2023.08.01.25
Files > Volume 8 > Vol 8 No 1 2023
Enhancing salinity tolerance in tomatoes at the reproductive stage by increasing pollen viability
Nasratullah Habibi1,2* , Mohammad Yosuf Fakoor2, Shah Mahomoud Faqiri2, Zarir Sharaf2, Mohammad Sadiq Hotak2, Nelofar Danishyar2, Mohammad Mustafa Haris2, Khuwaja Safiullah Osmani2, Takashi Shinohara1, Naoki Terada1, Atsushi Sanada1, and Kaihei Koshio1
, Mohammad Yosuf Fakoor2, Shah Mahomoud Faqiri2, Zarir Sharaf2, Mohammad Sadiq Hotak2, Nelofar Danishyar2, Mohammad Mustafa Haris2, Khuwaja Safiullah Osmani2, Takashi Shinohara1, Naoki Terada1, Atsushi Sanada1, and Kaihei Koshio1
1 Graduate School of Agriculture, Tokyo University of Agriculture, 1-1-1 Sakuragaoka, Setagaya-ku, Tokyo Japan 156-8502;
(N.H); [email protected] (T.S); [email protected] (N.T); [email protected] (A.S); [email protected] (K.K.).
2 Faculty of Agriculture, Balkh University, Balkh 1701, Afghanistan. [email protected] (M.Y.F); [email protected] (S.M.F); [email protected] (Z.S); [email protected] (M.S.H); [email protected] (N.D). [email protected] (M.M.H), [email protected] (K.S.U).
* Correspondence: [email protected]; Tel.: (+8180-4121-5020);
Available from: http://dx.doi.org/10.21931/RB/2023.08.01.25
ABSTRACT
This study was conducted to mitigate the adverse effects of sodium chloride stress on the reproductive parameters of tomatoes. This experiment was conducted in the greenhouse of the laboratory of tropical horticultural science, department of International agricultural development, Tokyo University of Agriculture. The design was a factorial CRD (completely randomized design) with five sodium chloride (0 mM, 50 mM, 100 mM, 150 mM, and 200 mM) treatments and four primings (0 MPa, 0.4 MPa, 0.8 MPa, and 1.2 MPa) treatments. Micro-Tom seeds were soaked in polyethylene glycol (PEG6000). Salinity was applied through irrigation water when the first flower bloomed. Reproductive-related parameters such as the number of flowers per plant, pollen viability, pollen germination, pollen tube length, number of fruits per plant, fruits size and yield per plant were measured. It was observed that salinity affected the tomato plants severely during the flowering stage, and many flowers did not bear fruit due to the decrease in pollen viability. In addition, electrolyte leakage increased under salt stress, while priming decreased this parameter. Priming improved the number of flowers, pollen viability, and fruits per plant. The best priming treatments were 0.8 MPa and 1.2 MPa for promoting and enhancing tolerance in the reproductive stage.
Keywords: physiology, priming, pollen viability, reproductive stage, and salinity.
INTRODUCTION
Tomato (Solanum lycopersicum L.) is one of the essential vegetables around the globe. Countries such as China, the United States, Turkey, Italy, India, and Egypt are the biggest producers of tomatoes1. Tomato producers face many challenges and many constraints that limit tomato production2. Among abiotic stress factors, salinity is one of the most important abiotic factors that can be found in almost half of the world's soils3,4. Salt-affected soils include 63.2 million hectares of topsoil (0-30 cm) and 120.0 million subsoils (30-100 cm)4. These soils are formed because of concentrated salts and ions like Na+, which causes degradation4.
The salt-affected soils are problematic for most plants, and salinity is a high injury factor for vegetable crops like tomatoes worldwide. In some areas, the irrigation source is only seawater. Therefore, further effort is needed to increase water efficiency or plant tolerance5, but it might be difficult to find a solution for this problem6 easily.
Salinity affects tomatoes' vegetative and reproductive growth because it slows the absorption of nutrients due to the accumulation of toxic ions like Na+ and Cl- 7. In vegetables, like tomatoes, the reproductive stage is susceptible, and flowers will be severely affected under salinity8. Furthermore, fruits per plant in tomatoes are also harmed due to this factor 9, 10, 11, which leads to high leaf surface temperature; thus, stomata start to close, and the cell wall and electrons start to leak from leaf cells12. During the reproductive stage, it has been proved that pollen viability and pollen germination are the ideal parameters to be assessed for studying the tolerance of plants against constraints13 like salinity or heat stress, and both pollen viability and pollen viability and pollen germination decreased under salt stress conditions14.
There are studies on the breeding and development of salt-tolerant varieties, but most were ineffective for all regions due to climatic fluctuations. Therefore, some easy and effective methods with low cost can increase salt tolerance in the plant before facing stress, one of them is seed priming9, and it has already been proved that various seed priming agents10, 38 such as polyethylene glycol (PEG) improved growth parameters in tomato plants under sodium chloride stress11.
In addition, osmo-priming by polyethylene glycol mitigates the toxicity of the ions that are accumulated due to severe salt stress by stabilizing the balance between cations and anions; therefore, polyethylene glycol diminishes the negative effects of Na+ and Cl- ions accumulated in leaves due to sodium chloride salinity stress13 by increasing the amount of K+ ions14.
Considering the adverse effects of salinity, tomato is susceptible during their vegetative and reproductive growth, especially in the flowering stage, which is critical against salinity stress15. There is no previous study on the comparative effects of PEG6000 and sodium chloride on the reproductive parameters of tomatoes. Thus, the current study was conducted to (a) evaluate the negative effects of salinity on the growth and physiological parameters of tomatoes, (b) evaluate the comparative effectiveness of PEG and salinity on the growth and physiological parameters of tomatoes during flowering and fruit-bearing stages, and (c) study the interaction between PEG and salinity.
MATERIALS AND METHODS
This experiment was conducted at the Tokyo University of Agriculture (Setagaya Campus) in 2021. The experiment was designed as a factorial complete randomized design with 6 replications, having 4 salinity levels and 4 priming treatments, and Micro-Tom seeds were used as plant material. The sodium chloride salinity treatments were (a) control (0 mM), (b) 50 mM, (c) 100 mM, (d) 150 mM, and (e) 200 mM, and priming treatments were (a) control (0 MPa), (b) 0.4 MPa, (c) 0.8 MPa, and (d) 1.2 MPa. PEG6000 was used to prime the seed before cultivation, and the seeds were soaked in the solution for 36 h. Then, the seeds were washed three times with pure water and dried for 3 hours at 25±5°C. The calculation for PEG6000 to be used in osmo-priming was according to Michel & Kaufmann16 and Aqvila & Carella17. The below formula was used to calculate the amount of PEG6000 to be dissolved in pure water to prepare priming treatments:
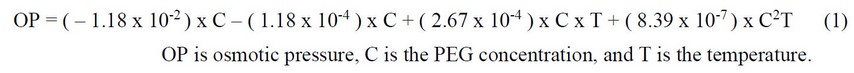
The seeds were cultivated in seed trays at the greenhouse (25±5°C temperature and 60±10 % humidity), and after 30 days, when the plants were 3 to 4 leaves (30 days after germination), they were transplanted to the plant bed made of wool and is called Rockwool.
Salinity was applied at the start of the flowering stage. Salt stress was applied within irrigation water. Irrigation was done using tap water, and we fixed the pH of irrigation to 5.8 – 6, and it was adjusted using sodium hydroxide and hydrochloric acid. Then NaCl was added to the irrigation water in the appropriate molarity and mixed and used for irrigation. Temperature also plays a vital role during salinity stress; therefore, we recorded temperature using a digital thermo-recorder (TR-72WB; ThermoWorks, American Fork, UT, USA). Data were collected hourly, then the average of every 24 hours was taken and represented as daily temperature. The temperature in the greenhouse was adjusted at 25±2 °C by the automatic electric heating system.
Temperature measurement
Temperature is an essential constraint on tomatoes during the reproductive stage. Many researchers have recently reported the negative effects of high and low temperatures on flowering and fruit yield. In the current experiment, temperature data were recorded on an hourly basis from cultivation to harvest. The digital thermometer was used to record the temperature, and the device was kept at least 1 meter from the ground.
Flowers per plant
The number of flowers per plant was counted daily, starting with the first flower blooming and then on the full flowering; the sum of all data was considered as the total number of flowers per plant.
Pollen viability
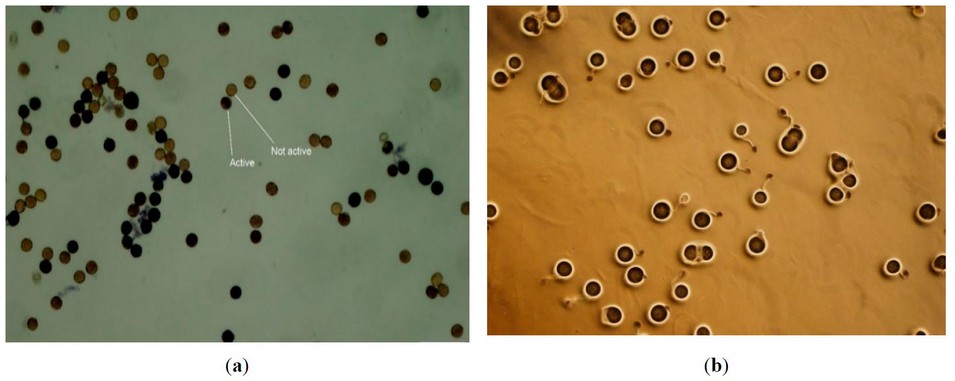
Pollen viability was determined by MTT (2,5-diphenyl mono-tetrazolium bromide) assay17. Some modifications were added to the method. Firstly, 500 mg of agar and 2.5 g of sucrose were poured into a 100 ml beaker, and 47 ml of pure water was added. Then, the solution was kept inside the heater under 70 °C temperature for one minute and mixed well using a strayer. The solution was poured into glassy Petri dishes and covered to avoid contamination. A circle was made in the middle of the medium to keep pollen grains. Then, 50 mg of MTT was taken in a 15 ml falcon, and 5 ml pure water was added and vortexed well. Flowers from all treatments were taken in the morning from 8:00 to 10:00 AM, and pollen was dropped on medium using a sterilized brush. Five flowers were taken from each treatment. The brush was washed using ethanol 100% to be used in each treatment. Then, 0.5 mL of MTT solution was quickly added to pollens using a single channel pipet made by thermos fisher, Japan. Then, the Petri dishes were closed and incubated at 25 °C for one hour. Pollen pictures were taken using CellSense software by microscope (Olympus) with 10x magnification. Pollen with dark colorations was counted as alive and light colorations as dead (Figure 1a). The pollen viability was calculated as below:
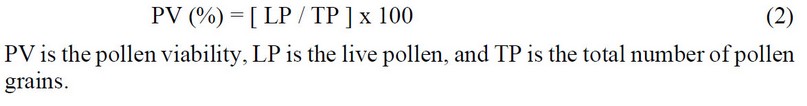
Figure 1. Represents live and dead pollens of tomato flowers (a), and germinated pollens (b).
Pollen germination
Germinated pollen grains were selected from the pictures taken by a microscope. The number of germinated and non-germinated were counted, and pollen germination percentage was calculated from them (Formula 3) and was measured at 4 hours, 6 hours, 8 hours, and 10 hours after incubation.

Pollen tube length
Pollen tube length was measured using ImageJ software. The pictures taken by the Olympus microscope were analyzed by imageJ for the pollen tube length. Pollen tube Pollen tube length was measured at 4 hours, 6 hours, 8 hours, and 10 hours after incubation.
Leaf injury rate
Leaf injury rate was concluded based on electrolyte leakage (EL) from leaf tissues. A handy meter was used to measure electric conductivity (LAQUATWIN-S070, Horiba Scientific Ltd., Japan), as per the method as described by Habibi12. The samples were taken from 15 leaves per treatment and stainless-steel cork-borer with one centimeter diameter was used to make the disk for electric conductivity. Electric conductivity (EC) was measured two times for calculating electrolyte leakage. The first electric conductivity (E1) was measured after keeping the disk inside a vial filled with two milli liter pure water at 25 ± 1 °C temperature for 20 minutes, then the samples were kept under 70 °C for 20 minutes and back cooled to room temperature and electricity was measured for the second time (E2). The following equation was used to calculate the leaf injury:
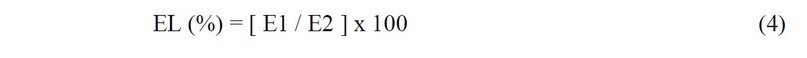
Yield attributes
The number of fruits per plant was, and these data were taken from six plants in each treatment. Fully ripened fruits were harvested from six plants in each treatment, and then the yield per plant (g) was measured using a digital balance.
Statistical analysis
We used a CRD (complete randomized design) to set up our experiment design. For the data analysis and explanation of the differences, we used two-way, Pearson's correlation analysis, and regression analysis. Analysis of variance was done using the R language, specifically RStudio v3.6.2 statistical software, and correlation analysis with regression analysis and visualization was done using Python software v3.7.4 (Jupyter Notebook, https://api.anaconda.org).
RESULTS
The flowering and reproductive stage was between April 5 to May 10; during this period, the temperature was between 18 to 30 °C, while the fruit growing or harvest stage occurred between May 11 to May 31 with a temperature between 23 to 37 °C. The average daily temperature during the flowering stage was 24 °C, while in the fruit growing and harvest stage, it was 27 °C (Figure 2). The temperature was not considered a factor and was kept constant in the range required for the tomato plant.
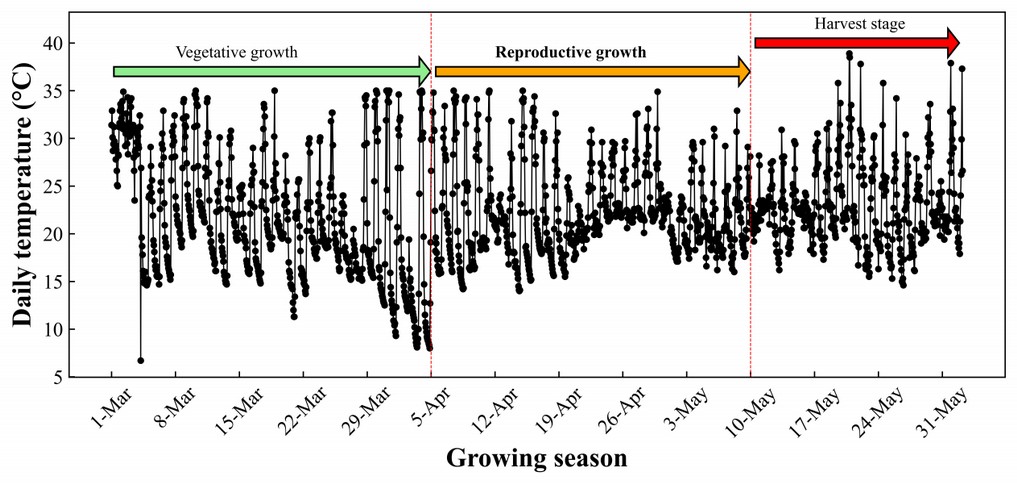
Figure 2. The average temperature during flowering and fruit-bearing stages.
Number of flowers per plant
The number of total flowers per plant was significantly affected by salinity and PEG. There was a strongly negative interaction between salinity and PEG; salinity decreased the number of absolute flowers per plant, but PEG increased. Plants under 0.8 MPa and 1.2 MPa PEG produced a more significant number of flowers per plant under all salinity treatments. There is a statistically significant difference between non-primed and primed treatments (Figure 3). The highest number of flowers per plant was recorded at 341 in 0.8 MPa under 50 mM sodium chloride salinity, and 1.2 MPa followed it under the same salinity level, producing 252 flowers per plant.
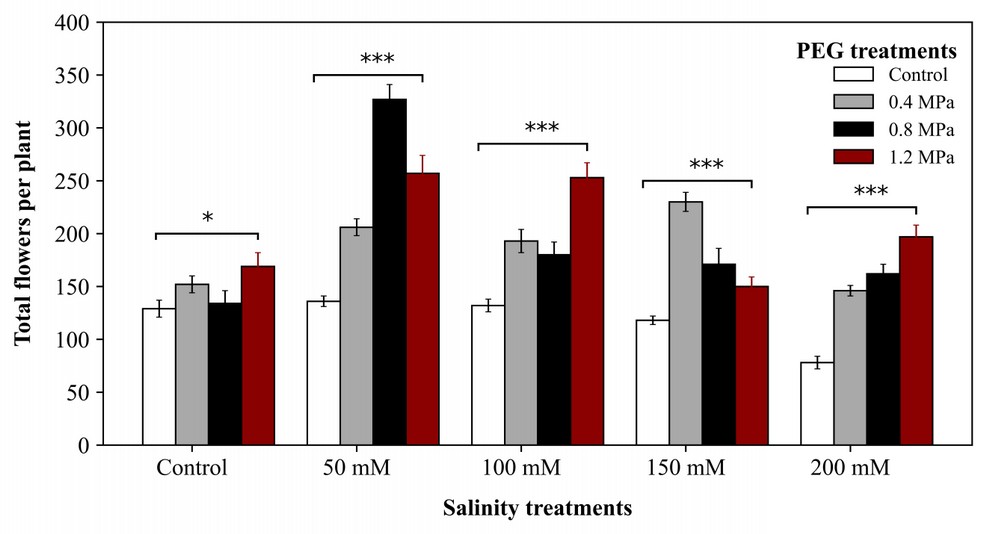
Figure 3. Effect of priming on the total number of flowers per plant under salt stress. *, **, and *** indicate significance levels of P <0.05, P < 0.01, and P < 0.001 respectively.
Pollen viability
In this research, flowers were the most sensitive organ against salinity in the tomato plant. When plants face high salinity stress, most flowers die, and some cannot produce fruits. The reason was that pollens were severely injured by salt stress. The number of life pollens was significantly decreased under salt stress treatments but significantly increased under priming treatments. Furthermore, the interaction between salinity and priming (PEG) was also significant. Only within the groups, the number of life pollen was not significantly different under 50 mM salinity, while for the 100 mM, 150 mM, and 200 mM salinity, 1.2 MPa priming treatment had a more significant number of life pollen compared to the control.
Furthermore, there were more dead pollen grains under salinity treatments with no priming, while the number of dead pollen grains was significantly decreased under priming treatments, and there was a significant negative interaction between salinity and priming. The total number of pollen grains also decreased under salinity, while priming significantly affected it. However, the all-over interaction between salinity and PEG was significant. Finally, pollen viability, considered an important factor for fruit production, was strongly decreased under salinity (p<0.001). However, PEG efficiently improved it; therefore, there was a significant negative interaction between salinity and priming. All PEG treatments had more pollen viability than the control (Table 1).
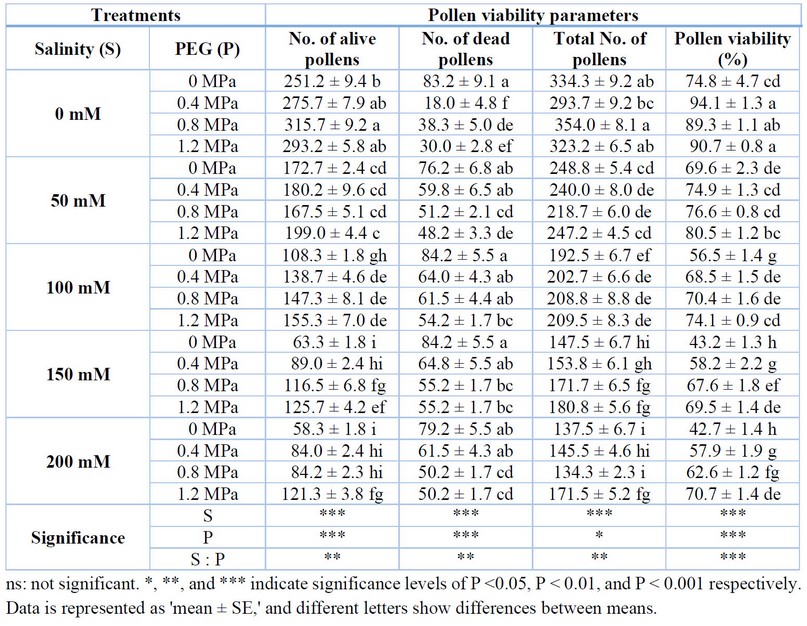
Table 1. Effect of PEG priming on pollen-related parameters of tomato plants under salt stress.
Figure 4 illustrates a visual comparison of pollen viability in our study. It shows the difference between primed and non-primed tomato plants regarding pollen viability. The top row shows the control (non-primed) plants under different salinity levels, whereas the bottom row shows the pollen viability belonging to the pre-sowing seed-primed plants. This visualization indicates that in non-primed plants, the total number of pollens is sometimes more than in primed ones. However, the flowers belonging to the primed plants had more pollen viability and more living pollen grains. Turning to salt stress conditions, priming treatments increased the total number of pollens compared to the control (Figure 4).
.
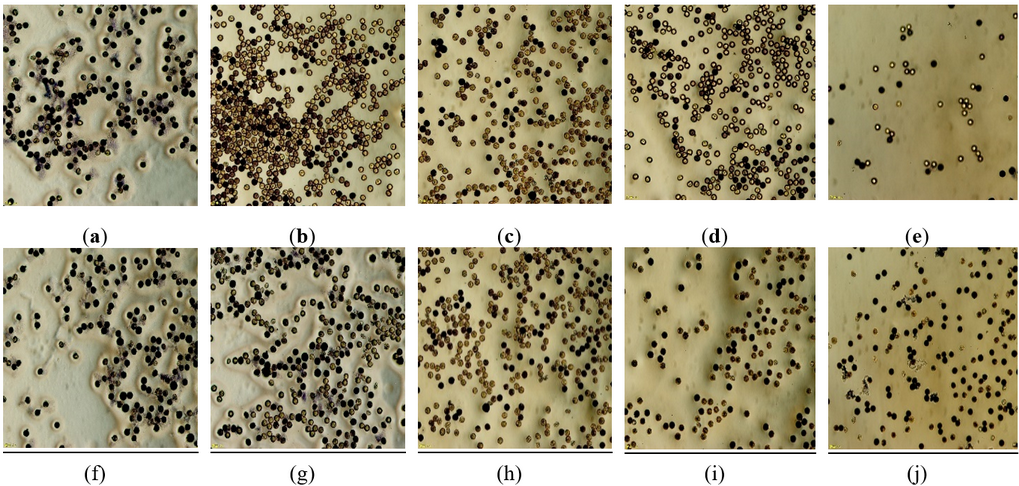
Figure 4. Represents the effects of priming treatments on pollen viability of tomatoes under salt stress compared to ambient conditions. Top row shows pollen viability in control – control (a), 50 mM – control (b), 100 mM – control (c), 150 mM – control (d), 200 mM – control (e) treatments, while the bottom row shows pollen viability in control – 1.2 MPa (f), 50 mM – 0.4 MPa (g), 100 mM – 1.2 MPa (h), 150 mM – 1.2 MPa (i), and 200 mM – 1.2 MPa (j) treatments.
Pollen germination
Pollen germination decreased under salt stress, while priming increased it. Overall, there was a significant difference in pollen germination within each salinity treatment. After 4 hours of incubation, 0.4 MPa and 0.8 MPa priming treatments showed better performance and the salinity and priming effects were strongly significant, while there was a negative interaction between salinity and priming. After 6 hours after incubation, pollen germination was also affected by both salinity and priming and there was a significant negative interaction between salinity and priming, however, the 0.8 MPa priming treatment was the best one. In addition, the interaction between salinity and priming was negatively significant. Finally, after 10 hours of incubation, primed plants had better pollen germination than the non-primed ones under all salinity treatments (Table 2).
The difference between the highest and lowest pollen germination was 58.4 %, showing a 78,1 % decrease in pollen germination in the salt-stressed plant compared to the normal plants (Table 2). The primed plants with 0.8 MPa of PEG 6000 under no salinity had the highest germination percentage, whereas non-primed plants with 200 mM salinity had the lowest value. The variation at 6, 8, and 10 hours after incubation was 69.6 %, 65.2 %, and 60.4 %, respectively. The highest pollen germination for 6, 8, and 10 hours after incubation belonged to 0.8 MPa, 1.2 MPa, and 1.2 MPa treatments, whereas under 200 mM salinity had the lowest values under 6, 8, and 10 hours after incubation.
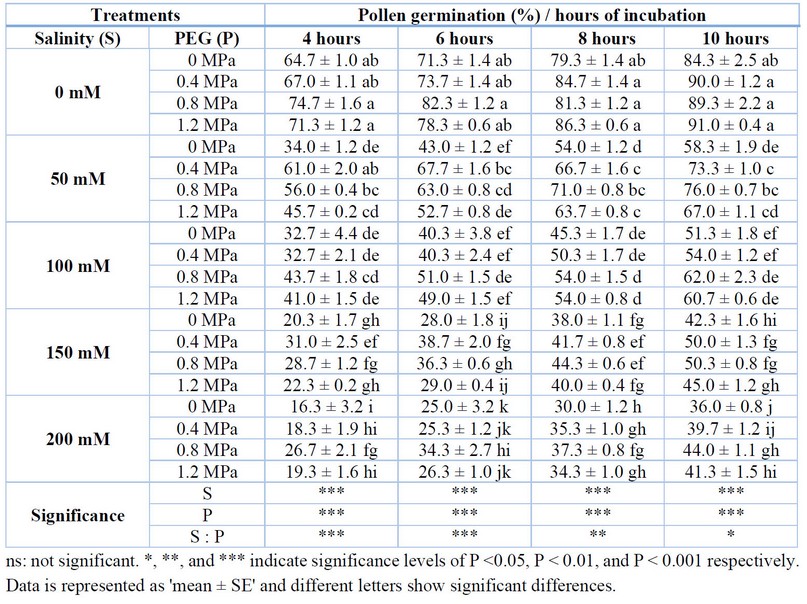
Table 2. Effect of PEG priming on pollen-related parameters of tomato plants under salt stress.
Pollen tube length
Pollen tube length was affected by salinity and priming, where salinity diminished the pollen tube length but oppositely, priming increased the pollen tube length, leading to a higher pollen tube growth speed. There was a significant effect of salinity on pollen tubes after four hours of incubation, while a positive impact of priming was found on pollen tubes. The statistical analysis showed an opposite interaction between salinity and priming treatments.
According to Table 3, after 4 hours of incubation, the 0.4 MPa priming treatment had higher pollen tube length (12.7 µm, 8.5 µm, 7.9 µm, and 6.9 µm) at 0, 50, 100, and 200 mM salinity treatments, while in 150 mM salinity there was no significant differences of pollen tube length between primed and non-primed plants. At 6 hours after incubation, all priming treatments affected the pollen tube length at no salinity treatment (0 mM), while the differences were not significant for salinity treatments. At 8 hours after incubation 0.4 and 0.8 MPa priming treatment had a good performance at 0 mM, 50 mM, 150 mM, and 200 mM salinity, while 1.2 MPa was good at 100 mM and 200 mM salinity compared to the non-primed plants. At 10 hours after incubation, 0.4 MPa priming treatment was the best at all salinity levels.
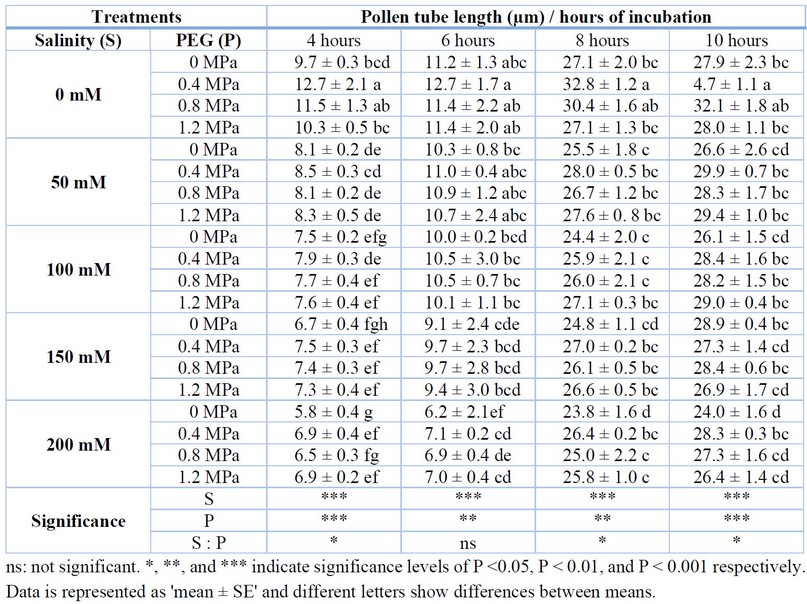
Table 3. Effect of priming on pollen tube length of tomato under salt stress.
Leaf injury and survival rate
Leaf injury was decided based on electrons (electrolyte leakage) that were leaked from leaf tissues. There was a positive relation between EL and salinity. A higher EL was observed in saline treatments compared to control during flower blooming and fruiting. Therefore, the flowers wilted, and fewer fruits were formed in saline treatments. Furthermore, salinity decreased the survival rate (%) in tomato plants under saline treatments compared to control, except for 50 mM NaCl stress (Table 4). Seed pre-sowing priming with PEG 6000 decreased EL during both flowering and fruit-bearing. In addition, there was a significant interaction between salinity and priming treatments. In ambient conditions, 0.4 and 1.2 MPa priming treatments significantly decreased the electrolyte leakage of leaves during the flowering stage, while in the fruit-bearing stage, 0.8 and 1.2 MPa priming treatments could dramatically decrease the leaf electrolyte leakage compared to non-primed plants. In 50 mM salinity, 0.4 and 1.2 MPa had the lowest electrolyte leakage in flowering and fruit-bearing stages. In 100 mM salinity, during the flowering stage 0.8 MPa priming treatment and flowering stage, 1.2 MPa priming treatment had lower electrolyte leakage compared to others. In the 200 mM salinity treatment, 0.4 MPa priming treatment was the best treatment during the flowering stage, but during the fruit-bearing stage, 1.2 MPa was the best priming treatment (Table 4).
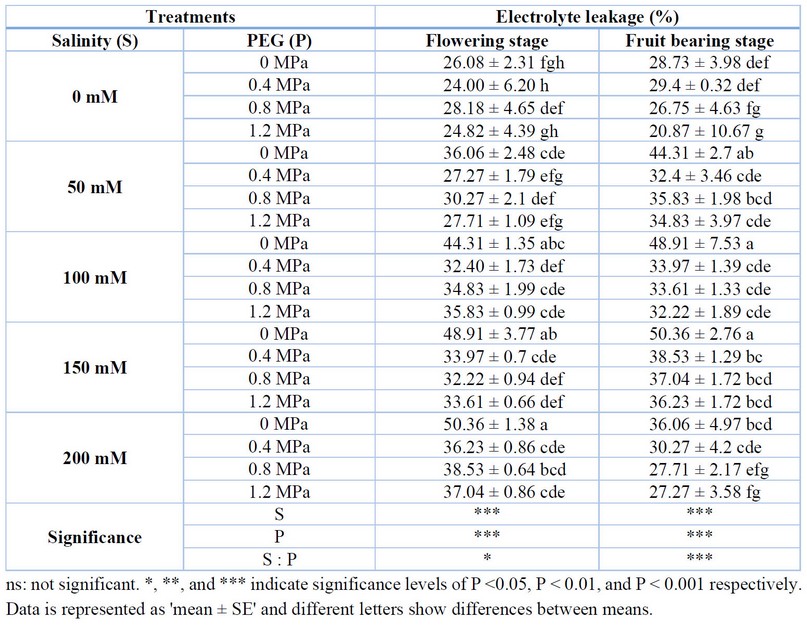
Table 4. Effect of PEG priming on leaf electrolyte leakage of tomato plants under salt stress.
Number of fruits per plant and plant yield
These two parameters were severely affected by salinity. In this study, the number of fruits per plant decreased under salinity levels, but priming increased them compared to non-primed plants. The treatment of 0 mM salinity and 1.2 MPa priming treatments had a higher number of fruits (45 fruits per plant), while 0.4 and 0.8 MPa were almost equal, but all priming treatments were better than non-primed treatments. Priming was effective in all salinity levels, like In 50 mM, 100 mM, and 200 mM salinity level, plants under 1.2 MPa priming treatment had more number fruits per plant, while in 150 mM salinity 0.8 MPa priming treatment was better than others (Figure 5A). Yield per plant was affected by salinity too. There was no significant effect of priming in control, but in 50 mM salinity, the differences between primed and non-primed plants were significant. The 1.2 MPa priming treatment produced more yield than others in all salinity levels (Figure 5B).
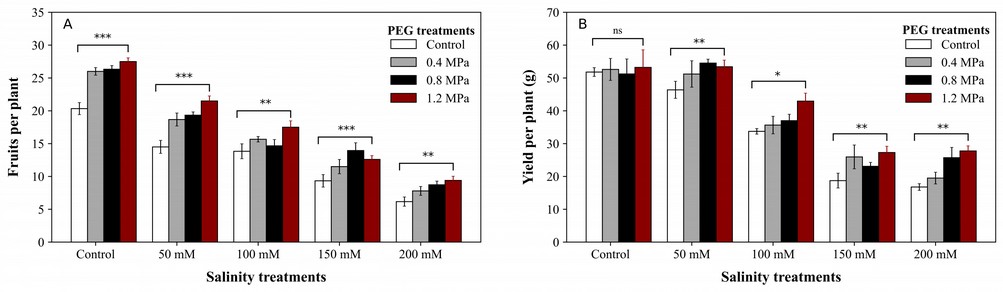
Figure 5. Effect of seed priming on fruits per plant (a) and yield per plant (b) of tomato under salt stress. *, **, and *** indicate significance levels of P <0.05, P < 0.01, and P < 0.001 respectively. ns: not significant.
Regression analysis
Based on linear regression analysis, the pollen viability and germination model explained 80 % of the variation of the data. Independently, pollen viability and the number of fruits per plant explained 79 %, and pollen viability and yield explained 69 %. It was found that there is a strong relationship between pollen viability and pollen germination, which means that an increment follows an increment in pollen viability in pollen germination. In addition, in flowers showing more alive, pollens also showed more germinated pollen grains. An increase in pollen viability caused a substantial increment in the number of fruits per plant, meaning that pollen viability directly affects the fruit set. Moreover, pollen viability is directly related to yield per plant (Figure 6).
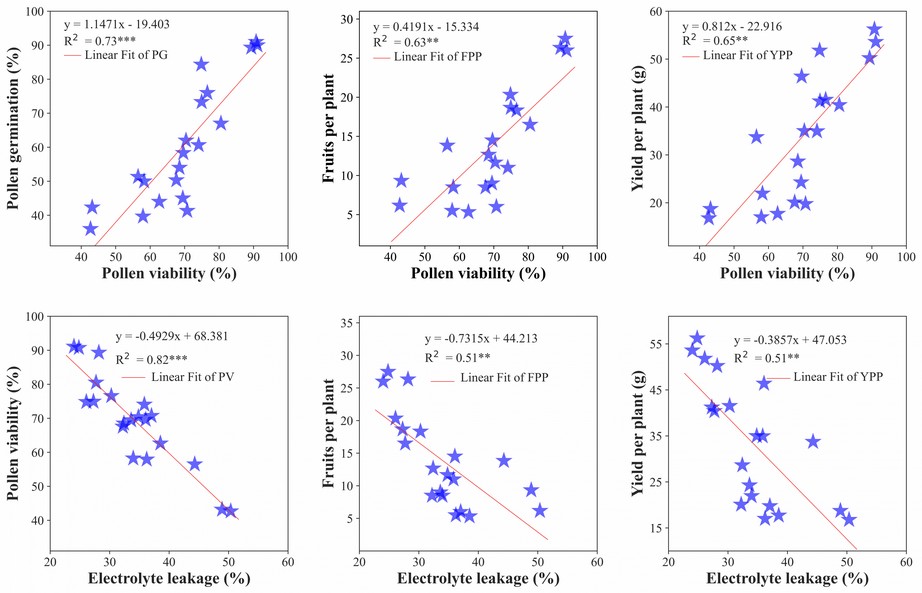
Figure 6. Regression analysis of pollen viability, fruits per plant and yield per plant. PV: pollen viability, PG: pollen germination, FPP: fruits per plant, and YPP: yield per plant.
In addition, there was a negative interaction between EL with pollen viability, number of fruits per plant, and yield. The linear models showed that EL gradually decreased pollen viability, leading to a lower number of fruits per plant and lower yield (Figure 6).
Correlation analysis
Pearson's correlation was conducted to study the relationship between pollen parameters and fruit set. The number of live pollen grains had a strong positive correlation with the total number of flowers, but in high salinity treatments, even if some flowers remained, the number of live pollen was less. Furthermore, the number of live pollens and death pollens had a significantly negative correlation. The total number of pollens had a strong positive correlation with the number of flowers per plant and the number of live pollens, which means that if there was a more significant number of flowers in a plant, there was more total number of pollen and more live pollen grains. Moreover, pollen viability had a positive correlation with the number of live pollen grains and the total number of pollen grains but had a negative correlation with the number of death pollen grains. Pollen germination had a strong correlation with flowers per plant, the number of live pollens, the total number of pollens, and pollen viability. Hence, the number of fruits per plant is an important yield parameter had a strong positive correlation with the number of flowers per plant, number of live pollen grains, total number of pollen grains, pollen viability, and pollen germination (Table 5).
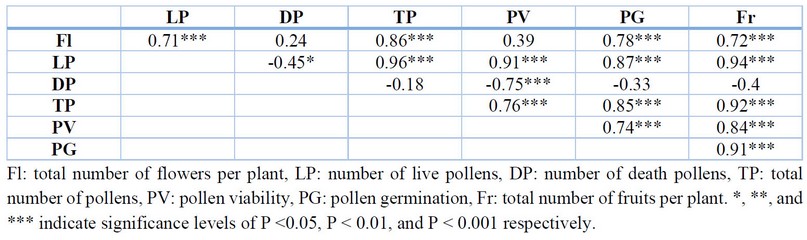
Table 5. Correlation Matrix of reproductive-related parameters.
DISCUSSION
Temperature is a very important factor besides other constraints on tomato plants during the reproductive stage. Many researchers early up to recently reported the negative effects of high and low temperature17 on flowering and fruit yield18.
Flowers are the sensitive organ of plants that can be harmed very quickly due to unboreable environmental conditions. Sodium ions accumulation due to salinity is very harmful to the number of flowers per plant, as previously reported too18,12. It's reported that salinity decreases the total number of flowers per plant. Furthermore, according to the previous reported result, sodium chloride salinity delays, the flowering date, and plants under salt stress took more days to produce flowers21,8. Moreover, also it's stated that plants under salt stress produce a smaller number of flowers per plant compared to the control plant where no salinity was applied22,8. In addition, a delay in production was also reported in tomatoes under salt stress23. All the reports are in parallel to our findings except for the effects of priming on the number of flowers per plant under salt stress, where there is very little information about this issue.
Moreover, it is important to be mentioned that some plants can produce flowers under salinity stress, but their flowers don't bear fruits due to inactive pollens. It is also been stated that pollen viability decreases as the salinity concentration increase24, and this research work was on the effects of salinity on pollen viability and pollen germination of different olive cultivars, and they believe that these parameters can be considered while testing the salt tolerance in plants. And a decrease in pollen viability, pollen germination, and pollen growth was 25 in Maize while the plants were grown under sodium chloride salinity conditions. Therefore, we conclude that pollen viability is an important factor in explaining the tolerance of plants against abiotic stresses. The difference between their study and ours is, firstly, the plant, and secondly, they just did the screening on the effects of salinity but nothing about increasing the tolerance. At the same time, our study provides enough evidence that seed priming with PEG6000 enhances tomato pollen viability under salt stress.
In high salt stress exposure, pollen viability and pollen germination decrement drastically, and it is reported that pollen viability and pollen germination in tomato plant decreases severely based on type and doses of salinity, and they reported that pollen germination is blocked in over 50 mM. Does of sodium chloride salinity23,26. Confirming this result, we also found the same result, but even under more than 50 mM sodium priming could improve our experiment's pollen viability and germination.
It was also found that high sodium chloride salinity decreases the pollen and pollen germination ability for the tomato plant, and they observed that more Na+ was accumulated in flowering organs like style, ovary, and anthers while K+ was decreased23. Besides, a significant decrease in pollen viability and pollen germination in chickpeas under 40 mM sodium chloride compared to control was observed 24,27.
Besides, 25 mM sodium chloride could significantly decrease the pollen germination percentage of four olive cultivars24. Moreover, it is reported that pollen germination of canola flowers was negatively affected by seawater, where the high salinity treatment decreased the pollen germination to 31-35 % based on varieties25,28. In addition, a significant decrease in pollen germination of tomato plant flowers is also reported under 25 and 50 mM of sodium chloride salinity37, which leads to a low fruit yield. The significance of our study is that we improved the pollen germination of the tomato plant's flowers by pre-sowing seed priming with PEG6000, which is a new finding, and there is no previous report about it. Based on reports26,23, salinity can decrease the pollen tube length, which confirms our finding, but the difference is that they used olive as plant material, and we used tomato. Furthermore, they used 0, 6.25, 12.5, 18.75 and 25mM of NaCl as salinity treatments, while we used higher concentrations (0, 50, 100, 150, and 200 mM NaCl) in our experiment.
It was observed that salt stress could increase electrolyte leakage in lettuce and spinach26 because under high salt stress, the cell membrane will be broken and more electrons will come out of tissues, hence, it can the electrolyte leakage could be concluded as cell injury indicator. It means as peroxidation happens in reproductive organs, the cell membrane will be damaged, and electrons will leak. Therefore, the pollens will dye, and pollen viability will be decreased. Habibi reported that a decrease follows an increase in electrolyte leakage in flowers per plant and yield per plant27,12. However, no research has been conducted before to show that seed priming with PEG6000 was effective on yield parameters of tomato plants und saline conditions, and our result is the first report in this regard.
Moreover, salt stress affects fruits per plant and yield per plant and it is reported that salinity decreases the number of fruits in tomatoes while salinization time was not significantly effective28,29, but in our study, we found that salinity substantially decreases the number of fruits per plant and fruit yield per plant too. Furthermore, it is reported that a salinity level of more than 150 mM is critical for the tomato plant, and they observe a huge reduction in tomato yield under 200 mM sodium chloride30. While we also found that salt stress can severely decrease the number of fruits per plant and the yield per plant, the results from Cano31 and Harel32 support our result that seed priming increases tomato yield under salt stress, but they primed the seeds with sodium chloride itself, while we primed the seeds with polyethylene glycol. A 9-10 % yield decrease in tomatoes under salt stress was reported, while seed priming with sodium chloride increased the yield compared to non-primed plants33.
In the current study, we did the regression analysis to find the relationship between pollen viability, fruits per plant, and yield per plant and consequently found that there was a strong relationship between pollen viability and fruits per plant and yield per plant which proves that more alive pollens will produce a greater number of fruits per plant which leads to higher yield production. Also, it is stated that pollen viability has a strong and direct relationship with fruit set and the number of fruits per plant34,35. In addition, it is also reported that a reduction in pollen viability and pollen germination caused the same reduction in fruit set of tomato plants under high temperature36, while a similar result was reported under high humidity and high temperature37. Therefore, the significance of our research is that there is no previous research work on the effect of seed priming by PEG6000 on the reproductive attributes of tomatoes under sodium chloride salinity.
CONCLUSION
Salinity severely affects the tomato plant during the reproductive stage. It damages the metabolism of the plant by harming physiological processes in the plant, and also it damages the cell wall and increases electrolyte leakage in leaves. Furthermore, the flower, which is a very sensitive organ of the plant, is also affected severely due to cell membrane damage through electrolyte leakage; therefore, many flowers dye under high salinity before bearing fruits, and they have low pollen viability because mostly pollens immediately dye when they face high salt stress. Moreover, the fruits under salinity stress will be smaller in size. However, seed pre-sowing priming with polyethylene glycol (PEG6000) improves the pre-tolerance in plants in a very early young stage. The polyethylene glycol produces a negative pressure around the seed while priming and sucks the inside content of the seed. The same process happens when the plant faces salinity stress. Therefore, the plant adapts to such stress and hereafter, when it faces such stress it can tolerate it.
Author Contributions:
Conceptualization, NH, KK, A. S, and NT; Methodology, NH, MYF, TS, and KK; Software, NH, SMF; Validation, NH, AS, ZS, and KK; Formal analysis, NH, MMH, MSH, ND.; Investigation, NH, KK, and TS; Resources, NH, MYF; Data curation, NH, MYF, NT, MMH, KSU, and SMF; Writing-original, N.H.; Writing-reviews and editing, ZS, MSH, SMF, MMH, KSU, and KK Visualization, NH, ND.; Project administration, NH, KK. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding
Institutional Review Board Statement: Not applicable.
Informed Consent Statement: Not applicable.
Acknowledgments: The authors thank the Japan International Cooperation Agency (JICA) for the financial support of the first author's study, and the Tokyo University of Agriculture, where the experiment was conducted.
Conflicts of Interest: The authors declare no conflict of interest.
REFERENCES
1. Sanower Hossain, M.; Sultan Ahmad Shah, J. Present scenario of global salt affected soils, its management and importance of salinity research . Int. Res. J. Biol. Sci. Pers. 2019, 1(1): 1-3.
2. Jaime D. P. V. Analytical model for the global consumption of tomatoes. Afr. J. Agr. Res 2012, 7(15). doi:10.5897/AJAR11.529
3. Bacha, H.; Tekaya, M.; Drine, S.; Guasmi, F.; Touil, L.; Enneb, H.; Triki, T.; Cheour, F.; Ferchichi. IMPact of salt stress on morpho-physiological and biochemical parameters of Solanum lycopersicum cv . Microtom leaves. Sou. Afr. J. Bot 2017, 108, 364–369. doi:10.1016/j.sajb.2016.08.018
4. Food and agriculture organization for the united nations (FAO). Global map of salt-affected soils. Intergovernmental Technical Panel on Soils (FAO USA) 2021, 1, 1-5.
5. Cuatero, J.; Bolarin, M. C.; Asins, M. J.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot 2006, 57(5), 1045–1058. doi: 10.1093/jxb/erj102
6. Yeo, A. R.; Flowers, T. J. Variability for some physiological characters affecting salt tolerance in tomato, J. Int. Horti. Sci 2002. 3, 435–441.
7. Caro, M.; Cruz, V.; Cuartero, J.; ESTAN, M. Salinity tolerance of normal-fruited and cherry tomato cultivars. Ind. J. Plant Physiol 1991, 1, 249–255.
8. Rahman, M. M.; Hossain, M.; Hossain, K. F. B.; Sikder, M. T.; Shammi, M.; Rasheduzzaman, M.; Hossain, M. A.; Alam, A. M.; Uddin, M. K. Effects of NaCl-salinity on tomato (Lycopersicon esculentum Mill.) plants in a pot experiment. J. Open Agr. 2018, 3, 578–585. doi:10.1515/opag-2018-0061
9. Pradhan, N.; Prakash, P.; Tiwari, S. K. Osmopriming of tomato genotypes with polyethylene glycol 6000 induces tolerance to salinity stress. J. Tren. Biosc 2014, 7(24): 4412-4417.
10. Hajer, A. S.; Malibari, A. A.; Al-Zahrani, H. S.; Almaghrabi, O. A. Responses of three tomato cultivars to sea water salinity, seedling growth. Afr. J. Biot. 2006, 5(10), 855–861.
11. Kumar, S. Comparative effect of NaCl and PEG on physiological and biochemical attributes during vegetative stage of tomato, Res. J. of Agri. Sci 2021, 12(3), 955-961.
12. Habibi, N.; Sediqui, N.; Naoki, T.; Atsushi, S.; Kaihei, K. Effects of salinity on growth , physiological and biochemical responses of tomato. J. ISSAAS 2021, 27(2), 14–28.
13. Sotomayor, A.; Merino, J.; Viera, W. Determining conditions for best pollen quality of red-purple tree tomato (Solanum betaceum Cav.) germplasm. Bionatura; Latin American J. Biot. Life Sci. 2021, 6(4), 2–7. doi: 10.21931/RB/2020.05.01.2
14. Balibrea, M. E.; Parra, M.; Bolarin, M. C.; Perez-Alfocca, F. PEG induced tomato adaptation to salt stress. Aus. J. Plant physiol 1999, 1, 781–786.
15. Farhoudi, R.; Saeedipour, S.; Mohammadreza, D. The effect of NaCl seed priming on salt tolerance , antioxidant enzyme activity, proline and carbohydrate accumulation of muskmelon (Cucumis melo L) under saline condition. Afr. J. Agric. Res. 2011, 6(6), 1363–1370. doi:10.5897/AJAR10.1007
16. Michel, B. E.; Kaufmann, M. R. The osmotic potential of polyethylene glycol 6000. J. Plant Physiol 1973, 51, 914-916.
17. Aqvila, D.; Carella, G. Polyethylene glycol 6000 priming effect on germination of aged wheat seed lots. J. Aur. Agr 1984, 26(3), 166–173.
18. Dafni, A. A new procedure to asses pollen viability. Sex Plant Reprod 2000, 12, 241–244.
19. Maisonneuve, B.; Den Nijs, A. P. M. In vitro pollen germination and tube growth of tomato (Lycopersicon esculentumMill.) and its relation with plant growth. J. Euphytica 1984, 33(3), 833–840.
20. Abdalla, A. A.; Verkerk, K. Growth, flowering and fruit-set of the tomato at high temperature. Neth. J. Agr. Sci 1968, 16(1), 71–76.
21. Panthee, D. R.; Jonathan, P. K.; Ann, P. Heritability of Flower Number and Fruit Set under Heat Stress in Tomato. J. Hort. Sci.2018, 53(9):1294–1299. doi: 10.21273/HORTSCI13317-18
22. Rosadi, R. A. B.; Senge, M.; Suhandy, D.; Tusi, A. The effect of EC levels of nutrient solution on the growth, yield, and quality of tomatoes (Solanum Lycopersicum) under the hydroponic system. J. Agr. Eng. and Biot. 2014, 2(1), 7–12.
23. Ghanem, M. E.; Elteren, J. V.; Albacete, A.; Quinet, M.; Martínez-andújar, C.; Kinet, J.; Pérez-alfocea, F.; Lutts, S. IMPact of salinity on early reproductive physiology of tomato (Solanum lycopersicum) in relation to a heterogeneous distribution of toxic ions in fl ower organs. J. Ind. Hort. 2009, 2, 125–136. doi: 10.1071/FP08256
24. Khaleghi, E.; Karamnezhad, F.; Moallemi, N. Study of pollen morphology and salinity effect on the pollen grains of four olive (Olea europaea) cultivars. Sou. Afr. J. Bot. 2019, 127, 51–57. doi: 10.1016/j.sajb.2019.08.031
25. Dhingra, H. R.; Varghese, T. M. Effect of Salt Stress on Viability, Germination and Endogenous Levels of Some Metabolites and Ions in Maize (Zea mays L.) Pollen. J. Ann. Bot 1985, 55, 415-420.
26. Yokaş, I.; Tuna, A. L.; Bürün, B.; Altunlu, H.; Altan, F.; Kaya, C. Responses of the tomato (Lycopersicon esculentum Mill.) plant to exposure to different salt forms and rates. Turkish J. Agri. Fores. 2008, 32(4), 319–329.
27. Turner, N. C.; Colmer, T. D.; Quealy, J.; Pushpavalli, R.; Krishnamurthy, L. Salinity tolerance and ion accumulation in chickpea (Cicer arietinum L.) subjected to salt stress. J. Int. Horti 2013, 2, 347–361. doi: 10.1007/s11104-012-1387-0
28. Gul, H.; Ahmad, R. Effect of salinity on pollen viability of different canola (Brassica napus L.) cultivars as reflected by the formation of fruits and seeds. Pak. J. Bot. 2006, 38(2), 237–247.
29. Del Amor, F. M.; Martinez, V.; Cerdá, A. Salt tolerance of tomato plants as affected by stage of plant development. J. Hort. Sci 2001, 36(7), 1260–1263.
30. Flowers, T. J. Improving crop salt tolerance. J. Exp. Bot. 2004, 55(396), 307–319. doi: 10.1093/jxb/erh003
31. Cano, E. A.; Moreno, V.; Bolarin, M. C. Pollen viability under salt stress. Plant Cell Reports 1996, 9. 791–794.
32. Harel, D.; Fadida, H.; Slepoy, A.; Gantz, S.; Shilo, K. The effect of mean daily temperature and relative humidity on pollen, fruit set and yield of tomato grown in commercial protected cultivation. Agron 2014, 4(1), 167–177. doi: 10.3390/agronomy4010167
33. Cuatero, J.; Rafael F. M. Tomato and salinity. J. Scientia Hort. 1999, 78, 83-125.
34. Tolessa, K.; Heuvelink, E. P. Pollen viability and fruit set of tomato introgression lines (Solanum esculentum XL. Chmielewskii) at Moderately High Temperature Regimes. J. Plant Physiol 2018, 36(1), 29–38.
35. Abdul-Baki, A. A.; Stommel, J. R. Pollen viability and fruit set of tomato genotypes under optimum- and high-temperature regimes. Hort. Sci 1995, 30(1), 115–117.
36. Wang, S. S.; Lia, Y. L.; Wen, X. Z. Effect of increasing humidity on flowering, fruit-setting and pollen characteristics of tomato under heat stress. Acta Hort. 2018., 1227, 305–311. doi:10.17660/ActaHortic.2018.1227.37
37. Hniličková, H.; Hnilička, F.; Orsák, M.; Hejnák, V. Effect of salt stress on growth, electrolyte leakage, Na + and K+ content in selected plant species. Plant, Soil and Environment 2019., 65(2), 90–96. doi:10.17221/620/2018-PSE
38. Safdary, A. J., Ahamdi, A. J., Habibi, N., Rahmani, Z., & Rasooli, S. (2020). The effect of different treatments on seed dormancy breaking and germination inducing in louisiana variety of okra (Abelmoschus esculentus l.). International Journal of Innovative Research and Scientific Studies, 3(4), 124–128. doi: 10.53894/ijirss.v3i4.45
Received: October 23, 2022 / Accepted: January 15, 2023 / Published:15 February 2023
Citation: Habibi N, Yosuf Fakoor M, Mahomoud Faqiri S, Sharaf Z, Sadiq Hotak M, Danishyar N, Mustafa Haris M, Safiullah Osmani K, Shinohara T, Terada N, Sanada A, Koshio K. Enhancing salinity tolerance in tomatoes at the reproductive stage by increasing pollen viability. Revis Bionatura 2023;8 (1)25. http://dx.doi.org/10.21931/RB/2023.08.01.25