2017.02.04.3
Files > Volume 2 > Vol 2 No 4 2017
INVESTIGATION / RESEARCH
Prevalence and major risk factors of diabetic retinopathy: A cross-sectional study in Ecuador
Prevalencia y factores de riesgo para retinopatía diabética: Un estudio transversal en Ecuador
Karen Sofía Flores-Mena1, *, Kory Naima Jara-Tamayo2,*, Paúl Herrera-González3, Enrique Gea-Izquierdo4
* Both authors contributed equally to this study.
http://dx.doi.org/10.21931/RB/2017.02.04.3
_______________________________________________________________________________________________________________________
ABSTRACT
Diabetes mellitus type 2 is one of the most prevalent diseases that cause dependency and disability, so its early diagnosis prevents future complications. More than 90% of blindness is preventable with strict systemic and ophthalmologic treatment. The objective of this study is to determine the prevalence and risk factors of diabetic retinopathy in type 2 diabetic patients between 30 and 60 years. Cross-sectional study analyzing age, sex, presence and duration of diabetes, unaware of being diabetic, high blood pressure, glycosylated hemoglobin, dyslipidemia, metabolic control and pharmacological treatment. Sample of 292 patients selected by simple random sampling from the Ophthalmology Service at San Francisco Hospital in Quito was determined. The prevalence of diabetic retinopathy was 21% (5% proliferative and 95% non-proliferative). There was a statistically significant association between diabetic retinopathy and age [prevalence ratio (95% CI): 8.14 (3.70-17.90), p=0.00], total cholesterol [prevalence ratio (95% CI): 7.43 (0.98-56.17), p=0.01], dyslipidemia [prevalence ratio (95% CI): 3.31 (0.96-11.38), p=0.04], metabolic control [prevalence ratio (95% CI): 4.57 (1.36-15.26), p=0.00], pharmacological treatment [prevalence ratio (95% CI): 46.88 (20.65-106.38), p=0.00] and use of insulin [prevalence ratio (95% CI): 41.10 (11.70-144.49), p=0.00]; on the other hand, there is no statistically significant association with sex, duration of disease, unaware of being diabetic, high blood pressure, glycosylated hemoglobin, triglycerides, HDL-C and LDL-C. The age, presence of dyslipidemia, metabolic control and diabetic treatment are risk factors that promote the development of diabetic retinopathy; so they must be taken into account from the first medical appointment for early detection, timely treatment and if necessary refer to the specialist.
Keywords: diabetic retinopathy, diabetes mellitus type 2, risk factors, metabolic control
_______________________________________________________________________________________________________________________
RESUMEN
La diabetes mellitus tipo 2 es una de las enfermedades más prevalentes que causa dependencia e incapacidad, por lo que su diagnóstico temprano evitará complicaciones futuras. Más del 90 % de ceguera por esta enfermedad es evitable, con un tratamiento sistémico y oftalmológico estricto. El objetivo de este estudio es determinar la prevalencia y los factores de riesgo de retinopatía diabética en pacientes diabéticos tipo 2 entre 30 y 60 años. Estudio transversal con análisis de edad, sexo, presencia y duración de diabetes, desconocimiento de la enfermedad, presión arterial alta, hemoglobina glicosilada, dislipidemia, control metabólico y tratamiento farmacológico de diabetes; en una muestra de 292 pacientes escogidos mediante muestreo aleatorio simple en el Servicio de Oftalmología del Hospital San Francisco de Quito. La prevalencia de retinopatía diabética encontrada fue del 21 % (5 % proliferativa y 95 % no proliferativa). Se evidenció una relación estadísticamente significativa entre retinopatía y edad [prevalence ratio (95% CI): 8.14 (3.70-17.90), p=0.00], colesterol total [prevalence ratio (95% CI): 7.43 (0.98-56.17), p=0.01], dislipidemia [prevalence ratio (95% CI): 3.31 (0.96-11.38), p=0.04], control metabólico [prevalence ratio (95% CI): 4.57 (1.36-15.26), p=0.00], tratamiento farmacológico [prevalence ratio (95% CI): 46.88 (20.65-106.38), p=0.00] y uso de insulina [prevalence ratio (95% CI): 41.10 (11.70-144.49), p=0.00]; por otra parte no se obtuvo asociación significativa con sexo, duración de enfermedad, desconocimiento de ser diabético, presión arterial alta, hemoglobina glicosilada, triglicéridos, HDL-C y LDL-C. La edad, dislipidemia, control metabólico y tratamiento de diabetes son factores de riesgo que promueven el desarrollo de retinopatía diabética; por lo que deben ser analizados desde la primera cita para una detección precoz, tratamiento oportuno y de ser necesario referir al especialista.
Palabras clave: retinopatía diabética, diabetes mellitus tipo 2, factores de riesgo, control metabólico
____________________________________________________________________________________________________________________
INTRODUCTION
Diabetes mellitus type 2 (DM2) is a chronic disease that has advanced in the last decade in occidental societies, causing variation in degrees of dependency. According to World Health Organization (WHO) the affected population during 2000 were 170 million people and for the year 2014, 422 million adult people were diabetic1.
Around 3.7 million diabetic patients passed away in 2012 and 43% affected people under 70 years old. In addition, the percentage of deaths attributable to DM2 in this group is higher in low and middle-income countries than in high-income. Based on the Ecuadorian Institute of Statistics and Censuses (INEC), since 2011 DM2 is the first cause of death in the country with a 7.15% of all of them2.
Diabetic retinopathy (DR) is a leading cause of new cases of visual loss among working-age worldwide. The prevalence for DR for adults with DM2 age 40 and older in the United States is 28.5% (4.2 million people); worldwide, the prevalence rate has been estimated at 34.6% (93 million people)3.
The Early Treatment Diabetic Retinopathy Study (ETDRS) has made an evolutional classification of DR that divided it into an early stage named as non-proliferative retinopathy (NPDR) and a severe level named proliferative retinopathy (PDR)4.
A quarter of the diabetic population is affected by any level of DR and 5% have a severe degree. DR is responsible of 10% of new cases of blindness every year, with a risk of development 25 times higher about this complication compared to the rest of the population.
Half of patients with DM2 presented PDR with more of 15 years of illness. According to the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), the prevalence of PDR is low during the first five years of DM2 diagnosis, although the risk of disease progression is directly proportional to the duration5.
The United Kingdom Prospective Diabetic Study (UKPDS)6 found that a level less than 7% of glycosylated hemoglobin is associated with a minor progression of the illness. Aditionally, the Diabetes Control and Complications Trial (DCCT)7 established that a controlled glycosylated hemoglobin reduced 76% risk of ocular damage, 50% renal complications and 60% neurological illness.
In consistency with UKPDS, controlled levels of blood pressure (BP) in hypertensive patients reduced 37% microvascular complications, 34% progression of the DR and 47% visual impairment. In addition, people with a systolic blood pressure (SBP) higher than 140 mmHg presented three times more risk of developing DR.
During the first years of DM2, no symptoms are presented, then most of the patients show complications at the time they present them. At 10 years of DM2, 35 to 40% of patients present DR. At 20 years, 80% of them have it.
The purpose of this study is to examine the prevalence and risk factors for DR in patients with DM2 between 30 and 60 years.
METHODS
Subjects: Between January 2013 and January 2015, a cross- sectional study was conducted in the Ophthalmology Service at San Francisco Hospital in Quito (Republic of Ecuador), located in a northern area of the city. A total of 1337 patients from 30 to 60 years old, including diabetic and non-diabetic people, who had been attended at this service and not presented any previous ophthalmological disease neither comorbidities as immunosuppression or collagenopathy, were analyzed. Medical records without complete information were excluded. 292 people were selected by random sampling, medical records were reviewed obtaining data like age, sex, presence and duration of DM2, presence or absence of DR according to the ocular fundus, unaware of being diabetic, blood pressure (analyzing the SBP and diastolic BP (DBP)), pharmacological treatment and personal background of hypertension and laboratory results such as glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C). Other variables were analyzed, as the duration of being diabetic with a cutoff > 10 years for longer diabetic status; and the ignorance of being diabetic at the moment of the DR diagnosis. All patients gave their informed consent prior to their inclusion in the study.
According to Adult Treatment Panel III (ATP III) and the American Heart Association, a person is diagnosed with dyslipidemia when presents a total cholesterol > 200 mg/dl, triglycerides > 150 mg/dl, low HDL-C < 40 mg/dl in males and < 50 mg/dl in female or LDL > 100 mg/dl. Patients with dyslipidemia were classified into four groups: isolated hypercholesterolemia when the patient only presented a total cholesterol higher than 200 mg/dl, isolated hypertriglyceridemia when the level of triglycerides was higher than 150 mg/dl, mixed dyslipidemia when the level of two of the followings were altered: total cholesterol, HDL-C, LDL-C and triglycerides; and HDL-C deficit when the value of this parameter was below the cutoff based on the sex.
Patients on antihypertensive drugs or with diagnosis of hypertension or BP level > 140 / > 90 mmHg were assumed to be hypertensive. Referring to glycosylated hemoglobin the cutoff for normal level was 7% (53 mmol/mol). Patients with abnormal levels of BP or laboratory tests were cataloged as bad metabolic control, otherwise as good metabolic control.
According to pharmacological treatment, the use of insulin was determining. It was not considering exercise and dietetic control. The type of DR were categorized by the severity scale of ETDRS4: NPDR and PDR.
Statistical analysis: All statistical analyses were performed with the software package SPSS version 21 for Windows and Epi Info version 7. Distribution of qualitative variables was compared by Pearson chi-square test and Fisher´s exact test.
Ethics statement: The study was conducted with the approval from the Research Ethics Committee of the Pontifical Catholic University of Ecuador. In addition, San Francisco de Quito Hospital delegated to the same Committee the approval of this study.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
RESULTS
Female constituted 51.37% of the sample and male 48.63%. People from 51 to 60 years old were the majority group representing 53.08%. Most of the patients presented normal blood pressure level (SBP < 140 mm Hg: 65.75%, DBP < 90 mm Hg: 72.60%), although 39.04% had a diagnosis of hypertension or were on an anti-hypertension treatment. 30.14% were diabetic and 21% of them had findings in their ocular fundus compatible with RD (5% PDR and 95% NPDR).
Regarding dyslipidemia, 76.37% of the patients presented it, being the most frequent mixed dyslipidemia with 43.50%, followed by isolated hypertriglyceridemia 24.21%, HDL-C deficit 17.49% and isolated hypercholesterolemia 14.80%.
In addition, 62.5% of the diabetic patients had high level of HbA1c; 46.59% showed a longer DM2 duration. Finally, 39.77% did not know their condition as diabetic at the time of the DR diagnosis.
According to the type of DR, all patients with PDR were older than 59 years old; on the other hand, patients with NPDR had an average age of 54 years old, even though it was found in patients since 30 years old.
Duration of 10 years or more of being diabetic is presented in 54.39% of patients with NPDR and 100% of PDR.
A higher level of HbA1c was found in 63.16% of patients with NPDR and the entire group of PDR.
NPDR patients with dyslipidemia were 85.97%. Meanwhile, patients with bad metabolic control were 94.74% of NPDR group and 100% of PDR group.
Female population presented 66.67% of the cases of PDR, 53.33% were diabetic for more than 10 years, 52.67% showed a high level of HbA1c and 82.67% had a bad metabolic control. On the other hand, male population showed 33.33% of the cases of PDR, 38.03% of them had DM2 for more than 10 years, 46.48% reported a high level of HbA1c and 84.51% had a bad metabolic control. In contrast, in NPDR the major group was represented by female (52.63%) versus male (47.37%).
Demographic data, selected clinical and laboratory findings for patients by sex, DM2 and DR are shown in Table 1.
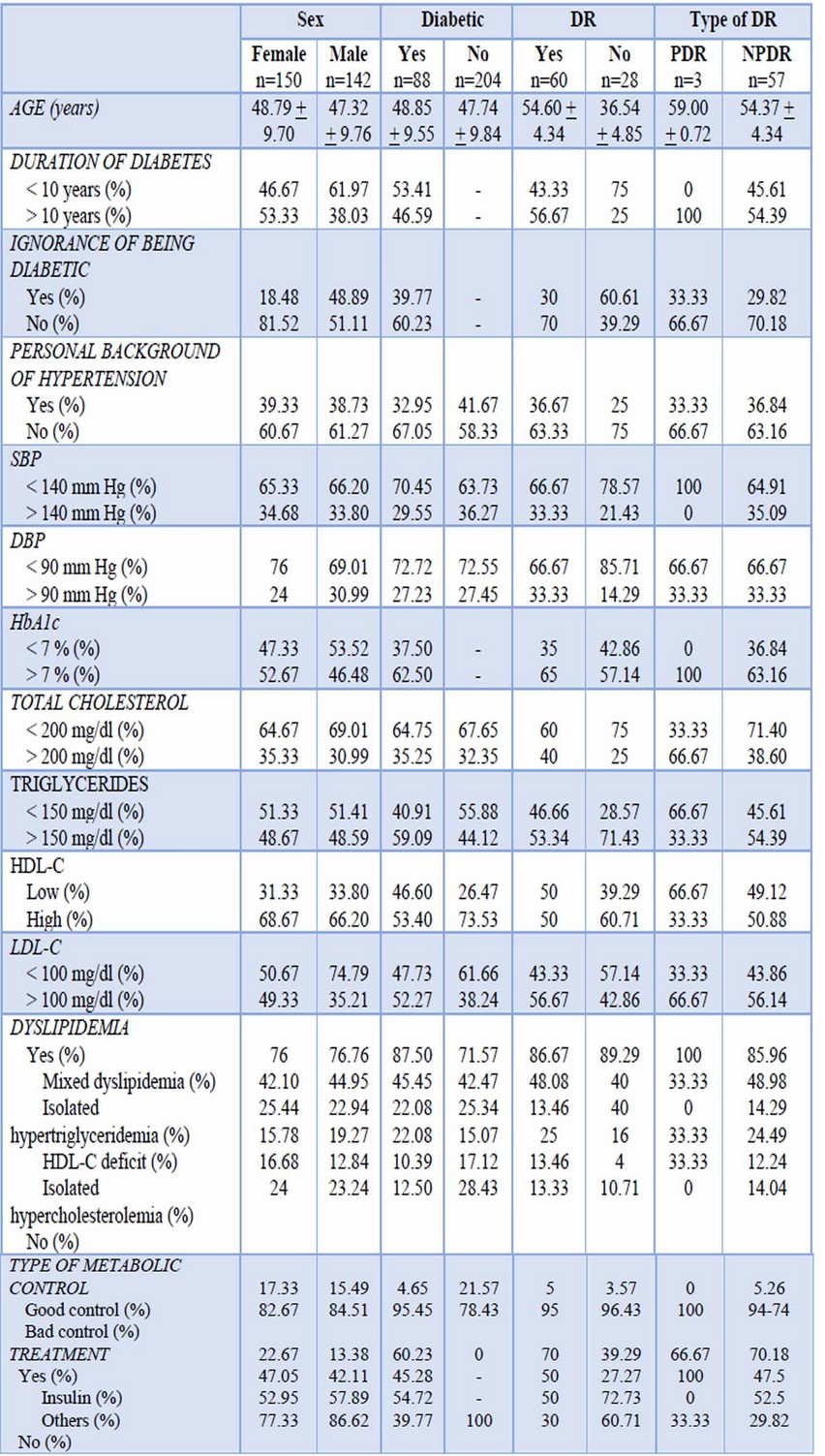
Table 1.- Demographic data and selected clinical and laboratory findings for patients by DR, sex and DM2
The prevalence of DR was 21%. There were a statistically significant association between DR and age [prevalence ratio (95% CI): 8.14 (3.70-17.90), p=0.00], total cholesterol [prevalence ratio (95% CI): 7.43 (0.98-56.17), p=0.01], dyslipidemia [prevalence ratio (95% CI): 3.31 (0.96-11.38), p=0.04], metabolic control [prevalence ratio (95% CI): 4.57 (1.36-15.26), p=0.00], pharmacological treatment [prevalence ratio (95% CI): 46.88 (20.65-106.38), p=0.00] and use of insulin [prevalence ratio (95% CI): 41.10 (11.70-144.49), p=0.00]. Table 2 shows the prevalence ratio, statistical test and significance for all the variables.
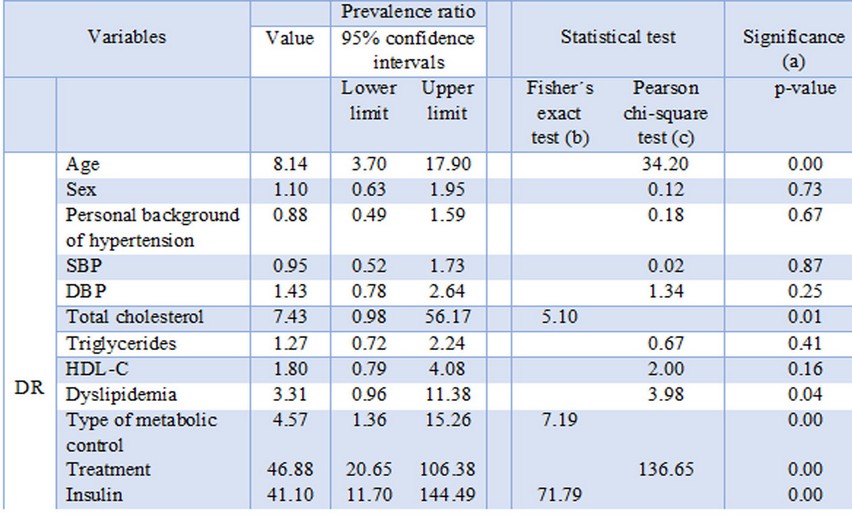
Table 2.- Prevalence ratio, statistical test and significance
(a) p < 0.05: statistically significant
(b) If the sample is too small and there are theoretical values under 5 in at least one box
(c) All boxes have an expected value higher than 5
DISCUSSION
DR is one of the most important causes of visual loss worldwide and is the principal cause of impaired vision in patients between 25 and 74 years old. Visual loss from DR may be secondary to macular edema (retinal thickening and edema involving the macula), hemorrhage from new vessels, retinal detachment, or neovascular glaucoma.
The results of this investigation clearly demonstrate that age, dyslipidemia, metabolic control and pharmacological treatment for DM2 are major risk factors that promote the development of DR.
Based on international literature, people between 50 to 70 years old represent 65% of DR and adults younger than 50 years old just a 10%8; this information has different results in the present investigation due to 51.02% of the sample were adults from 51 to 60 years old, representing the entire group of patients with PDR. Furthermore, 17.57% of patients between 30 to 49 years old, presented abnormal findings in the ocular fundus compatible with NPDR; showing that changes can be observed in younger people, highlighting the need to perform a fundus examination periodically.
Regarding dyslipidemia, there are several investigations that link it with the progression of DR because of its relation with the development of retinal hard exudates and diabetic maculopathy9,10, results that were affirmed in this study. Previous ETDRS analyses found elevated serum lipids are risk factors for concurrent presence and subsequent development of retinal hard exudates, which were associated with decreased visual acuity. The obtained results indicate that elevated lipids, most notably total cholesterol, are also a risk factor for the development of high-risk PDR with a statistically significant association. This finding may provide additional motivation for lowering elevated lipid levels in patients with DR.
According to the treatment, data in this study reveals that the use of insulin increase the progression of DR with a statistically significant association. This controversial topic is supported by some investigations that show that while hyperglycemia was a risk factor for the progression of retinopathy in all patients, change of treatment from oral drugs to insulin was associated with a 100% increased risk of retinopathy progression; on the other hand, insulin therapy is the indicated one for some patients and nowadays one of the alternative methods of delivering insulin that have been studied is insulin eye drops11, 12.
The other investigated variables did not show association with the development of DR, even though there are some important aspects to highlight. Based on DCCT and UKPDS the intensive glycemic control is associated with a minor prevalence of microvascular complications, so levels of HbA1c less than 7% (53 mmol/mol) reduce the DR progression to 54%13. The results in this investigation did not proved an association between these variables, thus to only one value of HbA1c during a year was taken into account. While lower glycosylated hemoglobin levels are associated with a decreased risk of retinopathy development and progression, good glycemic control does not guarantee that retinopathy will not develop or preclude regular screening for DR.
Duration of illness is another factor that is strongly related to the generation of DR14,15. Most investigations prove that 77.8% of DR cases are presented in people with more than 15 years of being diabetic; a duration less than 5 years shows that 24% of them have some suggestible changes for DR16. However, this study describes that 43.33% of diabetic patients showed changes in their ocular fundus in less than 10 years of illness duration, with an association that was not statistically significant. Therefore, when baseline retinopathy was carefully assessed and included in multivariate models, duration of diabetes was usually no longer a risk factor for progression demonstrating that it cannot develop DR by itself. All patients with PDR had 10 years’ duration of DM2, therefore the supreme importance of an adequate treatment and periodic ophthalmological controls.
A half of patients with DR worldwide did not know their diabetic condition, proportional with 58.33% obtained in this study. This data demonstrates that if a person ignores the illness state, exercise and nutritional measures will not be taken into account causing a fast progression of microvascular complications. Therefore, preventive medicine and screening are fundamental for the diagnosis and early treatment to avoid future complications.
Less than a half percent of patients was hypertensive with a normal BP at the moment of the diagnosis of DR. According to the physiopathology the maintained high BP by itself can produce vascular damage causing the progression of DR17,18. The available evidence supports a beneficial effect of intervention to reduce BP preventing DR for up to 4 to 5 years19, but unfortunately the association in this study was not relevant.
Present findings support the view that DR is developed by risk factors such as age, total cholesterol, dyslipidemia, type of metabolic control, treatment and use of insulin. All of them should be paramount factors from the first appointment in order to make early detection, timely treatment and if necessary refer to the specialist.
These findings also support the suggestion that the risk for retinopathy progression may be reduced by lowering elevated serum lipids.
Based on this research, the main motivation for further studies is to determine new risk factors that could promote the development of DR and, above all, be able to apply this knowledge in diabetic patients in order to prevent this microvascular complication.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
1. WHO. Global report on diabetes. 1st Ed. France: 2016, p 1-87.
2. American Diabetes Association. 2015. Statistics about Diabetes. Downloaded on November 7th 2016. (http://www.diabetes.org/diabetes-basics/statistics/?referrer=https://www.google.com.ec/?referrer=http://www.diabetes.org/diabetes-basics/statistics/).
3. INEC. 2013. Anuario de estadísticas vitales: nacimientos y defunciones 2013. Downloaded on November 8th 2016. (http://www.ecuadorencifras.gob.ec/documentos/web-inec/Poblacion_y_Demografia/Nacimientos_Defunciones/Publicaciones/Anuario_Nacimientos_y_Defunciones_2013.pdf).
4. Chew E, Klein M, Ferris F, Remaley N, Murphy R. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol 1996; 114(9):1079-84.
5. Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BEK. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008; 115(11):1859-68. DOI: 10.1016/j.ophtha.2008.08.023.
6. UKPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317(7160):703-13. DOI: 10.1136/bmj.317.7160.703
7. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329:977-86. DOI: 10.1056/NEJM199309303291401
8. Dehghan MH, Katibeh M, Ahmadieh H, Nourinia R, Yaseri M. Prevalence and risk factors for diabetic retinopathy in the 40 to 80-year-old population in Yazd, Iran. J Diabetes 2015; 7(1):139-41. DOI: 10.1111/1753-0407.12205
9. European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, De Backer G, et al., ESC Committee for Practice Guidelines (CPG) 2008-2010 and 2010-2012 Committees. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011; 32(14):1769-1818. DOI: 10.1093/eurheartj/ehr158
10. Liu L, Yue S, Wu J, et al. Prevalence and risk factors of retinopathy in patients with or without metabolic syndrome: a population-based study in Shenyang. BMJ open 2015; 5(12). DOI: 10.1136/bmjopen-2015-008855
11. Zhao C, Wang W, Xu D, Li H, Li M, Wang F. Insulin and risk of diabetic retinopathy in patients with type 2 diabetes mellitus: data from a meta-analysis of seven cohort studies. Diagnostic Pathology 2014; 9(2): 130. DOI: 10.1186/1746-1596-9-130.
12. Vieira-Potter V, Karamichos D, Lee D. Ocular Complications of Diabetes and Therapeutic Approaches. Biomed Res Int 2016; 20(16): 3. DOI: 10.1155/2016/3801570
13. Yau JW, Rogers SL, Kawasaki R, et al., Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35(3):556-64. DOI: 10.2337/dc11-1909
14. Hammes HP, Welp R, Kempe HP, Wagner C, Siegel E, Holl RW, DPV Initiative-German BMBF Competence Network Diabetes Mellitus. Risk factors for retinopathy and DME in type 2 diabetes-results from the German/Austrian DPV Database. PLoS One 2015; 10(7). DOI: 10.1371/journal.pone.0132492
15.Ahmed RA, Khalil SN, Al-Qahtani MA. Diabetic retinopathy and the associated risk factors in diabetes type 2 patients in Abha, Saudi Arabia. J Family Community Med 2016; 23(1):18-24. DOI: 10.4103/2230-8229.172225
16. Gao L, Xin Z, Yuan MX, Cao X, Feng JP, Shi J, et al. High prevalence of diabetic retinopathy in diabetic patient’s concomitant with metabolic syndrome. PLoS One 2016; 11(1). DOI: 10.1371/journal.pone.0145293
17. Arguedas JA, Leiva V, Wright JM. Blood pressure targets for hypertension in people with diabetes mellitus. Cochrane Database Syst Rev 2013; 10. DOI: 10.1002/14651858.CD008277.pub2
18. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eight Joint National Committee (JNC 8). JAMA 2014; 311(5):507-20. DOI: 10.1001/jama.2013.284427.
19. Iglesias R, Barutell L, Artola S, Serrano R. Resumen de las recomendaciones de la American Diabetes Association (ADA) 2014 para la práctica clínica en el manejo de la diabetes mellitus. Diabetes Práctica 2014; 5(2):124.
Recibido: 8 agost 2017
Aprobado: 1 novembre 2017
Karen Sofía Flores-Mena1,*, Kory Naima Jara-Tamayo2,*, Paúl Herrera-González3, Enrique Gea-Izquierdo4
* Both authors contributed equally to this study.
1 Sangolquí Hospital, Sangolquí, Ecuador.
2 Julio Andrade Health Center, Tulcán-Carchi, Ecuador.
3 San Francisco de Quito Hospital, Quito, Ecuador.
4 Pontifical Catholic University of Ecuador, Research Unit & Faculty of Medicine, Quito, Ecuador.
Correspondence: PhD Enrique Gea-Izquierdo, Faculty of Medicine, Avenida 12 de octubre 1076, Vicente Ramón Roca, Quito, Ecuador. Email: [email protected]