2020.05.02.3
Files > Volume 5 > Vol 5 No 2 2020
INVESTIGATION / RESEARCH
Development of an ecological strategy for the control of downy mildew (Pseudoperonospora cubensis) in cucumber cultivation (Cucumis sativus L.)
Julio Gabriel-Ortega1*, Edwin Pereira-Murillo2, Fernando Ayón-Villao3, Carlos Castro-Piguave4, Isaías Delvalle-García5, José A. Castillo6*
ABSTRACT
Downy mildew is a severe disease of cucumber worldwide. The oomycete Pseudoperonospora cubensis cause it and once it is established in a region, the infection spreads rapidly, causing significant loss of yield and fruit quality. The objective of the research was to develop an ecological strategy for the control of downy mildew in cucumber. The treatments were arranged in a completely randomized experimental design with an alternation of chemical and biological fungicides. The treatments were: T1: systemic fungicide (Ridomil Gold, 2.5 g/l) alternating with a contact fungicide (Bravo 2.5 ml/l), T2: CustomBio 5 (Bacillus-based fungicide, 3ml/l), T3: control (water), T4: Trichoderma sp. (3 ml/l), T5: systemic fungicide (Ridomil Gold 2.5g/l) alternating with CustomBio 5 (3 ml/l), and T6: systemic fungicide (Ridomil Gold 2.5g/l) alternating with Trichoderma sp. (3 ml/l). The following variables were evaluated: stem thickness, plant height, number and weight of fruits, yield, the area under the relative progress curve (AUDPCr), and economic analysis of each treatment. The results showed that the best treatments were T1 and T6, with an AUDPCr of 11.89% and 12.10%, respectively. Treatments T6 and T1 showed the best yield, as well. The profitability analysis showed that all the alternatives were profitable with a Benefit/Cost>1 ratio. However, the treatments T6 and T1 were the most useful. We recommend this control strategy to reduce the use of chemical fungicides and, at the same time, obtain an efficient control of the disease, which guarantees a significant yield of high-quality fruit.
Keywords. downy mildew; ecological control; cucumber; AUDPCr, biological fungicide
INTRODUCTION
Cucumber (Cucumis sativus L.) is the fourth most important vegetable in the world1. It was originated in India, where two botanical cultivars arose in the Himalayas, the domesticated (Cucumis sativus var. sativus), and wild cucumber (Cucumis sativus var. hardwickii Royle, Alef)2. Cucumber is a short-cycle crop (3-4 months) that could be grown in the open field, or under greenhouse conditions 3. Although the cucumber can be reproduced by vegetative propagation, it has substantial genetic diversity because it also spreads sexually 4. Current cultivars show a remarkable variety of agricultural features, including the color, shape, rib, diameter, spines, and the brightness of the fruit and the overall resistance to stress. Some cucumber cultivars are well adapted to specific environments because of genetic selection 5. In Ecuador, this vegetable is cultivated in the warm valleys of the Andean mountains and the dry tropics of the coastline corresponding to 87.20% and 12.80% of total land devoted to this vegetable, respectively 6. The Ecuadorian production reaches an annual average of 3,045 tons, from an area of 364 hectares with an average yearly growth of 1.03% 6.
Various diseases affect cucumber. Among the most critical conditions is downy mildew caused by the oomycete Pseudoperonospora cubensis, which is one of the most severe threats to cucumber production worldwide and in Ecuador. It is spread in temperate and semiarid regions, and the pathogen infects plants of all ages, although the disease is more severe at early stages. Usually, the disease infects foliage, causing a reduction in early photosynthetic activity, which affects plant development, delaying growth, and reducing yield. The inflicted chlorotic lesions on the leaf surface could progress until they become necrotic, engaging the entire leave, which may die within days after the infection 7. The disease cause premature defoliation and then the sunburn of the fruit due to overexposure to direct sunlight. In the United States, host resistance in past decades successfully controlled the downy mildew; however, the pathogen has overcome this resistance and now has become a very severe threat to cucumber growers, mainly because it is also evolving fungicide resistance 8. In Ecuador, the disease severely affects cucumber production damaging more than 60% of the output 7, 9.
The application of chemical pesticides is the most commonly used control method, either through using contact and systemic fungicides or the combination of both 10. However, the new trends are directed towards the integrated pest management (IPM) 11, which includes the application of alternative products such as resistance inductors (i.e., Trichoderma atroviride)12,13 or beneficial microorganisms such as Bacillus subtilis, Pseudomonas fluorescens, Derxia gummosa and Trichoderma harzianum 12,14. These microorganisms show the potential to interact with local microflora, and usually, their products are biodegradable in situ to non-toxic compounds by environmental organisms. The search for new and varied products of natural origin for disease management programs represents an essential alternative in sustainable agriculture15. Therefore, the objective of this work is to develop an ecological strategy for the control of downy mildew (Ps. cubensis) in cucumber farming.
There is a need to develop strategies for the control of Ps. cubensis in an ecological and environmentally friendly way, thereby safeguarding people's health, and contributing to reducing production costs due to the lower application of pesticides. Here, we present preliminary data on the use of a combination of chemical fungicides and microorganisms suitable for control of Ps. cubensis in cucumber production under greenhouse conditions16.
MATERIALS AND METHODS
Study site. This investigation was carried out from June to November 2017 in the ‘Puerto La Boca’ community in Manabi province, Ecuador (1°18'20''S, 80°45'42" W, 53 m altitude). Its climate has of 24.8°C and 298 mm of average temperature and annual rainfall, respectively, corresponding to dry tropical forest ecosystems.
Treatments, growth conditions and research management. Sexual seeds of the hybrid Humocaro were pre-germinated in blotting paper moistened with distilled water. The seeds were placed in a closed environment with white light, RH > 80% and a temperature of 20°C±2. For the transplanting of the seedlings, plastic trays with 128 alveoli (15 x 20 10 cm/alveolus) were used. The seedbed was prepared with topsoil (60%), Biocompost (30%), and Earthworm Humus (10%). The pre-germinated seeds were transplanted and then sprayed to field capacity. Nine days after the seedlings were put on plastic trays, they were transplanted to soil beds (36 x 1.20 x 0.60 m), containing topsoil supplemented with Biocompost in a 3:1 ratio. The transplant was performed to holes 0.15 m deep, at a distance of 1.2 m and 0.20 m between rows and plants, respectively, totaling 12 rows. Each experimental unit had an area of 26 m2 with 55 plants/units. The total area of the experiment was hosted in a 500 m2 greenhouse with a photoperiod of 12h light:12h dark, an average temperature of 22°C, and RH>60%. The chemical fungicides and biological organisms that we tested in various treatments are described in Table 1. The organic fungicide CustomBio 5 contains five species of the genus Bacillus (B. subtilis. B. laterosporus. B. licheniformus. B. megaterium y B. pumilus at 8x108 CFU/ml) and is produced by Ecuadorian company Naturalite S.A., Guayaquil, Ecuador.
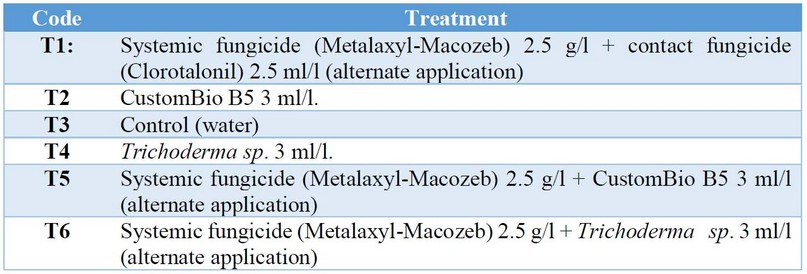
Table 1. Treatments analyzed in this work.
The treatments followed a completely randomized design (DCA) 17, and the experimental units of each treatment consisted of 330 plants, totaling about 1980 plants. Twenty-five random plants were chosen from each experimental group in each treatment for evaluation.
Plants were fertilized with Biocompost Organic Fertilizer (Organicgreen S.A., Ecuador) (50 g/hole), and EcoFungi (EcoMicrobials, Florida, USA) a mix of mycorrhizae (20 g/20 l of water) and Starlite bioregulator (Organicgreen S.A., Ecuador) (50 ml/20 l of water) applied to the ground at the first day of transplanting and along the crop cycle totaling six fertilizations.
Nine harvests were made at intervals of four to five days for each one. This began when the cucumber fruits presented the right color and size. The second, third, fourth, fifth, sixth, and seventh applications of the treatments were performed at 27, 34, 41, 48, 55, 62, and 68 days after the transplant, respectively. In total, seven crops were made throughout the crop cycle.
Response Variables. The area under the relative disease progress curve (AUDPCr) was calculated using five affected leaves of the upper middle third of the plant and a -100% damage scale 18.
Stem diameter (SD, in mm) was measured with a Vernier caliper at 30 cm from the ground when the crop had 50% flowering. Fruit weight (FW, in g) was measured by weighting fruits in each harvest. The number of fruits (NF) was measured by counting all fruits of each harvest. The number of not-set fruits (NNSF) was assessed, by counting the number of dead fruits in each treatment.
Plant height (PH, in cm) was measured when the plant had 50% of the flowering.
Profit/cost analysis was estimated using the methodology suggested by CIMMYT19 that analyzes all the benefits and costs of the different treatments.
Statistical analysis. We performed an analysis of variance to test hypotheses about the fixed effects of treatments and the comparisons of means to determine the response of the best treatment using SAS University software, Cary, NC. For this, we contrasted ways using one degree of freedom and α=0.01. Likewise, the analysis of variance allowed estimating the variance components for random effects. The comparison of means was performed using the multiple Tukey tests at α = 0.05, and the association between the variables studied were analyzed using Pearson's correlation test 17.
RESULTS
Before the analysis of variance, analysis of normality and homogeneity of variances of the variables and the treatments were made. This analysis showed that the variables AUDPCr, NF, FW, and NNSF were not normally distributed, so the data was transformed to square root to normalize it. For AUDPCr, severity data were arcsine transformed 17.
The analysis of variance of the following variables, AUDPCr, SD, NF, and FW, showed that the coefficients of variation (CV) are within the ranges allowed for this type of research (CV of 7.61 to 35.43%) (Table 2). Highly significant differences were observed at the probability of p <0.01 for all the variables evaluated. This result indicates that at least one of the treatments was different from the others (Table 2). The analysis of variance of the variables NNSF and PH showed that the CV, are within the ranges allowed for this type of investigation (CV from 8.08 to 29.44 %) (Table 2). Highly significant differences were observed at the probability of p <0.01 only for PH. This indicated that at least one of the treatments is different in PH. In contrast, there were no significant differences in NNSF (Table 2).
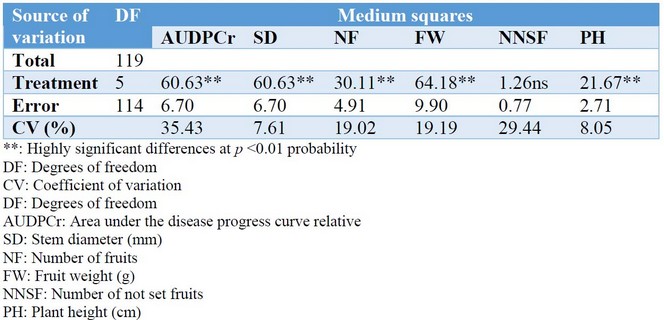
Table 2. Analysis of variance for AUDPCr, SD, NF, FW, NNSF and PH in cucumber plants, Puerto La Boca, Ecuador, 2017.
The analysis of means performed by the Tukey test at p <0.05 probability for the variables AUDPCr, SD, NF, FW, NNSF, and PH, showed significant differences in all cases except for NNSF, where all treatments are statistically equal (Table 3).
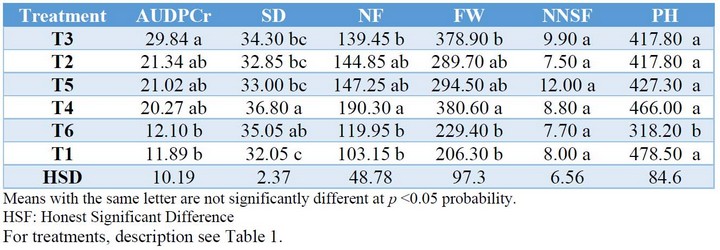
Table 3. Analysis of means for AUDPCr, SD, NF, FW, NNSF and PH, Puerto la Boca, Ecuador, 2017.
The best disease reduction was achieved with treatments T1 and T6 relative to T3 (control), observing AUDPCr of 11.89%, 12.10%, and 29.84 %, respectively. It is worthy to note that other secondary diseases caused by different fungi were also controlled. Regarding the SD, results indicate that the T4 treatment showed a higher diameter (36.80 mm) compared to the T1 treatment (32.01 mm), being significantly different at a p<0.05 probability. The T3 and T6 treatments were statistically the same instead;, treatments T4, T2, T5, and T6 were not statistically different from each other. About NF, the T4 treatment was significantly different from the T3, T6, and T1 treatments at p <0.05 probability, but not substantially different from the T2 and T5 treatments. The T3 treatment was not statistically different from the T2, T5, T6, and T1 treatments for this variable. Regarding the FW, the T4 treatment was significantly different from the T3, T6, and T1 treatments at p <0.05 probability. The treatments T2, T5, and T4 were not significantly different from each other, nor treatments T3, T2, T5, T6, and T1. Concerning PH, it was found (Table 4) that treatments T3, T2, T5, T4, and T1 were not significantly different from each other, but were significantly different from the T6 treatment. There were no significant differences for NNSF for any of the treatments. The Pearson's correlation analysis (Table 4) showed a high and significant negative correlation for AUDPCr concerning to NF (-0.94) and FW (-0.94).
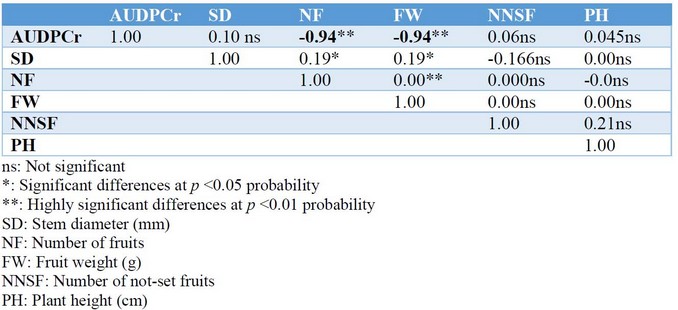
Table 4. Correlation analysis
The profitability analysis (Table 5) showed that, in general, all the alternatives were profitable with a benefit to cost ratio (B/C> 1). However, the treatments T6 (Systemic fungicide + Trichoderma sp) and T1 (Systemic fungicide + contact fungicide) were distinguished, as the most profitable with USD 1606 and USD 1600 respectively.
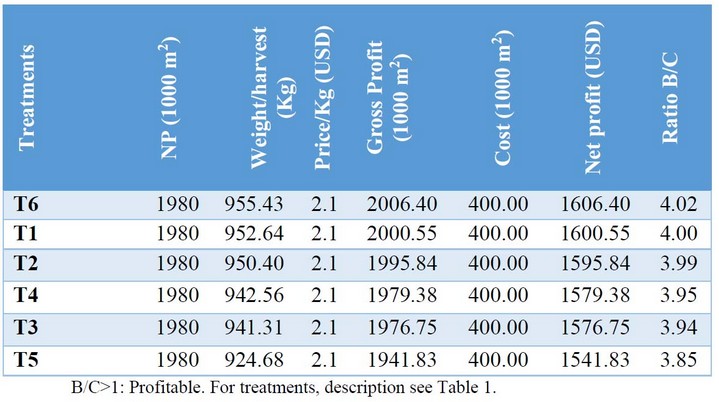
Table 5. The profitability of treatments for the control of downy mildew in cucumber, Puerto La Boca, Ecuador, 2017.
DISCUSSION
The downy mildew of cucumber is traditionally controlled with chemical fungicides; however, chemical control is not always feasible because of the high costs associated with the application especially for poor farmers of South American countries and the high environmental liabilities that the use of chemical fungicides means. In this work, we developed an ecological control strategy for Ps. cubensis based on the alternation of systemic and contact fungicides and the replacement of the contact fungicide by a microorganisms-based one. This strategy allowed satisfactorily controlling the pathogen and reaching a high production of fruits. Likewise, it helped to reduce production costs due to the lower application of pesticides. Although we present preliminary results, we undoubtedly think this is a promising strategy to control fungal pathogens in cucumber that can be adopted in IPM programs because of their affability with the environment and lost cost20,21. We also determined that all control alternatives evaluated here were profitable with a benefit/cost ratio greater than 1 (B/C> 1). Still, particularly the treatments alternating systemic fungicide with Trichoderma, as well as systemic with contact fungicide, were the most profitable. This suggests that applying an ecological strategy with microorganisms is useful and beneficial.
The contact fungicides that we used (Chlorothalonil), acted in the first hours after the application. Still, once oomycete penetrates the plant, systemic fungicides (Metalaxyl) should be used to control fungal infection because these are mobilized in the internal tissues and organs of the plant after their application 22,23. In our trials, the alternate form of Bacillus-based fungicide (CustomBio 5) with chemical counterparts allowed reducing the use of pesticides at least 50% of the applications. The mode of action may rely on the production of antibiotic molecules because it is well known that the Bacillus species contained in CustomBio 5 are efficient producers of antibiotic molecules 24,25 and antifungal volatiles 26. Likewise, Trichoderma sp. worked properly when it was applied alone or in alternate applications with the systemic and contact fungicide27. Adnan et al.27 mention that the species of the genus Trichoderma are the most commonly used antagonists for the control of plant diseases caused by fungi, due to their rapid growth in a large number of substrates, ample abiotic stress tolerance, quick ability to colonize, easy establishment after inoculation and sufficient capacity to compete for space and nutrients with pathogens. Besides, Trichoderma can stimulate plant growth due to its ability to produce plant growth regulators, vitamins, and recycle nutrients such as P (phytate) and Zn in the soil 28. In our experiments, the systemic fungicide did not harm Trichoderma since the applications were alternate, letting at least one week pass between applications. Additionally, some Trichoderma strains show resistance or tolerance to fungicides 29,30, although this particular property was not tested in the Trichoderma strains used in this work.
CONCLUSION
Ps. cubensis is a significant fungal disease for cucumber farming in Ecuador and worldwide, causing the disease called downy mildew. Traditionally, the application of fungicides has helped to hinder the spreading of the disease; however, chemical control is not always feasible because of the high costs associated with the implementation of fungicides and the implied environmental damage. In this work, we developed an ecological control strategy for Ps. cubensis based on the alternation of systemic and contact fungicides and the replacement of the contact fungicide by a microorganisms-based one. This strategy allowed satisfactorily controlling the pathogen and reaching a high production of fruits. Likewise, it helped to reduce production costs due to the lower application of pesticides. Undoubtedly, this strategy constitutes an excellent alternative to control pathogens in cucumber, and it can be adopted in IPM programs because of their affability with the environment and lost cost
Acknowledge
The authors are grateful for the financing support and facilities provided by the State University of Southern Manabí (UNESUM) for the realization of this research that is part of the project entitled "Development of technological alternatives for the sustainable production of high-quality vegetables under greenhouse conditions" (Grant PROG-003-PROY-001-DIP-2017 to JGO). We also thank the farmers of Puerto La Boca and all the students involved in this research.
Funding information
This study was funded by the State University of Southern Manabí (UNESUM), Ecuador (grant PROG-003-PROY-001-DIP-2017 to JGO).
Conflict of interest
All authors declare that they have no conflict of interest.
REFERENCES
1. Lv, J., Qi, J., Shi, Q., Shen, D., Zhang, S., Shao, G., Li, H., Sun, Z., Weng, Y., Shang, Y., Gu, X., Li, X., Zhu, X., Zhang, J., van Treuren, R., van Doijeweert, W., Zhang, Z. and Huang, S. Genetic diversity and population structure of cucumber (Cucumis sativus L.). PLoS ONE. 2012. 7:e46919. https://doi.org/10.1371/journal.pone.0046919
2. Sebastian, P., Schaefer, H., Telford, I.R.H. and Renner, S.S. Cucumber (Cucumis sativus) and melon (C. melo) have numerous wild relatives in Asia and Australia, and the sister species of melon is from Australia. Proc Natl Acad Sci USA. 2010. 107:14269–73. https://doi.org/10.1073/pnas.1005338107
3. Gruda, N., Sallaku, G. and Balliu, A. Cucumber. In: Baudoin, W., Nersisyan, A., Shamilov, A., Hodder, A., Gutierrez, D., de Pascale, S., Nicola, S., Gruda, N., Urban, L., Tanny Good, J. (eds) Agricultural Practices for greenhouse vegetable production in the South East European countries - Principles for sustainable intensification of smallholder farms. FAO Plant Production and Protection Paper 230. 2017. pp 287-300. ISBN 978-92-5-109622-2
4. Innark, P., Khanobdee, C., Samipak, S. and Jantasuriyarat, C. Evaluation of genetic diversity in cucumber (Cucumis sativus L.) germplasm using agro-economic traits and microsatellite markers. Sci Hortic. 2013. 162:278–84. https://doi.org/10.1016/j.scienta.2013.08.029
5. Naegele, R.P. and Wehner, T.C. Genetic Resources of Cucumber. In: Grumet R, Katzir N, Garcia-Mas J, (eds). Genetics and genomics of Cucurbitaceae. Springer International Publishing. 2016. pp. 61–86
6. Brussil, C.S. Proyecto de factibilidad para la exportación de pepino a los Países Bajos. Thesis. Universidad Tecnológica Equinoccial, Ecuador. 2012.
7. Savory, E.A., Granke, L.L., Quesada-Ocampo, L.M., Varbanova, M., Hausbeck, M.K. and Day, B. The cucurbit downy mildew pathogen Pseudoperonospora cubensis: Cucurbit downy mildew. Mol Plant Pathol. 2011. 12:217–26. https://doi.org/1,0.1111/j.1364-3703.2010.00670.x
8. Burkhardt, A. and Day, B. A genomics perspective on cucurbit-oomycete interactions. Plant Biotech. 2013. 30:265–71. https://doi.org/10.5511/plantbiotechnology.13.0315a
9. Lebeda, A., Pavelková, J., Urban, J.and Sedláková, B. Distribution, host range and disease severity of Pseudoperonospora cubensis on cucurbits in the Czech Republic. J Phytopathol. 2011. 159:589–596. doi: 10.1111/j.1439-0434.2011.01811.x
10. Khare, C.P., Srivastava, J.N., Tiwari, P.K., Kotesthane, A. and Thrimurthi, V.S. Downy Mildew of Cucurbits and Their Management. In: Awasthi LP. (ed) Recent Advances in the Diagnosis and Management of Plant Diseases. Springer, New Delhi. 2015. pp 47-54. https://doi.org/10.1007/978-81-322-2571-3_5
11. Haveri, N., Thulasiram, K. and Shashidhar, K.R. Integrated approach for management of downy mildew of cucumber caused by Pseudoperonospora cubensis. J Pharmacogn Phytochem. 2019. 8:510-513.
12. Scherf, A., Schuster, C., Marx, P., Gärber, U., Konstantinidou-Doltsinis, S. and Schmitt, A. Control of downy mildew (Pseudoperonospora cubensis) of greenhouse grown cucumbers with alternative biological agents. Commun Agric Appl Biol Sci. 2010. 75:541-54.
13. Szczech, M., Nawrocka, J., Felczyński, K., Małolepsza, U., Sobolewski, U., Kowalska, B., Maciorowski, B., Jas, K. and Kancelista, A. Trichoderma atroviride TRS25 isolate reduces downy mildew and induces systemic defence responses in cucumber in field conditions. Sci Hortic. 2017. 224:17–26. https://doi.org/10.1016/j.scienta.2017.05.035
14. Elsharkawy, M.M., Kamel, S.M., Nagwa, M., El-Khateeb, M. Biological control of powdery and downy mildews of cucumber under greenhouse conditions. Egypt J Biol Pest Co. 2014. 24:407-414.
15. Brzozowski, L. and Mazourek, M.A. Sustainable agricultural future relies on the transition to organic agroecological pest management. Sustainability. 2018. 10:2023. https://doi.org/10.3390/su10062023
16. Cohen, Y. and Coffey, M.D. Systemic fungicides and the control of oomycetes. Ann. Rev. Phytopathol. 1986, 24:311-338. https://doi.org/10.1146/annurev.py.24.090186.001523
17. Gabriel, J., Castro, C., Valverde, A and Indacochea, B. Diseños experimentales: Teoría y práctica para experimentos agropecuarios. Grupo COMPAS, Universidad Estatal del Sur de Manabí (UNESUM), Jipijapa, Ecuador. 2017. 116 p. ISBN 978-9942-750-50-1. http://142.93.18.15:8080/jspui/handle/123456789/116
18. Gabriel, J., Ortuño, N., Vera, M., Castro, C., Narváez, W. and Manobanda, M. Manual para evaluación de daños de enfermedades en cultivos agrícolas. Grupo COMPAS, Universidad Estatal del Sur de Manabí, Jipijapa, Ecuador. 2017b. 53 p. ISBN 978-9942-760-04-3. https://www.researchgate.net/publication/317356316_Manual_para_evaluacion_de_danos_de_enfermedades_en_cultivos_agricolas
19. CIMMYT. La formulación de recomendaciones a partir de datos agronómicos: Un manual metodológico de evaluación económica. México D.F., México: CIMMYT. 1988. ISBN 968-6127-24-0
20. Colucci, S.J. and Holmes, G.J. Downy mildew of cucurbits. The Plant Health Instructor. 2010. https://doi.org/10.1094/PHI-I-2010-0825-01
21. Sharma, A., Katoch, V. and Rana, C. Important Diseases of Cucurbitaceous Crops and Their Management. Important diseases of cucurbitaceous crops and their management. In: Pessarakli M. (ed). Handbook of cucurbits growth, cultural practices, and physiology. CRC press. 2016. pp. 301-324.
22. Fernández-Northcote, E.N., Navia, O. and Gandarillas, A. Bases de las estrategias de control químico del tizón tardío de la papa desarrolladas por PROINPA en Bolivia. Rev Latinoam Papa. 1999. 11:1-25.
23. Ruiz-Sánchez, E., Tún-Suárez, J.M., Pinzón-López, L.L., Valerio-Hernández, G. and Zavala-León, M.J. Evaluación de fungicidas sistémicos para el control del mildiú velloso (Pseudoperonospora cubensis Berk. & Curt.) Rost. en el cultivo del melón (Cucumis melo L.). Rev Chapingo. 2008. 14(1): 79-84.
24. Raaijmakers, J.M., Vlami, M. and de Souza, J.T. Antibiotic production by bacterial biocontrol agents. Anton Leeuw. 2002. 81:537–547. https://doi.org/10.1023/A:1020501420831
25. Stein, T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005. 56:845–57. https://doi.org/10.1111/j.1365-2958.2005.04587.x
26. Kai, M., Haustein, M., Molina, F., Petri, A., Scholz, B. and Piechulla, B. Bacterial volatiles and their action potential. Appl Microbiol Biot. 2009. 81:1001–12. https://doi.org/10.1007/s00253-008-1760-3
27. Adnan, M., Islam, W., Shabbir, A., Khan, K.A., Ghramh, H.A., Huang, Z., Chen, H.Y.H. and Lu, G.D. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb Pathogenesis. 2019. 129:7–18. https://doi.org/10.1016/j.micpath.2019.01.042
28. Li, R.X., Cai, F., Pang, G., Shen, Q.R., Li, R. and Chen, W. Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE. 2015. 10:e0130081. https://doi.org/10.1371/journal.pone.0130081
29. Chaparro, A.P., Carvajal, L.H. and Orduz, S. Fungicide tolerance of Trichoderma asperelloides and T. harzianum strains. Agr Sci. 2011. 2:301–307. https://doi.org/10.4236/as.2011.23040
30. Silva, M.A.F. da, Moura, K.E. de, Moura, K.E. de, Salomão, D. and Patricio, F.R.A. Compatibility of Trichoderma isolates with pesticides used in lettuce crop. Summa Phytopathol. 2018. 44:137–42. https://doi.org/10.1590/0100-5405/176873
Received: 7 March 2020
Accepted: 2 April 2020
Julio Gabriel-Ortega1, Edwin Pereira-Murillo2, Fernando Ayón-Villao3, Carlos Castro-Piguave4, Isaías Delvalle-García5, José A. Castillo6*
1 Ph.D., Professor, Faculty of Natural and Agricultural Sciences, State University of Southern Manabí. Via Noboa s/n, Los Angeles Campus. Jipijapa, Manabi, Ecuador. [email protected] ID ORCID: 0000-0001-9776-9235
2 Agricultural engineer, Independent Agricultural Consultant, Santo Domingo, Ecuador. [email protected] ID ORCID: 0000-0003-1680-4524
3 M.Sc., Professor, Faculty of Natural and Agricultural Sciences, State University of Southern Manabí. Via Noboa s/n, Los Angeles Campus. Jipijapa, Manabi, Ecuador. [email protected] ID ORCID: 0000-0003-4772-9344
4 M.Sc., Professor, Faculty of Natural and Agricultural Sciences, State University of Southern Manabí. Via Noboa s/n, Los Angeles Campus. Jipijapa, Manabi, Ecuador. carlos. [email protected] ID ORCID: 0000-0003-3180-2359
5 M.Sc., Representative, Organicgreen Co., Jipijapa, Ecuador. [email protected] ID ORCID: 0000-0002-4248-8134
6 Ph.D., Professor, School of Biological Sciences and Engineering, Yachay Tech University, Urcuqui, Ecuador.
* Corresponding author, [email protected]
ORCID: 0000-0001-8941-9040
