Monoaminoxidase activity
F. Manna et al., have synthesized a series of 1-acetyl-3-(4-hydroxy- and 2,4-dihydroxyphenyl)-5-phenyl-4,5-dihydro-(1H)-pyrazole derivatives and investigated their ability to selectively inhibit the activity of the isoforms of MAO and created a novelty. The newly synthesized compound proved to be more reversible, potent, and selective inhibitors of MAO-A than of MAO-Compounds 6 and 11 were found to be most potent 36
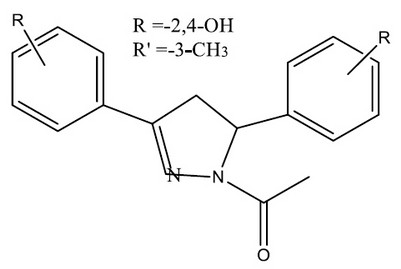
Figure 9. series of 1-acetyl-3-(4-hydroxy- and 2,4-dihydroxyphenyl)-5-phenyl-4,5-dihydro-(1H)-pyrazole derivatives for MAO activity
F. Chimenti et al., synthesized a coumarin-3-acyl derivatives as a series and investigated for the ability to inhibit selectively monoamine oxidases. The coumarin-3-carboxylic acids,2a–e, proved to be reversible and selective inhibitors of the MAO-B iso-form. The coumarin-3-acyl chlorides 3a–e showed high pIC50 values against both MAO-A and MAO-B isoforms,3d being the highest against MAO-B with a pIC value of 8.00. To rationalize the activity/selectivity results, molecular descriptors were generated. Further insight about enzyme–inhibitor interaction was obtained by docking experiments with the MAO-B isoform.37
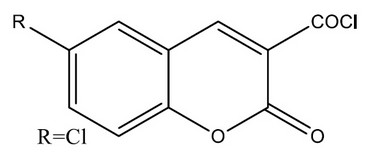
Figure 10. Series of coumarin-3-acyl derivatives
Antifungal activity
Sivakumar R et al., docked 14α-demethylase routinely used to understand drug-receptor interaction in modern drug design. The docking of benzimidazole containing pyrazoline-5-one derivatives as inhibitors of 14α-demethylase. The inhibitory activities against 14α-demethylase were investigated by molecular docking using the HEX docking software. These compounds docked into the active site of the receptor (PDB code, 1E9X) using Hex docking tools software, which showed good affinity for the enzyme when compared with the binding energies of standard drugs such as clotrimazole (-24.05) and griseofulvin (-36.57). Among all the designed compounds, compound 7 shows more binding energy values (-59.85). 38
Kumar R and Joshi Y. C. synthesized β-diketones/β-ketoesters, 4a–e on condensation with different β-diketones/β-ketoesters, 3a–e in the presence of sodium hydroxide from the diazonium salt of 4-amino-1-methyl-3-propyl-1H-pyrazole-5-carboxamide .The β-diketones/β-ketoesters 4a–e were condensed with o-phenylenediamine (o-PDA) in the presence of p-toluene sulfonic acid/SiO2 to give biologically active 3H-1,5-benzodiazepines, 5a–e. Crofloxin and ciclopirox olamine were used as reference standards for comparison of the antibacterial and antifungal activities, respectively. Compound 5c exhibited greater antimicrobial and antifungal activities than the standard drugs, whereas compounds 5d and 5e showed significant anthelmintic activity All the newly synthesized compounds were characterized by elemental analysis and spectral studies and screened for their antimicrobial, antifungal and anthelmintic activities.39
Deng et al., synthesized a series of 1,3,5-trisubstituted-2-pyrazoline derivatives by introducing the furan rings. Among all compounds, compounds 4,7,9,12,18 and 19 displayed excellent antifungal activity against Rhizoctonia solani and tried to discover some more potent antifungal compounds. Additionally, at site 3 and site 5of the pyrazoline in the compounds 9 and 19 bearing two furan rings respectively and with the help of bioactivity results pyrazoline derivatives receives a template for the further structural optimization 40
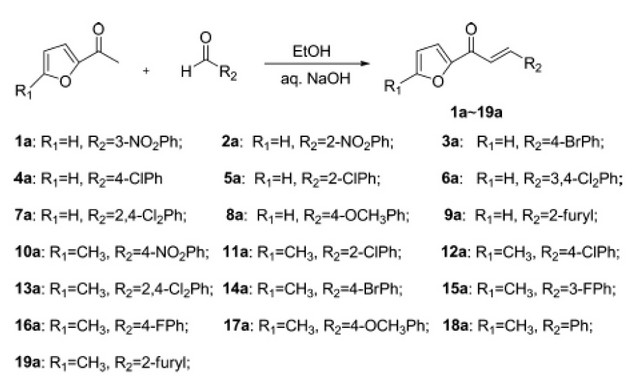
Figure 11. Series of 1,3,5-trisubstituted-2-pyrazoline derivatives as Antifungal
Antiepileptic activity
Maruthi Rao B et al., prepared two varieties of acetophenones were condensed with two varieties of aromatic benzaldehydes to get four chalcone derivatives by undergoing condensation followed by cyclisation with isoniazid to get the final four 2-pyrazoline derivatives. Compounds T1 and T2 having 2-furyl derivatives names of T2 (5-(furan-2-yl)-4,5-dihydro-3-(4-hydroxyphenyl) pyrazol-1-yl) (pyridin-4-yl) methanone and T1(3-(4-chlorophenyl)-5-(furan-2-yl)-4,5-dihydropyrazol-1-yl) (pyridin-4-yl) methanone has prominent anti-epileptic activity on hydroxy-2 and furyl have the most potential anti-epileptic activity41
Anticancer Activity
M. Shaharyar et al. synthesized series of benzimidazolewhich carries 2-pyrazolines and then tested as well as belonging to different panels these compounds against various cancer cell lines such as renal, breast, colon, melanoma, prostate, and so on. The most active compound of the series was found to be 2-[5-(3,4-dimethoxyphenyl)-1-phenyl-4,5-dihydro-1H-3-pyrazolyl]-1Hbenzimidazole. Based on close examination of the substituent it was concluded that the role of electron donating group on the phenyl ring at C5 of the phenyl ring had a great influence on anticancer activity.42
Eman M. Flefel et al. primarily synthesized a newly substituted pyrazole, thiazole, and 1, 2, 4-triazole derivatives and then reported. Among all the sugar hydrazones and their acetylated derivatives as yet derived acyclic C-nucleoside analogs, and the thioglycosides of the 1, 2, 4-traizole derivatives were also prepared. Compounds that were synthesized as well as studied and several compounds showed significant antitumor activities in the tested results.43
CONCLUSION
Pyrazoline, a heterocyclic compound that exhibits a two nitrogen in the ring nucleus which synthesized via cyclization of chalcone from the reaction of substituted aldehydes and ketones in the presence of basic conditions. Medicinally pyrazoline and its derivatives showed the diversity of biological activities such as antibacterial, antimicrobial, anti-inflammatory, antioxidant, antidiabetic, anticancer, antifungal, antidepressant, anticonvulsant, analgesic, and monoamine oxidases (MAOs) and elucidated by spectral analysis and also characterized by elemental analysis.
REFERENCES
1. Manna K. et.al, A review on synthesis and pharmacological diversity of isoxazoles & pyrazolines, NirmaUniv J Pharm Sci; 2014, 1(1) 37-49
2. DD Perrin. Dissociation Constants of Organic Bases in Aqueous Solution. London: Butterworths; 1972.
3. Ehrlich H W, The Crystal and Molecular Structure of Pyrazole, Acta Cryst. (1960). 13, 946.
4. Hauptmann S, Eicher T, The Chemistry of Heterocycles: Structure, Reactions, Synthesis and Applications. 2nd ed. New York: Wiley-VCH; 2003.
5. Jagdaleet al., A Review on Synthetic Approaches of Pyrazoline Derivatives, world journal of pharmacy and pharmaceutical sciences, Volume 4, Issue 11, 505-514.
6. Solankee A.et al., Synthesis and Antibacterial Evaluation of Some Novel Isoxazole And Pyrazoline Derivatives, Rasayan J. Chem Vol.1, No.3 (2008), 581-585
7. Desai N.C et al., Facile synthesis of novel fluorine containing pyrazole based thiazole derivatives and evaluation of antimicrobial activity Journal of Fluorine Chemistry 142 (2012) 67–78
8. Venkataraman.S. et al. Synthesis and biological activity of some novel pyrazolines, Acta PoloniaePharmaceutica n Drug Research, Polish Pharmaceutical Society, Vol. 67 No. 4 pp. 361-366, 2010 ISSN 0001-6837.
9. JOIS, Kalluraya and Girisha , Synthesis and antioxidant activity study of pyrazoline carrying an arylfuran/arylthiophene moiety, J. Serb. Chem. Soc. 79 (12) 1469–1475 (2014)
10. B. Cottineauet al. , Synthesis and Hypoglycemic Evaluation of Substituted Pyrazole-4-carboxylic Acids Bioorg. Med. Chem. Lett. 12 (2002) 2105–2108.
11. Alka Pradhan and Meghna Mehndiratta, Pyridine based pyrazolines: synthesis and its impact on cytotoxic environment in spleenocytes, Journal of Environmental Research and Development Vol. 1 No. 1, July-September 2006
12. R. Sivakumar et al., International Journal of Health & Nutrition,A Computational Approach of Benzimidazole Containing Pyrazoline-5-one Derivatives as Targeted Antifungal Activity,1-1 (2010) 1-6
13. B. K. Kaymakcıoğlu et al., Antidepressant-like Activity of 2-Pyrazoline Derivatives, Journal of Marmara University Institute of Health Sciences Volume: 3, Number: 3, 2013 - http://musbed.marmara.edu.tr
14. S. A Siddiqui.et al., Synthesis and Anticonvulsant Activity of Some Substituted 3,5-Diphenyl-2-Pyrazoline-1-Carboxamide Derivatives, Chemical Sciences Journal, Volume 2010: CSJ-8.
15. Velmurugan et al., Synthesis, characterisation and evaluation of analgesic activity of 3, 5-disubstituted pyrazoline derivatives, International Journal of Pharmacy and Pharmaceutical Sciences Vol 4, Issue 2, 2012, ISSN- 0975-1491 Pg no -189-191.
16. Manna F et al,Inhibition of Amine Oxidases Activity by 1-Acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole Derivatives, Bioorganic & Medicinal Chemistry Letters 12 (2002) 3629–3633
17. Md. Jahangir Alam et al., A review on pyrazole chemical entity and biological activity, International Journal of Pharma Sciences and Research (IJPSR), ISSN: 0975-9492, Vol 6 No 12 Dec 2015
18. Barthakur MG, Saikia A, Borthakur M, Saikia CJ, Bora U, Boruah RC. Conjugate base catalysed one-pot synthesis of pyrazoles from β-formyl enamides. Tetrahedron Letts 2006; 47:43-6.
19. Leonelli C,Corradi A, Rizzuti A, Rosa R, Veronesi P, Grandi R, et al. New “green” approaches to the synthesis of pyrazole derivatives. Molecules 2007; 12:1482-95.
20. Sun Y, Hu J, Chen S, Yang J, Rao Y. Synthesis of tri- and tetra substituted pyrazoles via Ru(II) catalysis: Intramolecular aerobic oxidative C-N coupling. Org Lett 2012; 14:5030-3.
21. Jena AK ,Panda N,. Fe-catalyzed one-pot synthesis of 1,3-di- and 1,3,5-trisubstituted pyrazoles from hydrazones and vicinal diols. J Org Chem 2012; 77:9401-6.
22. Yadav SK, Kumar SV, Raghava B, Saraiah B, Ila H, Rangappa KS, et al., Cyclocondensation of arylhydrazines with 1,3-bis(het)arylmonothio-1,3-diketones and 1,3-bis(het)aryl-3-(methylthio)-2-propenones: Synthesis of 1-aryl-3,5-bis(het)arylpyrazoles with complementary Regio selectivity. J Org Chem 2013; 78:4960-73.
23. Abdel-Hady, Badawy, Eid, New heterocyclic synthesis, convenient synthesis of fused pyrimidines, pyrazoles, and triazines, Journal of Islamic Academy of Sciences 2:4, 241-243, 1989
24. Goda et al ., Synthesis and antimicrobial evaluation of new Isoxazoles and pyrazole derivatives ,Saudi Pharmaceutical Journal , Vol 11, No 3,July 2003
25. Zaki M. E. A., Synthesis of Novel Fused Heterocycles Based on Pyrano[2,3-c]pyrazole , Molecules 1998, 3, 71-79
26. M. C. FOTI et al.Biopolyphenolics as antioxidants:Studies under an Indo-Italian CSIR-CNR project, Pure Appl. Chem., Vol. 77, No. 1, pp. 91–101, 2005.
27. Bonacorsoet al. , Regiospecific Synthesis of New Non-Condensed Heteropolycyclic Systems from β-Heteroaryl-β-methoxyvinylTrihalomethyl Ketones, J. Braz. Chem. Soc., Vol. 16, No. 4, 868-873, 2005.
28. Sahu S K . et al., Synthesis, Analgesic, Anti-inflammatory and Antimicrobial Activities of Some Novel Pyrazoline Derivatives , Tropical Journal of Pharmaceutical Research, June 2008; 7 (2): 961-968
29. KENDRE & BASEER, Synthesis and Evaluation of Some New Pyrazoline Derivatives as Antimicrobial Agents ,Orient. J. Chem., Vol. 29(1), 253-256 (2013) , Pg. 253-256
30. A. O¨ zdemir et al., Synthesis and Biological Evaluation of Pyrazoline Derivatives Bearing an Indole Moiety as New Antimicrobial Agents, Arch. Pharm. Chem. Life Sci. 2013, 346, 463–469.
31. Dipankar et al. SYNTHESIS, CHARACTERIZATION AND ANTIMICROBIAL ACTIVITIES OF SOME 2-PYRAZOLINE DERIVATIVES, Asian J Pharm Clin Res, Vol 5, Issue 4, 2012, 42-46
32. N.C. Desai. et al., Facile synthesis of novel fluorine containing pyrazole based thiazole derivatives and evaluation of antimicrobial activity Journal of Fluorine Chemistry 142 (2012) 67–78
33. B. K. Kaymakcıoğlu, S. Gumru, N. Beyhan, F. Aricioglu , Antidepressant-like Activity of 2-Pyrazoline Derivatives , Journal of Marmara University Institute of Health Sciences Volume: 3, Number: 3, 2013
34. Ahsan MJ. Anticonvulsant activity and neuroprotection assay of 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3 H-indeno [1,2-c] pyrazole-2-carboxamide analogues. Arab J Chem 2013; 3:644-50.
35. Ds
36. F. Manna et al. Inhibition of Amine Oxidases Activity by 1-Acetyl-3,5-diphenyl-4,5-dihydro-(1H)-pyrazole Derivatives Bioorg. Med. Chem. Lett. 12 (2002) 3629–3633
37. F. Chimenti et al, Inhibition of monoamine oxidases(MAO) by coumarin-3-acyl derivatives: biological activity and computational study, Bioorganic & Medicinal Chemistry Letters 14 (2004) 3697–3703.
38. SIVAKUMAR R et al., A Computational Approach of Benzimidazole Containing Pyrazoline-5-one Derivatives as Targeted Antifungal Activity, IJHN 1-1 (2010) 1-6
39. KUMAR R and JOSHI Y. C., Synthesis and antimicrobial, antifungal and anthelmintic activities of 3H-1,5-benzodiazepine derivatives, J. Serb. Chem. Soc. 73 (10) 937–943 (2008)
40. Deng et al., Synthesis and In Vitro Antifungal Evaluation of 1,3,5-Trisubstituted-2-Pyrazoline Derivatives, Chem Biol Drug Des 2012; 79: 279–289
41. Maruthi Rao B et al., Synthesis, characterization and evaluation of anti-epileptic activity of four new 2-pyrazoline derivatives compounds , Scholars Journal of Applied Medical Sciences (SJAMS) ISSN 2320-6691,Sch. J. App. Med. Sci., 2013; 1(1):20-27
42. Mohammad Shaharyar MMA, Bakht MA, Majeed J. Pyrazoline bearing benzimidazoles: search for anticancer agent. Eur J Med Chem 2010;45:114–19.
43. Waled A. Tantawy, Eman M. Flefel, Wael A. El-Sayed, Hayam H. Sayed, Nahed M. Fathy Journal ofHeterocyclic Chemistry Volume, 2013, pages 344–350
Received: 14 May 2019
Accepted: 20 July 2019
Gurinderdeep Singh1*, Anju Goyal2, R S Bhatti3 & Sandeep Arora2
1Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, Punjab, India
2Chitkara College of Pharmacy, Chitkara University, Rajpura, Punjab, India
3Government College of Pharmacy for Girls, Patiala, Punjab, India
* Author for Correspondence:
Mr. Gurinderdeep Singh
Assistant Professor (Pharmaceutical Chemistry)
Department of Pharmaceutical Sciences and Drug Research, Punjabi University Patiala
Punjabi University Patiala
Mob.94178-62535,+91-96464-99252
