2023.08.01.23
Files > Volume 8 > Vol 8 No 1 2023
Analysis of the Genetic Distance of Several Generations of Barley (Hordeum valulgare L) by RAPD-PCR Technique
1Department of Biology, Faculty of Science, University of Mosul
*Corresponding author. [email protected]
http://dx.doi.org/10.21931/RB/2023.08.01.23
ABSTRACT
Random-amplified-polymorphic-DNA(RAPD) was assayed to detect the genetic variation of 6 barley generations from Iraq. Four primers generated a total of 17 scoreable bands in RAPD analysis) and resolving power, the three polymorphic primers differed (Rp). The use of RAPD marker systems to detect the genetic distance among barley generation was discovered to be beneficial. The RAPD dendrograms indicate a diverse grouping of 6 barely specimens, although we did see that certain groups were identical in several cases. As a result, the RAPD molecular markers reveal two genetic groups in the few specimens examined.
Keywords. Barley, Genetic variation, RAPD-PCR.
INTRODUCTION
The Barley plant (Hordeum vulgare L.) is the most important crop in the world, and it has been the focus of extensive genetic research. It is considered a diploid plant (2n) species with much DNA that is mostly self-fertilizing1.
In Iraq, barley is grown on around 450.000 hectares. Early domestication and local expertise have resulted in varied local barley that is mainly utilized for feed. Poorly in terms of food2.
Sheep owners mainly plant barley in semiarid locations, and it is harvested once or twice as an early winter crop when food and pasture are scarce. Plant genetic resources must be conserved and used and needed for the long-term upkeep and growth of production of agricultural products3,4.
The preservation of breeders' interests and the documentation of genetic resources have grown increasingly dependent on identifying agricultural plant types. The malting and brewing sectors, respectively, Are critical since various kinds of barley plants have vastly varied characteristics & applications5,6.
It requires farmers and gardeners to be careful in choosing the seeds of the varieties they want to plant. They must be pure seeds that are not genetically treated and not subject to any additions in order not to affect their genetic sequence.5,8. Various biochemical methods have been employed to supplement the phenotypic analysis of barley plant cultivation, most of which depend on differences in iso-enzymes and protein storage in the seed seeds. Nonetheless, because of the weak levels of allelic variation for multi-iso-enzymatic loci, the great rank of heredity connection in the middle of the various assortment, and the great extent of variation inside barley plant varieties, characterization with these indicators was not particularly effective for barley varieties6,7,9.
Molecular and genetic markers have been shown to be effective methods for identifying and assessing genetic variation indoors and across types and societies. Distinct characteristics have been shown to display, unlike types of variation4,10. It's linked to the proportion of the DNA surveyed from every kind of genetic indicator, their portioning across the chromosomes, and the size of the purpose target examined by each technique11.
The adaptation of several molecular methods like RAPD, SSR, STS, RAMP and ISSR was made by the essential technique called the polymerase-chain-reaction-(PCR)12,13.
In barley, these molecular markers were utilized to identify genetic distance, detection of genotype and chromosome mapping. The RAPD technique has numerous benefits over other methods, including ease of application, affordable expense, and the utilization of a small quantity of plant matter. Self-pollinating species with little intraspecific diversity, like hexaploid wheat and cultivated barley, are examples., RAPDs have been shown to be helpful as genetic markers10,14.
Bernard et al. 15 utilized the RAPD technique and discovered a lot of variation between wild barley genotypes. They found that comes from the overall diversity of the genetic predicted, 75 percent of the variation was partitioned within genotypes. In contrast, 25% was found to be partitioned within populations., with no significant variations between nations16.
The ISSR technique shows and detects the genetic sequences of different varieties belonging to certain plants, such as potatoes, through PCR analysis18.
We use RAPD markers to assess the degree and structure of genetic variation and relationships in complex samples produced in Iraq to provide a baseline for the species' future conservation and breeding initiatives. We also want to show how RAPD may be used to analyze genetic diversity and connections among barley specimens3,19,20,21.
MATERIAL AND METHODS
Plant-based material In this study, six barley (Hordeum vulgare L.) specimens from Iraq's "Ebaa Grain Receiving Center" were utilized.
With few adjustments, total DNA was isolated from fresh leaves using the Genaid kit. And DNA concentration and purity measurement by nanodrop and separated by 2% agarose gel electrophoresis.
Extraction of DNA and Analyzed with RAPD-PCR. Operon molecular for life provided ten decamer random primers for RAPD analysis (Table 1). Total DNA was extracted from grains of 6 Barley using generated kit, and the extracted DNA was diluted to 25 ng /mic by nanodrop technique. Primers were diluted to 10 ng /mic, used for PCR amplification, and separated by 2% agarose gel electrophoresis1.
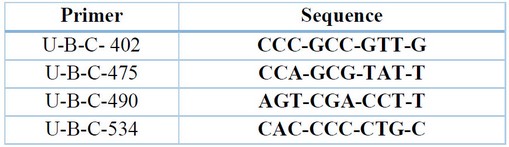
Table 1. sequence of primers used in the RAPD technique15
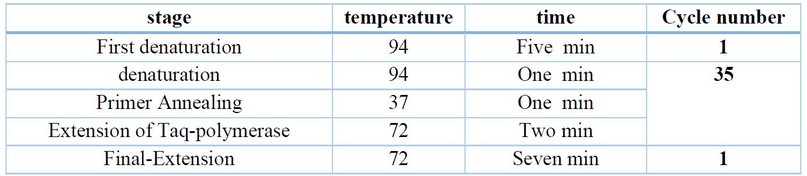
Table 2. The PCR condition used in the RAPD technique
RESULT AND DISCUSSION
Out of many universal primers utilized for early detection with typical genotypes, just four primers replicate polymorphic specimens. Then these 4 primers were used in the RAPD-PCR technique to analyze all 6 barley plant generations. The PCR bands of the 6 generations with these 4 primers give an overall of 15 different bands. The elevated digit of the band (7) was received by UBC-475 primer, while the little number (3) was received by UBC-490 primer. Several denote the identic distance in their aptitude to determine the genetic variation (100 percent). The 4 polymorphic primers visualize the polymorphism concerning the average band in informativeness (AvIb) and Resolving power (Rp).
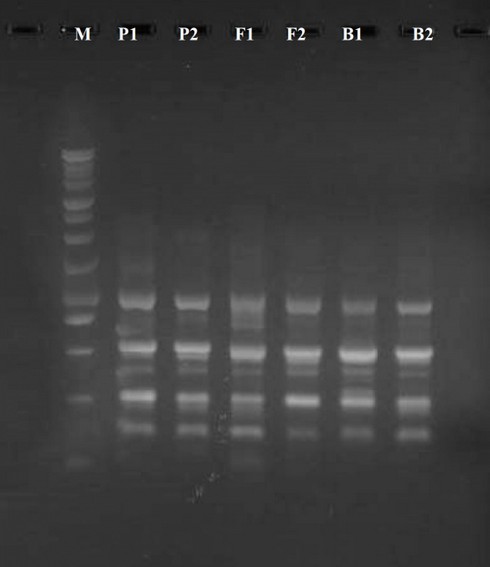
Figure 1. Raped-PCR product for UBC-402 primer. M marker 100 bp
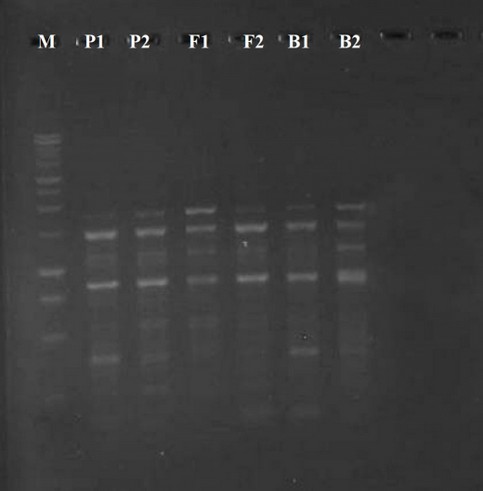
Figure 2. Raped-PCR product for UBC-475 primer. M marker 100 bp
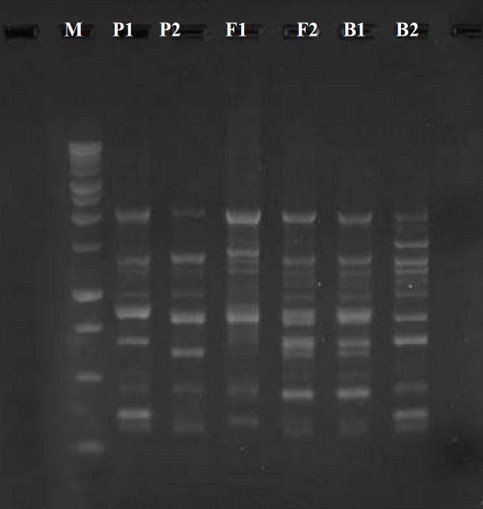
Figure 3. Raped-PCR product for UBC-490 primer. M marker 100 bp
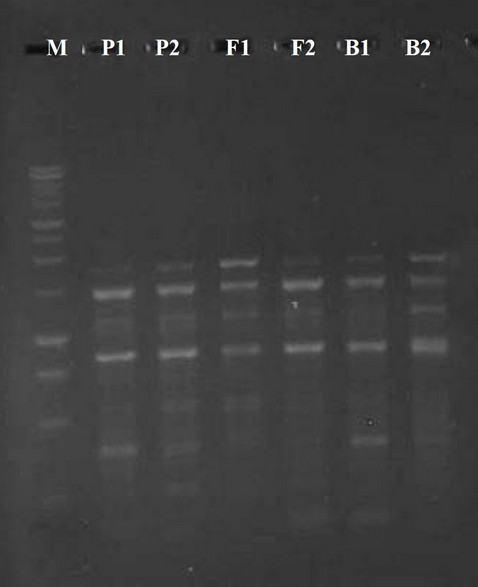
Figure 4. Raped-PCR product for UBC-534 primer. M marker 100 bp
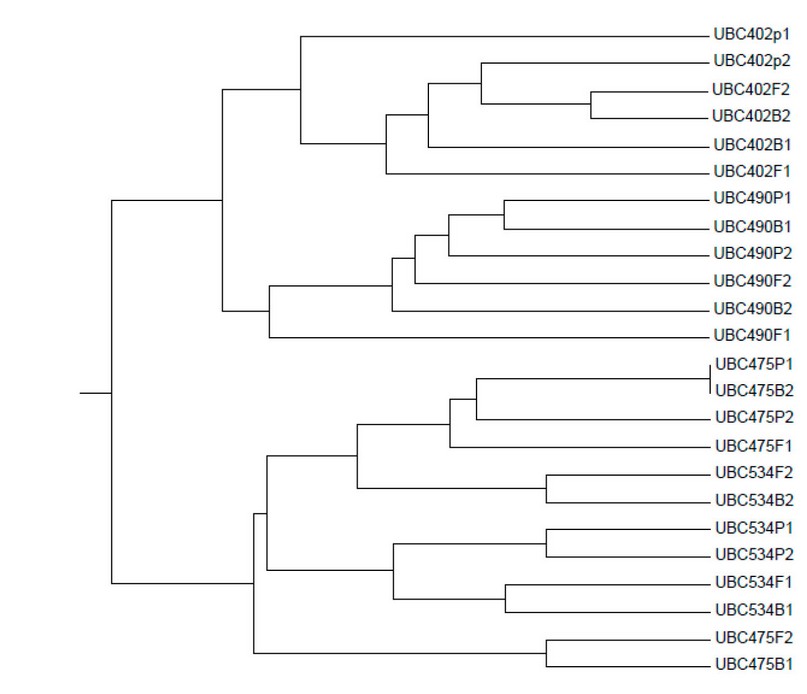
Figure 5. Dendrogram constellates were revealed by six barley plant samples using an RAPD-PCR marker.
RAPD-PCR data acquired from the dendrogram show 23 chief clusters (Figure 5).
Because of its global spread, the genetic variation of barley plant germ cells from divergence homeland has been valuator7,8,12,22,23. Bernard et al.15 used RAPD markers to examine the genetic distance of 6 generations from 121 wild barley plant societies from Iraq, Turkey, and Iran. When the overall genetic distance was calculated, 75 percent of the variance was split between the six genotypes and 25% was divided between the social. Because the majority of the very discovered (97 percent) was to part among the 20 societies and the residue across the nations, there were no significant differences between countries when the variation was evaluated11. Therefore, the few specimens were joined together regardless of their geographic origin. Dendrograms using RAPD-PCR and ISSR techniques do not indicate geographic profiling among barely plant samples in this investigation23.
Only a few clusters had comparable classifications based on the makeup of clusters derived using RAPD and ISSR markers separately. Various metrics like the relation of polymorphism, mean band informativeness, resolving power, and clusters produced in the dendrogram were used to assess the performance of these markers1,7,13,22.
The findings of this study's genetic distance were compared to reports from other RAPD research, such as an assessment of gene distance in the outer covering of a seed of barley plant using phenotypic characteristics and RAPD markers, as well as stored protein analyses 9,14.
Furthermore, it has been observed that the dendrogram created by the RAPD phylogeny meshes best with the barley plant cultivars' genealogy and known pedigree than the dendrogram obtained by the ISSR findings.
On the other hand, data derived from RAPD were shown to be more directly connected to the geographic distribution of the genus Houttuynia Thunb, whilst data derived from the ISSRs technique were found to be intimately related to chromosome number 8-13. It might be accounted for partly by the disparity in the number of informative PCR products. They emphasized the relevance of the real number of locus and their covering of the entire genome once more, as well as obtaining reasonable estimations of genetic relationships among the materials investigated. RAPD-PCR has proven to be the majority polymorphic technique in barley plants, making them extremely useful for various barley applications 19.20. RAPD techniques are a marker used to identify and were also linked to multiple characteristics, including the leaf rust resistance gene Lr 28 in Aegilops speltoides17,22.
As a result, RAPD analysis might be beneficial for selecting ancestors to cross to generate acceptable populations for genome mapping and breeding 20. We reach the following deduction depending on the findings of this study: The results showed that RAPD-PCR analysis might be used to assess genetic diversity among Iraqi barley varieties. The findings of this study, based on genetic diversity, will aid breeders in selecting parents for hybridization programs and determining the kind of variation in barley.
CONCLUSION
Despite the narrow genetic base among the six species used here, RAPD analysis was quite effective in determining the genetic variation among Barley species. It can be used to generate DNA fingerprints for variety identification.
Funding: self-funding
Acknowledgments: In this section, we acknowledge any person who supports us in completing this project.
Conflicts of Interest: there is no conflict
REFERENCES
1. S. Laugesen, K. S. Bak-Jensen, P. Hagglund et al. "Barley ¨ peroxidase isozymes. Expression and post-translational modification in mature seeds as identified by two-dimensional gel electrophoresis and mass spectrometry," International Journal of Mass Spectrometry, 2007, vol. 268, no. 2-3, pp. 244–253.
2. P. C. Canci, L. M. Nduulu, R. Dill-Macky, G. J. Muehlbauer, D. C. Rasmusson, and K. P. Smith. "Genetic relationship between kernel discoloration and grain protein concentration in barley," Crop Science, 2003, vol. 43, no. 5, pp. 1671–1679.
3. X. P. Chen, L. Yan, and Y. Ding. "RAPD analysis and the probable evolutionary route of wild relatives of barley from China," Acta Botanica Sinica, 2000, vol. 42, no. 2, pp. 179–183,.
4. Z. Y. Feng, Y. Z. Zhang, L. L. Zhang, and H. Q. Ling. "Genetic diversity and geographical differentiation of Hordeum vulgare ssp. spontaneum in Tibet using microsatellite markers," High Technology Letters, 2003, vol. 10, pp. 46–53.
5. F. Guasmi, L. Touil, K. Feres, N. Marzougui, W. Elfalleh, and ´ A. Ferchichi. "Variety identification and genetic relationship of some South Tunisian barley accessions using agronomic parameters," Journal of Food, Agriculture and Environment, 2009,vol. 7, no. 2, pp. 522–527.
6. Korol, and E. Nevo. "Microscale ecological stress causes RAPD molecular selection in wild barley, Neve Yaar microsite, Israel," Genetic Resources and Crop Evolution, 2003, vol. 50, no. 2, pp. 213– 224.
7. E.-H. Dakir, M.-L. Ruiz, P. Garc´ıa, and M. Perez de la Vega. "Genetic variability evaluation in a Moroccan collection of barley, Hordeum vulgare L., by means of storage proteins and RAPDS," Genetic Resources and Crop Evolution, 2002, vol. 49, no. 6, pp. 619–631.
8. Y. Q. Yin, D. Q. Ma, and Y. Ding. "Analysis of genetic diversity of hordein in wild close relatives of barley from Tibet," Theoretical and Applied Genetics, 2003,vol. 107, no. 5, pp. 837–842.
9. Zeina S. M. Al-Hadeithi, *Abdul Kareem A. Al-Kazaz, ** Bilal K. Al-Obaidi. Genetic Diversity And Relationships Among Iraqi Barley Cultivars Using RAPD – PCR Technique. The Iraqi Journal of Agricultural Sciences, 2012, 43(6): 117-124.
10. Karim, K.; A. Rawda and C.M. Hatem,. Genetic diversity in barley genetic diversity in local Tunisian barley based on RAPD and SSR analysis. Biological Diversity and Conservation, 2010, Tunisia. ISSN 1308-5301 Print; ISSN 1308-8084 Online.
11. Ciulca, A.; E. Madosa; S. Mihacea, and C. Petolescu. RAPD analysis of genetic variation among some barley cultivars. Romanian Biotechnological Letters, 2010, 15(1):19-24.
12. Mahmood, T.; A.Siddiqua; A.Rasheed, and N.Nazar. Evaluation of genetic diversity in different Pakistani Wheat landraces. Pakistan .Pak. J. Bot., 2011, 43(2): 1233-1239.
13. Eshghi, R. and E. Akhundova. Genetic diversity in hulless barley based on agromorphological traits and RAPD markers and comparison with storage protein analysis. African Journal of Agricultural Research , 2010,5 (1): 97-107.
14. Goswami, S. and V. Tripathi. The use ISSR and RAPD marker for detecting DNA polymorphism, genotype identification and genetic diversity among Trichosanthes dioicaRoxb. Cultivars International Journal of Bio diversityand Conservation. India, 2010,2(12):405-413.
15. R. B. Bernard, E. Nevo, A. J. Douglas, and A. Beiles. "Genetic diversity in wild barley (Hordeum spontaneum C. Koch) in the near east: a molecular analysis using random amplified polymorphic DNA (RAPD) markers," Genetic Resources and Crop Evolution, 1997, vol. 44, no. 2, pp. 147–157.
16. Al- Assei, A. H.A. Analysis of genetic variation of barley cultivated in Iraq using DNA markers. PhD thesis. Biology Department, College of Ibn AlHaithm Education –University of Baghdad, 2002,p:111.
17. Süleyman CENKCİ*, Nevra DOĞAN. Random amplified polymorphic DNA as a method to screen metal-tolerant barley (Hordeum vulgare L.) genotypes. Turk J Bot ,2015, 39: 747-756.
18. s Agarwal M, Shrivastava N, Padh H. Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep, 2008, 27: 617–631.
19. Amabile RF, Faleiro FG, Capettini F, Júnior WQR, Peixoto JR, de Almeida BC. Genetic variability of elite barley genotypes for Brazilian savanna irrigated systems based on RAPD markers. Biosci J, 2014, 30: 1118–1126.
20. Aras S, Aydın SS, Körpe DA, Dönmez Ç. Comparative genotoxicity analysis of heavy metal contamination in higher plants. In: Begum G, editor, Ecotoxicology. Rijeka, Croatia: InTech Publications, 2012,pp. 107–124.
21. Cenkci S, Yıldız M, Ciğerci İH, Bozdağ A, Terzi H, Terzi ESA. Evaluation of 2,4-D and Dicamba genotoxicity in bean seedlings using comet and RAPD assays. Ecotox Environ Safe, 2010b, 73: 1558–1564.
22. Cenkci S, Yıldız M, Ciğerci İH, Konuk M, Bozdağ A. Toxic chemicals-induced genotoxicity detected by random amplified polymorphic DNA (RAPD) in bean (Phaseolus vulgaris L.) seedlings. Chemosphere, 2009, 76: 900–906.
23. Cenkci S, Yıldız M, Konuk M, Eren Y. RAPD analyses of some wild Triticum L. and Aegilops L. species and wheat cultivars in Turkey. Acta Biol Cracov Bot, 2008, 50: 35–42.
Received: October 23, 2022 / Accepted: January 15, 2023 / Published:15 February 2023
Citation: Salem Alsaffar R. Analysis of the Genetic Distance of Several Generations of Barley (Hordeum valulgare L) by RAPD-PCR Technique. Revis Bionatura 2023;8 (1)23. http://dx.doi.org/10.21931/RB/2023.08.01.23
