2020.05.04.11
Files > Volume 5 > Vol 5 No 4 2020
INVESTIGATION / RESEARCH
Antimicrobial Activity of Herbal Mixture Extract Combination on Microorganisms Isolated from Urinary Tract infection
Risala H Allami1, Raghad S. Mouhamad2*, Sura A. Abdulateef3 , Khlood abedalelah al-Khafaji4
Available from: http://dx.doi.org/10.21931/RB/2020.05.04.11
ABSTRACT
Urinary tract infection (UTI) is the second most common infection after respiratory tract infection. Its prevalence is more in women as compared to men. Approximately 50% of women have an infection of the Urinary tract in their life-time. The bacterial infection is one of the most important bioactivity; using their ability to imitate evanish then distributes international fitness problems into the 21st centenary. Thus a recent study was undertaken to investigate the antibacterial activity of a mixture of three medicinal plants against UTI infectious isolates. The three considered plants were (Aloe vera, Artemisia herba alba and Teucrium polium), which were used in Iraqi medicine for many centuries. The effectiveness of this combination was investigated using in vitro well diffusion method. The extract was tested against four isolated pathogenic bacteria (Staphylococcus aureus, Klebsiella spp, and Proteus spp). The aqueous extract exhibited antibacterial activity against gram-positive and gram-negative bacteria. The mixture extract had the highest effect against S. aureus and Proteus spp, followed by a lower effect on Klebsiella spp. In conclusion, the antibacterial effect of the tested plant extracts confirmed a higher impact on Gram-positive bacteria as compared to Gram-negative bacteria. Therefore, it can be concluded that the usage of these plants as a traditional medicine form can be considered as a strong assistant to regular medicine drugs and treatments.
Keywords: Urinary tract infection (UTI), Antimicrobial Activity, Herbal Mixture Extract,
INTRODUCTION
The herbal remedy is a developing sector of health care and demands attention1. Plants served as a valuable source of traditional treatment over the years2. World Health Organization (WHO) estimated that around 80% of the worldwide population remember at least one traditional remedy by extracting active components3. This, due to medicinal flora, bears various advantages (a low price or much fewer facet effects) compared to modern conventional drugs, as are expensive yet acknowledged in hazardous side effects4.
The prevalence of Urinary tract infections (UTI) by bacteria is problematic worldwide and among all age range and gender. Several pathogens belong to gram-positive and gram-negative bacteria regard as a fundamental everyday health hazards5to cause UTI; the most pathogens for UTI infection include strains of uropathogenic Escherichia coli, Klebsiella pneumonia, Enterococcus spp, Staphylococcus saprophyticus, St. aureus, group B Streptococcus, proteus mirabilis 6-10 Among all bacterial species E. coli is known to be the most common in complicated and uncomplicated UTI especially in diabetic patients 6.
Treatment of UTI subordinate the severity of infection; it can be ranging from a single-dose antibiotic treatment to rescue nephrectomy for pyonephrosis in diabetic patients with septic shock11. Third-generation beta-lactam antibiotics such as ciprofloxacin, cefixime, and ceftriaxone are commonly used in UTI. Indiscriminate antibiotic use resulted in the development of resistance to one or multiple antibiotics that give a severe challenge upon disease treatment or even treatment failure12beside other adverse effects on the liver and bone marrow13,14. The tremendous use of antimicrobial has induced resistances among various bacterial kinds and, as much be counted concerning fact1, the efficacy of these compounds is remarkably decreased. A long time put in appearances concerning antibiotic stopping pathogens has been a global problem in the latest. According to inquire, the undesirable facet consequences about half of antibiotics instituted us because of latter sources in conformity will combat these problems15. Literature cited that many strains of E. coli and K. pneumoniae isolated from UTI have extended-spectrum to Beta-lactam antibiotics, carbapenem-resistant, and polymyxin; moreover, the resist could be transferred to other infectious bacteria through horizontal gene transfer systems as transformation, transduction, and conjugation16.
This necessitates relies on a safe and low-cost medicinal plant having antibacterial activities with a promising future. More than half a million plants worldwide have medical issues essential to treat or prevent many infections17. A variety of secondary metabolite produces in plant tissues with therapeutic values with less toxicity and side effects and could be a good substitute for traditional synthetic or semisynthetic chemical antibiotics and overcome multidrug-resistant bacteria18. Since several plant antimicrobial contains different functional groups, their antibacterial activity attributed to multiple mechanisms. Therefore, the prospect of developing resistance to plant constitutes is relatively smaller19. The antibiotic resistance phenomenon exhibited by the pathogenic microorganisms were not reported in medicinal flora because of their strong antimicrobial activity20. Phytochemical compounds and small secondary metabolites have a significant value for medicinal plants. The most important of these bioactive constituents are alkaloids, tannins, flavonoids, and phenol, all of which are accoutered for new antibacterial agents21,22. It is believed that crude extracts from some medicinal plants are more biologically active than isolated compounds due to their synergistic effects23.
The researchers are showing interest in natural products with bactericidal activity24- 26. Humans use plant extracts for a wide variety of diseases, of dense developing countries; it depends on traditional medical practitioners or their collections regarding medicinal vegetation in conformity with treatment to them27. Herbals execute apply for an important position in conserving biodiversity. These plants are genuinely familiar to bucolic human beings anybody according to their scarcity yet their disappearance28. Indeed, medicinal plants lead the very necessary health ponderous position and then symbolize a giant source concerning profits for many families within the countryside and cities29.
Urinary tract infection is the second most common infection after respiratory tract infection worldwide. Its prevalence is more in women than men30; approximately 50% of women have an infection of the Urinary tract in their life. The current s study focuses on discovering antibacterial outcomes regarding aqueous extracts of a natural combination of about 3 medicinal plants (Aloe vera, Artemisia herba alba, and Teucrium polium ) against UTI causing bacteria.
MATERIAL AND METHODS
Isolation and identification of bacteria from UTI.
Twenty out samples of urine were collected from patients with suspicious clinical symptoms like dysuria, loin pain, fever, frequent urination, and need to urinate with an empty bladder7; patients were visited Ibn Al-Nafees Hospital/ Baghdad and asked about taken antibiotic prior visiting the hospital during last seven days. The ethical committee approved the study of the University.
All urine samples were cultured over blood agar, MacConkey agar, and mannitol salt agar. Bacterial characters were identified using Gram stain, urease test, oxidase, catalase, hemolysis of RBCs, and Indole Methyl red Vokes Proskauer (IMVC)31.
Aqueous extraction of Plant Material
This research was conducted from January to February 2020. Plants of (Aloe vera, an Herbal (Sheh), and Teucrium polium (Algeada) toughness were obtained from the local Iraqi market and were identified at the College of Agriculture Engineering of Baghdad University - Iraq. The plants were kept at room temperature (20-25ºC) till usage. Equal weights of each force in (Aloe vera, Artemisia herba alba (sheh), and Teucrium polium (Algeada) were ground then mixed. The aqueous extract regarding that combination was prepared along with the useful resource; 2 g regarding mixture, including 200 ml of sterilized distilled water for 15 minutes, left overnight in a refrigerator. Meanwhile, the extract panel was filtered and refrigerated in a glass container as described 32 with modification.
Screening of the active compound
Many tests were applied to screen the different active compounds in the aqueous extract of plant mixture; these tests include the detection of each flavonoid, tannins, glycosides, saponin, and terpin and steroid. The procedures were briefly described below:
Flavonoids were detected in cold aqueous extract; the detecting solution composed of an equal volume of 50% ethanol and 1M KOH. About 5 ml of extract was mixed with 5 ml of detecting solution at 25C, and the color was changed to the yellow indicating presence of flavonoid33.
The system below34 was used to detect tannins; in its procedure, 50 ml of each ban was back in conformity withstand equally broken on couple conical flasks. For the first one, administration lead acetate (CH3COOPb) (1%; w/v) was once introduced to plant extract afterward; the jelly pellet was used following keep viewed a direct reaction. The second flask, ferric chloride (FeCl2) (1%; w/v) was used for screening tannins. Navy-blue shade was involved in the emergence concerning tannins.
Glycosides: These techniques were once taken according to the method described by35; non hydrolyzed extract: Equal aggregation concerning the bury recover was once as soon as blended with the Fehling test in the test tube; development of purple precipitate shows a quality result for glycosides.
Hydrolyzed extract: Few drops of HCl were added to 5ml of the aqueous extract of the plant since they were left at a water bath of 20 minutes, the acidity was once neutralized using NaOH solution, amount of aggregation respecting the Fehling test was added. The improvement concerning crimson precipitate suggests a positive result.Saponins: This method was made under conformity with35. Couple methods detected saponins:
The first method was applied by shaking the tube containing extract; the formation of foams standing indicates a first-rate result.
While the second method was done by adding five ml of plant extract over 1-3 ml of 3% ferric chloride solution, positive results were pointed out upon bright precipitate formation33.
Terpenes and steroids: few drops of chloroform were drop wised to 1 ml of the, then a decline over acetic anhydride and a decline on sulfuric acid; brown precipitate appeared that represents the availability of terpenes. While dark blue color appeared after about five minutes, it suggests the availability of steroids33.
Antibacterial activity of aqueous herbal extracts
Agar well diffusion approach on Mueller -Hinton agar was once used in imitation of the search for antibacterial activity36. Bacterial cultures were crashed out from the nutrient agar plate and were suspended in sterilized peptone water. Turbidity was evaluated and compared with McFarland standard tube number 1, which equivalents approximately to 1X108 CFU/mL. The cotton swab was immersed in bacterial suspension and spread over Muller Hinton agar, which let for 10 minutes to ensure bacterial adherence. Meanwhile, the borer applicator was sterilized by flame, cooled, and pressed on the top of seeded Muller Hinton agar to make well with a 6 mm radius, let distance about 15 mm between wells the aspect of the plate. Each well was filled with 25, 50, 75, and 100 µl, plates were stand for 10 minutes and were incubated for 24 h at 37 °C. Three replicas of each plate were prepared, and the diameter of the inhibition zone was recorded from the edge of the well.
RESULTS AND DISCUSSION
Isolation and identification of bacteria
The recent result of isolation and identification regarding bacterial isolates from patients with UTI symptoms showed the prevalence of Klebsiella spp, Proteus, and S. aureus; the characters of each isolate listed in the table (1). Although this was a preliminary study with a small number of UTI patients, the study showed that G-ve bacterial species were more prevalent than G+ve bacteria. Many other researches referred to that different bacterial species might encounter with the UTI inpatients, and some of these bacteria develop antibiotic-resistant to one or more of the traditional and extended-spectrum of beta-lactam antibiotic37 Mohamed H. Mourad and one of the most important reason is the recurrent uses of antibiotic even without a physician prescription. This following 38 found that 54.8% of isolates were gram-negative, while 45.2 % were gram-positive.
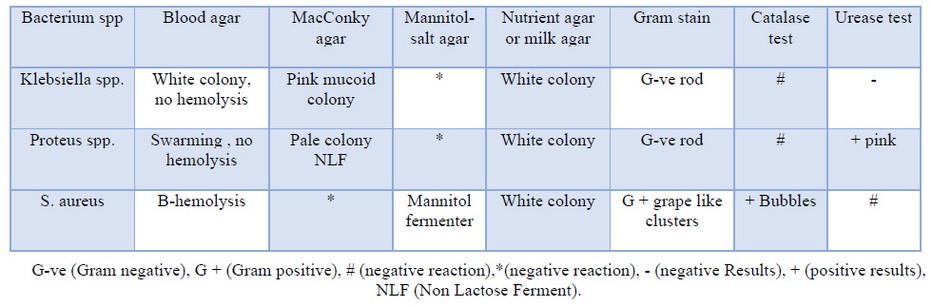
Table 1. the morphological and characteristics of bacteria on different cultural media with some biochemical test
Chemical analysis of the aqueous extract of plant mixture under study showed that a weight of 1.58g representing 5.95%, could achieve from 25g regarding the extracted plant material. Screening of bioactive components revealed that the extract contains some flavonoids, phenols, tannins, saponins, glycosides, and terpenes have been detected. Aqueous extracts were low in steroids (Table 2). A. herba-alba is a prosperous source of flavonoids certain as like hispidulin as well as cirsilineol. Flavonoids isolated out of some medicinal vegetation have been established in conformity with possessing anti-inflammatory effect39.
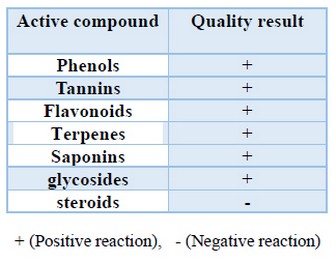
Table 2. Chemical analysis of active compounds in aqueous extract of a plant mixture.
Antibacterial activity
Each extract was tested against bacterial isolates (S. aureus, Klebsilla.spp, and Proteus.spp). The extracts' different concentrations illustrated increased effectiveness against the studied microorganisms represented by the increased inhibition zones in figure (1).
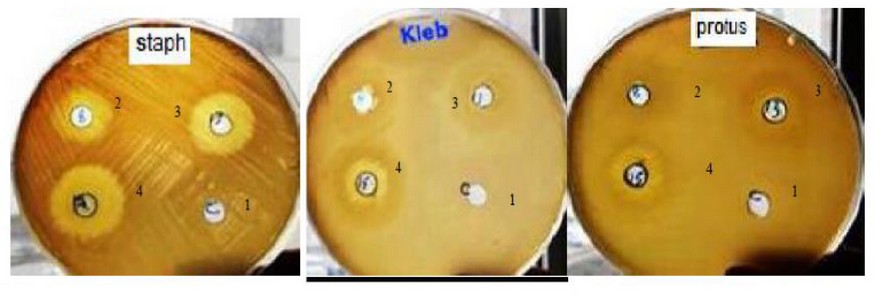
Figure 1. Bacterial inhibition zone with different concentration of aqueous plants extract (1= 25µl, 2= 50 µl, 3=75 µl, 4=100 µl): Muller Hinton agar
The measurement of the average diameter of inhibition zone indicated that highest bacterial inhibition reached with higher concentration of plant mixture extract for all bacterial isolates under study. Also, study found that Staph. aureus was more susceptible than other bacterial species followed by Klebsiella spp. (figure2).
An increase rate of re-emerging infections, has carried an inquire for instant and more safe natural antimicrobial compounds; however, these compound need more investigation for their effecting mechanism. Plants are valuable supply of pharmaceutical substances, due to the fact they have an almost limitless potential according to synthesize compounds including one of a kind antimicrobial endeavor against more than a few pathogenic as well as opportunistic microorganisms40.
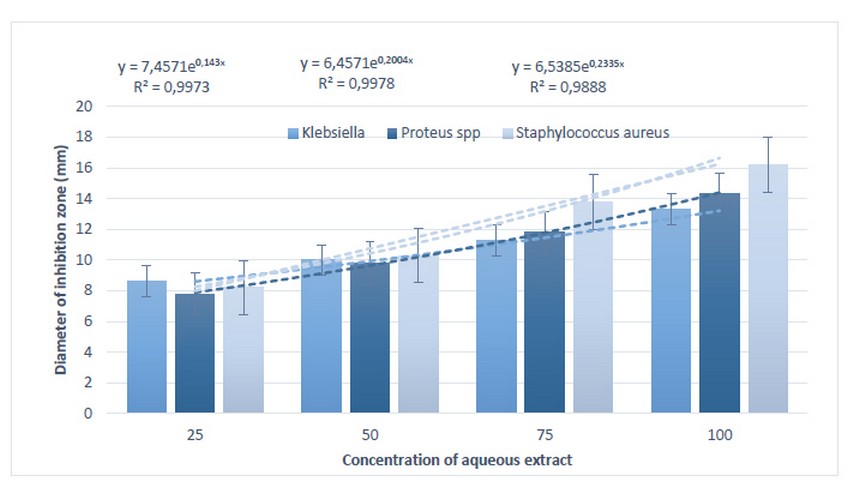
Figure 2. the differences in bacterial susceptibility in response to aqueous plant mixture extract
The essential factors up to expectation to antibacterial reactivity are the type of diffusion strategies of active substances used and the tendency to inter bacterial cell, pH, and temperature of the surroundings41. The pH may also result in the antibacterial inhibitory effect of R. sativus had an excellent antibacterial effect at acidic pH, which has declined by increasing pH toward alkaline. This might be because antibacterial compounds in cationic forms can also engage the negatively charged bacterial cells42.
Successful extraction concerning bioactive compounds beside row material depends on the solvent back into the extraction procedure. The recent study's main success is using aqueous extract for three plant material that contained different active compounds with a synergistic effect in one preparation, a phenolic compound known to alter microbial cell permeability, leading to change damage the macromolecules inside the bacterial cell. Many enzymes responsible for either the bacterial reproduction and growth or virulence factor might lose their activity in response to phenolic compounds43,44,45. The saponin activity against monocrystalline sugar; reduces sugar within the bacteria reducing power intake to the bacterial cell, leading to growth decline and death46. The presence of tannins and flavonoids caused a toxic effect and an inhibition of different types of enzymes and transporter proteins found in the cell membrane and inside cell 47,48.
Many researchers observed that the extraction of the plant active ingredients and the organic solvents methanol, ethyl acetate, and chloroform resulted in a more substantial antibacterial effect, but aqueous extraction, cheaper and need less requirements to prepare 26=32. Gram-negative microorganisms are among the resistant organisms against chemotherapeutic antibiotics than gram-positive bacteria; a survey concerning currently observed antibacterial activity of herbal takes place, showing that >90% of herbal extracts lacked activity against E. coli, in compassion to gram-positive bacteria49.
The mechanisms of bacterial resistance against antibiotic might be equipped through changing membrane permeability; drug molecules in accordance with a mobilephase can keep transferred through membrane via porins, diffusion through the bilayer then through self-uptake. The porin channels are located among OM (outer membrane) about Gram-negative bacteria. The little hydrophilic molecules (β-lactams then quinolones) can pass the OM only through porins. The minimize among quantity over porin channels leads to decreased access regarding β-lactam antibiotics between the cells, subsequently resistance50. G+ve microorganisms like Staphylococcus might harbor transmissible genetic element encoding for antibiotic resistance such as plasmids encoding the penicillinase genes, namely in collecting negative bacteria; conjugation is an essential mechanism regarding drug transfer and effect occur into supreme genera50. The main barrier for Gram-positive bacteria towards antibiotics is thick peptidoglycan that protects against osmotic rupture. The simple subunit over the peptidoglycan thing is a disaccharide monomer regarding N -acetylglucosamine (NAG) and N -acetylmuramic (NAM) pentapeptide. The pentapeptide consists of amino water brash residues alternating into L- and D-stereoisomers, then terminating within D-alanyl-D-alanine. A stem peptide regarding moving measure then contract is given to the third amino water brash over it pentapeptide. Pentapeptides are then same, including stem peptides, according to form a cross-link between polysaccharide chains. This reaction is catalyzed by a transpeptidase. This transpeptidation response is sensitive to prohibition via β-lactams. The penicillin-sensitive reactions are catalyzed by using a family of closely associated proteins, penicillin-binding proteins (PBPs). β-Lactam antibiotics out turn their lethal impact regarding bacteria through inactivation concerning more than one PBPs simultaneously, then for that reason inhibiting cell wall synthesis. The inhibition of PBPs additionally leads to imitation of breakdown on an ideal match, probably at the time over cell division. This agitated morphogenesis is hypothesized after provoking cell dying51,52.
CONCLUSIONS
Different active compounds were detected in the aqueous mixed extract of the 3 medicinal vegetation (Aloe vera, Artemisia herba alba and Teucrium polium) permanency life, including phenols and flavonoids tannins, saponins, glycosides afterward terpenes. Aqueous extrusion concerning an herbal aggregate upon 3 medicinal vegetation (Aloe vera, Artemisia herba alba and Teucrium polium ) undergo antibacterial effect against positive but village Gram negative microorganism. Our consequences aid that the aqueous extract regarding this three plant combination well-known shows the synergistic effect as antibacterial activity compared to previous studies that examined each plant alone.
REFERENCES
1. Ekor M. (2014). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in pharmacology, 4: 177. https://doi.org/10.3389/fphar.2013.00177
2. Center for Substance Abuse Treatment. Substance Abuse Treatment for Persons with HIV/AIDS. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2000. (Treatment Improvement Protocol (TIP) Series, No. 37.) Chapter 8—Ethical Issues. Available from: https://www.ncbi.nlm.nih.gov/books/NBK64933/
3. World Health Organization (WHO). 1992. Our planet, our health. Report of WHO Commission on Health and Environment. Geneva: World Health Organization.
4. http://www.ciesin.org/docs/001-012/001-012.html
5. Sung, B., Chung, H. S., Kim, M., Kang, Y. J., Kim, D. H., Hwang, S. Y., Kim, M. J., Kim, C. M., Chung, H. Y. and Kim, N. D. (2015). Cytotoxic effects of solvent-extracted active components of Salvia miltiorrhiza Bunge on human cancer cell lines. Experimental and Therapeutic Medicine, 9(4): 1421–1428. https://doi.org/10.3892/etm.2015.2252
6. Flores-Mireles A. L., Walker J. N., Caparon M., and Hultgren S. J.( 2015). Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nature Reviews Microbiology, 13(5): 269–284.
7. Foxman B. ( 2014). Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infectious Disease Clinics of North America, 28(1): 1–13.
8. Kline K. A., Schwartz D. J., Lewis W. G., Hultgren S. J. and Lewis A. L.( 2011). Immune activation and suppression by group B Streptococcus in a murine model of urinary tract infection. Infection and Immunity, 79(9): 3588–3595.
9. Nielubowicz G. R. and Mobley H. L.(2019). Host-pathogen interactions in urinary tract infection. Nature Reviews Urology, 7(8): 430.
10. Ronald A. (2003). Etiology of urinary tract infection: traditional and emerging pathogens. American Journal of Medicine, 113(1): 14–19.
11. Kang C. I., Chung D. R., Son J. S., Ko K. S,. Peck K. R. and Song J. H. (2011): Clinical significance of nosocomial acquisition in urinary tract-related bacteremia caused by gram-negative bacilli. Amer. J. Infection Control, 39(2): 135-140.
12. Foxman B.[2003], Epidemiology of urinary tract infections: incidence, morbidity and economic costs. Dis Mon.; 49:53-70.
13. Nicki R.Colledge et al. (2010). Davidson'S Principles and Practice of Medicine 21sted. Churchill Livingstone Elsevier publication; p-471-472
14. Vincent C., Boerlin P., Daignault D., Dozois C. M., Dutil L., Galanakis C., Reid-Smith R.J., Tellier P. P., Tellis P. A., Ziebell K. and Manges A. R. (2010). Food reservoir for Escherichia coli causing urinary tract infections. Emerg. Infect Dis. 16: 88-95.
15. Weam B., Abraham M., Doiphode S., Peters K., Ibrahim E., Sultan A., and Mohammed H. O. (2016). Foodborne Bacterial Pathogens Associated with the Risk of Gastroenteritis in the State of Qatar. International Journal of Health Sciences, 10(2), 197–207.
16. Liu Y.-Y., Wang Y., Walsh T. R. et al., (2016). Emergence of plasmid mediated colistin resistance mechanism mcr-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infectious Diseases, 16( 2): 161–168.
17. Hassan B. and Abdul R.(2012). Medicinal Plants (Importance and Uses)., Pharmaceut Anal Acta, 3: 1 .
18. Rath S., Dubey D., Sahu M.C., Debata N.K. and Padhy R.N. (2012). Antibacterial activity of 25 medicinal plants used by aborigines of India against six uropathogens with surveillance of multidrug resistance. Asian Pacif. J. Trop. Biomed. 2: S846-S854.
19. Tepe B., Daferera D., Sokmen N., Polissiou M. and Sokmen A. (2004). Invitro antimicrobial and antioxidant activities of the essential oils and various extracts of Thymus eigii Journal of Agricultural and Food Chemistry, 52: 1132-1137.
20. Shihata I.M. A pharmacological study of Anagallis arvensis. MD. Vet, Thesis, Cairo University (cited by Nehia Hussein, Noor A. Hanon).
21. Abubakar M.C., Ukwuani A.N. and Shehu RA (2008). Phytochemical screening and Antibacterial activity of Tamarindus indica pulp extract. Asian J. Biochem. 3(2):134-138.
22. Kalimuthu K., Vijayakumar S. and Senthilkumar R. (2010). Antimicrobial Activity of the Biodiesel Plant, Jatropha curcas L. International Journal of Pharma and Biosciences, 1(3):1-5.
23. Soković M., Glamočlija J., Marin P. D., Brkić D. D., van Griensven L. J. (2010). Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules, 15 (11): 7532-7546.
24. Voon H. C., Bhat R., and Rusul G. (2012). Flower extracts and their essential oils as potential antimicrobial agents for food uses and pharmaceutical applications. Comprehensive Reviews in Food Science and Food Safety, 11( 1): 34–55, 2012.
25. Kalwij J. M. (2012). Review of the Plant List, a working list of all plant species. Journal of Vegetation Science, 23( 5): 998–1002.
26. Trinh P-C. , Thao T. Le-T., Ha H.-T.-Viet, and Nguyen T. (2020). DPPH-Scavenging and antimicrobial Activities of Asteraceae Medicinal Plants on Uropathogenic Bacteria Evidence-Based Complementary and Alternative Medicine, 2020: 1-9. ID 7807026, https://doi.org/10.1155/2020/7807026.
27. Chen S. L., Yu H., Luo H. M., Wu Q., Li C. F., and Steinmetz A. (2016). Conservation and sustainable use of medicinal plants: problems, progress, and prospects. Chinese Medicine, 11:37. https://doi.org/10.1186/s13020-016-0108-7
28. Sofowora A., Ogunbodede E., and Onayade A. (2013). The role and place of medicinal plants in the strategies for disease prevention. African journal of traditional, complementary, and alternative medicines : AJTCAM, 10(5): 210–229. https://doi.org/10.4314/ajtcam.v10i5.2
29. Scheunemann L. P., and White D. B. (2011). The ethics and reality of rationing in medicine. Chest, 140(6):1625–1632. https://doi.org/10.1378/chest.11-0622.
30. Najar M. S., Saldanha C. L., and Banday K. A. (2009). Approach to urinary tract infections. Indian Journal of Nephrology, 19(4): 129–139. https://doi.org/10.4103/0971-4065.59333
31. MacFaddin J. F.(2000). Biochemical tests for identification of medical bacteria, Williams and Wilkins. Philadelphia, PA, page 113.
32. Al- Khafaji K. A., Hammood S. A., Omran S. G., Hassan M.A., Yaseen H. J., Abood H., Ali S. G. (2014). Biological activity and protease inhibitor from watery extract of lentil (Lens culinaris) against some bacterial species. J. Baghdad Science, 2:781-788.
33. Harbone J. B. (Editor) (1984). Phytochemical Methods, 2nd Edition. Chapman and Hall, U.K., pp. 37-99
34. Lucera A., Costa C., Conte A. and Del Nobile M. A. (2012). Food applications of natural antimicrobial compounds. Frontiers in Microbiology, 3:287. https://doi. org/10.3389/fmicb. 2012. 00287
35. Barréro A., Herrador M. M., Arteaga P., Quitz J., Aksira M., Mellouki F. and Akkad S. (2005). Chemical composition of essential oils of leaves and wood of Tetraclinis articulata (Vahl) Masters. J. Essent. Oil Res. 17: 166-167.
36. Shaheen A. Y., Sheikh A. A., Rabbani M., Aslam A., Bibi T., Liaqat F., Muhammad J. and Rehmani S. F. (2015). Antibacterial activity of herbal extracts against multi-drug resistant Escherichia coli recovered from retail chicken meat. Pak. J. Pharm. Sci. 28 (4) :1295-1300.
37. Mourad M. H., Salih S. A-R. , Elaasser M. M., Safwat N. A. and Ibrahim M. Y..(2016). Antibacterial activity of certain medicinal plant and their essential oils on the isolated bacteria from UTI patients. Int. J. Adv. Res. 4(12): 1510-1530.
38. Ahmed M. E, Al-lami M. Q., and Abd Ali D. M. (2020). Evaluation of Antimicrobial Activity of Plants Extract Against Bacterial Pathogens Isolated from Urinary Tract Infection among Males Patients. Al-Anbar Medical Journal DOI: 10.33091/AMJ.0701622020/ http://doi.org/10.33091/AMJ.0701622020
39. Houari M., Ferchichi A. (2009). Essential oil composition of Artemisia herba-alba from southern Tunisia. Molecules, 14: 1585-1594.
40. Moghtader M. (2009). Chemical composition of the essential oil of Teucrium polium L. from Iran. Am Euras. J. Agric. Environ. Sci. 5, 843–846.
41. Nikaido H. and Vaara M. (1985). Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 49:1–32.
42. C 1995. GC-MS analysis of Artemisia herba-alba Asso essential oils from Algeria. Dev Food Sci 37: 147-205.
43. Bajpai V.K., Rahman A., Dung N.T., Huh M.K. and Kang S.C. (2008). In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. Journal of Food Science, 73:M314.
44. La Storia A., Ercolini D., Marinello F., di Pasqua R., Villani F. and Mauriello G. (2011). Atomic force microscopy analysis shows surface structure changes in carvacrol-treated bacterial cells. Res. Microbiol. 162: 164-172.
45. Tako M., Kerekes E. B.,Zambrano C., Kotogan A., Pap T., Krisch J.and Vagvolgyi C.(2020). Plant phenolic and phenolic enriched extract as antimicrobial agents against food- contaminating microorganisms. Antioxidants, 9: 165-186. Doi:10.3390/antiox9020165.
46. Saba T.H., Isam SH.H. and Manal AH (2013). Identification of quantitative chemical compounds of ethanolic extracts of Q.infectoria infectoria and studies its inhibitory effect in some bacteria. Indian Journal of Research, 2: 125-128.
47. Cowan M.M. (1999). Plant products as antimicrobial agent. Clin. Microbiol Review, 12(4): 564-582.
48. Ramanathan R., Tan C. and Das N. (1992).Cytotoxic effect of plant polyphenols and fat soluble vitamins on malignant human cultured cells. Cancer letters, 62: 217-224.
49. Ple' siat P. and Nikaido H. 91992). Outer membranes of gram-negative bacteria are permeable to steroid probes. Mol Microbiol. 6:1323–1333.
50. Rhodes P.L., Mitchell J.W., Wilson M.W. and Melton L.D. (2006). Antilisterial activity of grape juice and grape extracts derived from Vitis vinifera variety Ribier. Int J Food Microbiol. 107:281–286. doi: 10.1016/j.ijfoodmicro.2005.10.022.
51. Beevi S. S., Mangamoori L. N. and Anabrolu N. (2009). Comparative activity against pathogenic bacteria of the root, stem, and leaf of Raphanus sativus grown in India. World J. Microbiol. Biotechnol. 25:465–473.DOI 10.1007/s11274-008-9911-3.
52. Nejatzadel- Barandozi F. (2013). Antibacterial activities and antioxidant capacity of Aloe vera. NejatzadehBarandozi Organic and Medicinal Chemistry Letters, 3:, 5.
Received: 8 september 2020
Accepted: 10 october 2020
Risala H Allami1, Raghad S. Mouhamad2*, Sura A. Abdulateef3 , Khlood abedalelah al-Khafaji4
1 College of Biotechnology, Al-Nahrain University, Baghdad, Iraq,[email protected],
https://orcid.org/0000-0002-3000-4614
2 Ministry of Science and Technology, Baghdad, Iraq, [email protected]
https://orcid.org/0000-0003-3335-254X
3 Dept. of Applied Science, Division of Biotech. Univ. of Tech.-Iraq
https://orcid.org/0000-0003-2481-911X
Risala H. Allami
4 College of Biotechnology, Al-Nahrain University, Baghdad, Iraq,
https://orcid.org/0000-0003-3659-8641
