2022.07.04.64
Files > Volume 7 > Vol 7 No 4 2022
Phenotypic and genotypic investigation of three weeds residues allelopathic effect on the growth of three hybrid wheat cultivars
Enas Kuosay Al-Doree1, Raed Salem Al-Sffar2, Iman radha Jasim3*
1 Department of Biology, College of Sciences, University of Mosul, Mosul, Iraq
2 Department of Biology, College of Sciences, University of Mosul, Mosul, Iraq
3 Department of Biology, College of Sciences, University of Mosul, Mosul, Iraq
* Correspondence: [email protected]
Available from: http://dx.doi.org/10.21931/RB/2022.07.04.64
ABSTRACT
The present study was carried out to determine the allelopathic effect of extraction of three herbs (Silybum marianum L. and Malva parviflora L. and Loliuim rigidum L. ) on three wheat cultivars (Rashid, Abo Ghrib and IPA99) on germination, growth and ear formation by classical research methods (laboratory and greenhouse experiments and molecular detection of GA3-oxase2 (TaGA3ox2-1) gene expression and its crucial role in wheat growth and Ta14S gene expression as a gene responsible for ear development. The study analyzed the influence of weed residues on the germination and growth of three wheat cultivars. According to the mean effect of aqueous weed extract on the cultivars, the laboratory experiments revealed a significant difference in all the characteristics studied.
Keywords. Weeds, hybrid wheat cultivars, allelopathy, RT-PCR.
INTRODUCTION
Wheat (Triticum aestivum) is a grass that belongs to the Poaceae family and is a widely cultivated agronomic crop for grains used in household consumption and the creation of a multitude of processed materials, which was labeled as a profitable agricultural venture; the crop has a long and illustrious history in human culture and civilization. It is essential to the world economy and food security1. Weeds can produce many allelochemicals; they have a range of effects on other plants, including reducing or activating the germination and growth of receiving plants2. Allelopathy from specific weed species impacts crop development; allelochemicals from allelopathic weed products can harm budding crop seedlings by disrupting root and shoot growth3. Allelochemicals secreted from Chenopodium murale L. root hairs were found to be responsible for cell cycle disruption and oxidative damage in wheat4. Allelochemicals are a type of secondary metabolite that isn't necessary for metabolism; they have been linked to decreased seed germination and seedling growth; their inhibition is complicated, involving interactions between various chemical classes such as phenolic chemicals, flavonoids, terpenoids, alkaloids, steroids, carbohydrates, and amino acids3.
Allelopathic water extracts from weed species, such as Malva parviflora L., were also found to limit barley growth and photosynthetic activity5. While found that adding aqueous extract vegetative sections of Malva rotundifolia L, Silybum marianum, and Sonchus oleracens to wheat seedlings reduced plumule and extreme length and weight6. Allelopathic weeds have been reported in enormous numbers; they harm crop plants from emergence through maturity, causing significant economic losses7. A varied range of weeds in wheat-growing fields is one of the critical limiting factors in wheat output8. Compared to wheat infested with weeds (A. fatua and S. Marianum), the results showed that wheat grown in weed-free circumstances had the highest biomass and leaf area index during both growing seasons. To reduce crop-weed conflict and increase crop output, control of certain weeds, namely A. fatua and S. marianum, is particularly desirable9. Many kinds of research were achieved overall the world. In a seed bioassay,10 utilized aqueous extracts of different extracts of four common weeds (A. fatua, Phalaris minor, Melilotus alba, and Chenopodium album) and found allelopathic effects on wheat at levels of 6,8,10, and 12g/100ml. The allelopathic effect of weeds (Avena fatua, Melilotus officinalis, and Polypogon hissaricus) on germination, growth, dry biomass and chlorophyll concentration of three wheat cultivars was discovered by 11.
Gibberellins (GAs) are plant hormones that control many growths and developmental processes in plants12. They are especially critical in stem elongation regulation13,14. As a result, it's reasonable to look for a link between GAs and plant height heterosis. In previous research, GA levels have been linked to the robust plant development seen in hybrid F1 plants. There are three lines of evidence that support this relationship. In maize, hybrids have greater GA levels than parental inbreds15, while an interspecific hybrid between Liriodendron chinense and L. tulipifera has higher GA levels than parental inbreds15. There are three phases in the production of GA in higher plants. As a molecular biomarker, we chose the GA20ox gene, which is involved in the third stage of GA biosynthesis and is linked to a critical role in the corrosion precursor of bioactive GAs to GA1. The bioactive GA is a factor in to increase in hybrid shoot cylinders than in inbred shoot cylinders16. Many experiments, on the other hand, focus on molecular mechanisms of developmental regulation that have the potential to accelerate wheat improvement. Even though 14-3-3 proteins are increasingly being linked to developmental control15. In the formation of wheat seeds, little is known about such genes. Because hexaploid There are three types of wheat chromosomes. We focused on the relevance of the Ta14S gene in wheat cultivars under study to better understand a regulatory function in wheat seed development17.In many scientific domains, molecular research utilizing quantitative polymerase chain reaction (qPCR) is regarded as a gold standard technology for confirming traditional results. The activation of relevant genes for numerous metabolic pathways is detected by screening at the molecular level of allelopathic action, which leads to increased plant germination, growth, seedling, flower, and fruit formation18.
One of the most severe issues in agricultural productivity is weeds. They have an impact on crops through direct resource competition as well as allelopathic consequences. Allelochemicals can be found in almost every part of a plant, including the leaves, flowers, fruits, roots, rhizomes, seeds, and pollen
MATERIAL AND METHODS
In 2021,the research was carried out at Mosul University's Biology Department College of Science. Three weeds(Silybum marianum L., Lolium rigidum L., and Malva sylvestris L.) were collected and transported to the laboratory for allelopathic testing after harvesting. Wheat seeds were obtained from the sources testing and confirmation center in Ninava governorate/Iraq.
Laboratory assay
Preparation of aqueous extracts
Weeds were mixed and stored for 24 hours at room temperature in distilled water (5gm per 100ml). After that, the solutions were filtered twice: once through a double layer of muslin gauze and then again through Whatman No.1 filter paper. These water extracts include 5% (w/v) water.The effect of water extracts at concentrations 5 %(w/v) was evaluated in a Petri dish bioassay. Ten wheat cultivars (Rashid, Abu Graib and IPA99) seeds were placed in sterilized Petri dishes lined with twice-folded filter paper. In each Petri dish, 8 ml of (5%,w/v) of three weed extracts was added, while distilled water was used in control. There were three frequencies in all of the treated samples. The dishes were incubated at 22 °C(±2) for 7 days in a Completely Randomized (CR)Design. The percent of seeds germination was calculated in the following equation19.
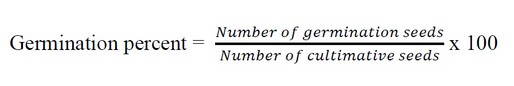
Seedling (cm), plumule and radical length (cm), in addition to plumule and radical fresh weight (mg), were measured at the end of this study.
Preparation of weed residues:
In 2021, a pot experiment was carried out at the Department of Biology's greenhouse. The effect of incubated dry weed residues(5%,w/w)during one week in pots with soil was determined, 10 seeds were placed in Plastics pots with a diameter of 20cm for each of the wheat cultivars (Rashid, Abo Ghrib, and IPA-99) at 0.5cm depth from the surface of the soil, then irrigated with water and placed in a greenhouse at a temperature of 22 (±2)°C, 10 wheat seeds were sowed in the soil without residues as a control. After 60 days of seedling, the germination percent was assessed using a complete randomized block design, with each pot having three frequencies20.
Phenotypic characteristics investigation:
Germination percent, shoot length(cm), fresh weight of shoot(gm), spike length, number of leaves per plant, spike weight(g), and weight of 100 seeds. The water content (WC) can be expressed on a dry weight (DW) or fresh weight (FW) basis, as listed in the following equation 21.
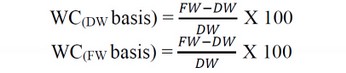
Molecular investigation assay
RNA isolation
RNA was extracted according to the manufacturer's instructions for the RNA kit (Geneaid, Taiwan).
Single-strand DNA synthesis
After RNA isolation, the cDNA synthesis phase was started. Amplification mixture(GeneAll,S.Korea)was composed of 10X Reaction Buffer2 µl,20X dNT P(1µl),Rtase(1µl),RNase Inhibitor(0.5µl),RNase free Water (3.5µl), RNA Sample (6µl), primers (3µl) and RNA Sample(10µl).Amplification steps included 25°C (10min),27°C (120min) and 85°C(5min).
Real-Time qPCR profile
Amplification mixture was used in this step composed of 2X MasterMix(S YBR Green)10μl, primers 2μl,DNA template2μl and RNase free distilled water 6 μl(GeneAll,S.Korea),primers that used were, wheat growth indicator,TaGA3ox2-1-S-5'-GTACATGGGCGTGCGCAAGAAG-3',TaGA3ox2-1-A-5'-GCACGCATCC ACCAGCATCATC-3 (26), spike development,Ta14S-R1-5'-CGCCT GCTACGCTACAAGGAC-3', Ta14S-F2- 5'-GTCAATGACCGTTG CAATGTG3', in addition to β-actin-F-5'-TTTGAAGAGTCGGTGAAGGG-3',β-actin-R-5'-TTT CATACAGCAGGCAAGCA-3'17. Amplification mixture plate was placed in Applied Biosystem 7500 fast thermocycler; an amplification program was included, initial unwinding at 95°C(5min), then 40cycles of unwinding at 95 °C(30 sec), primer hybridization at 60°C, and 57°C(30sec) respectively and extension 72°C (30sec).
Statistical analysis
Phenotypic outcomes analysis
The data were statistically evaluated by SPSS Program, and differences in treatment means were compared using the Completely Randomize Design (CRD) test at a probability level of 0.0523
Molecular outcomes analysis
The quantification findings were expressed in terms of the cycle threshold value calculated using the manually modified baseline. The method mentioned earlier was used to determine relative gene expressions. To detect the difference between control and treated cases, differences in the amplification values of target and reference genes were estimated as expression levels(fold change) for each sample18,24.
RESULT
The current findings (table1) revealed a substantial difference in wheat germination growth characteristics after being treated with an aqueous solution of three weed residues(5%,w/v). According to the mean effect of cultivar, the results showed the highest effect of seed germination percent in IPA-99(91.67%). After treatment with Malva parviflora L.aqueous extracts, the highest increase in seed germination percent (96.67%) was reported in the Rashid cultivar. According to the mean effect of weeds, the highest seed germination percent (93.33%) was noticed after treatment with Malva parviflora L.
Regarding the mean effect of cultivar, the outcomes revealed the highest impact of plumule length in Abo Garib (4.38cm). The treatment with Silybum marianum L., aqueous extracts showed the highest increase in plumule length (4.93cm) reported in the Abo Garib cultivar. The means effect of weeds showed the highest value in plumule length (4.04cm)after treatment with Malva parviflora L.
The mean effect of cultivar outcomes showed the highest impact of plumule weight in Rashid(0.07gm). Due to treatment with Silybum marianum L., aqueous extracts' highest increase in plumule weight(0.092gm) was documented in the Ras-hid cultivar. The mean effect of weeds showed the highest value in plumule weight (0.054gm)after treatment with Silybum marianum L. The mean effect of cultivar outcomes showed the highest impact of radical length in Abo Garib (6.19cm). Because of therapy with Loliuim rigidum L.,aqueous extracts, the highest reducing effect of radical length(2.97cm) was noticed in the Rashid cultivar. The mean effect of weeds showed the highest value in radical length (4.93 cm)after treatment with Silybum marianum L.
The mean effect of cultivar outcomes showed the highest effect of radicale weight in Rashid(0.039gm).because of treatment with Silybum marianum L.,aqueous extracts, the highest increasing effect of radicale length(0.045cm)was noticed in Abo Garib cultivar. The mean impact showed the highest value in weight(0.038gm)after treating with Loliuim rigidum L.
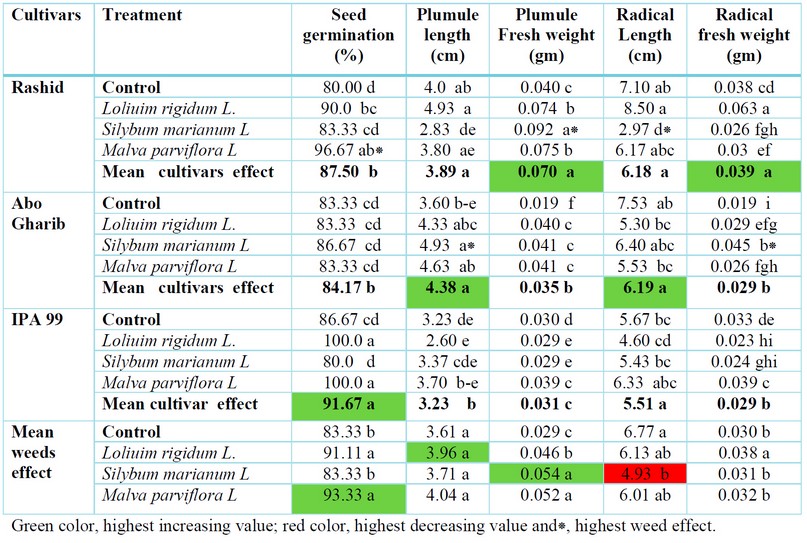
Table 1. Effect of aqueous extracts of weeds in germination and growth of three wheat cultivars.
Figure 1. Shows that the three weed residues were affected in the seed germination percent of three wheat cultivars. The highest increasing value was linked with Abo Ghraib cultivar after cultivation in the soil incubated with Silybum marianum L. and Malva parviflora L. residues. In contrast, the highest reduction percent of IPA99 seed germination was documented after cultivation in soil with Silybum marianum L. and Malva parviflora L.
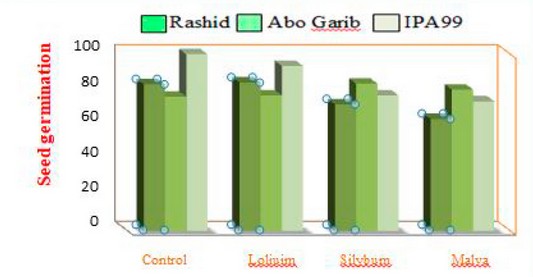
Figure 1. Histogram of seed germination of three cultivars of wheat plants treated with three weeds (Silybum marianum L., Malva parviflora L., and Loliuim rigidum L.).
The three cultivars that were cultivated in the soils incubated with weeds residues showed variant increasing values in spike length. However, the remainder of the treatments varied in spike length with different cultivars; the highest spike length was linked with Abo Garib after treatment with Malva parviflora L., as represented in the histogram (figure2).
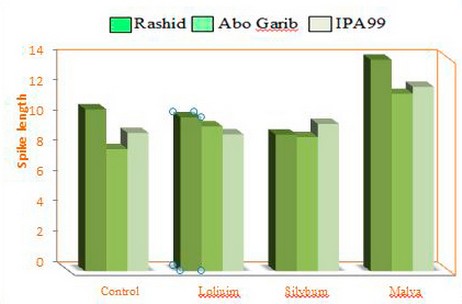
Figure 2. Histogram of spike length of three cultivars of wheat plants treated with three weeds( Silybum marianum L. and Malva parviflora L. and Loliuim rigidum L.).
Figure (3) demonstrated a variance in the number of leaves of the three wheat cultivars cultivated in the weed-containing soil compared to the number of plants grown in the control soil(without weeds). The number of leaves of the cultivar IPA99 treated with Loliuim rigidum L. revealed the highest increasing value.
Figure 3. Histogram of the number of leaves of three cultivars of wheat plants treated with three weeds( Silybum marianum L. and Malva parviflora L. and Loliuim rigidum L.).
The present results demonstrated that the weight of 100grains was differed according to the three wheat cultivars after treated with soil weed residues. Cultivar Rashid treated with soil having Malva parviflora L. residues showed the most significant value of growth (figure 4).
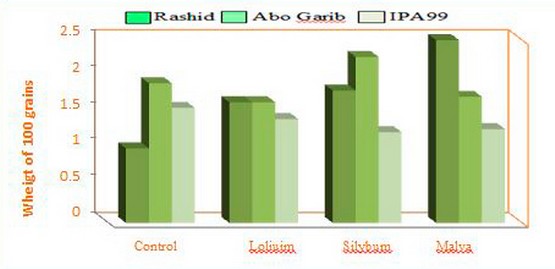
Figure 4. Histogram of the weight of 100 grains of three cultivars of wheat plants, treated with three weeds( Silybum marianum L. and Malva parviflora L. and Loliuim rigidum L.).
In the soil incubated with weed residues, there were changes in the relative water content in wheat cultivars, as shown in Figure(5). The relative water content of Abo Ghraib plants grown in soils incubated with Malva parviflora L. residues showed the highest increasing value.
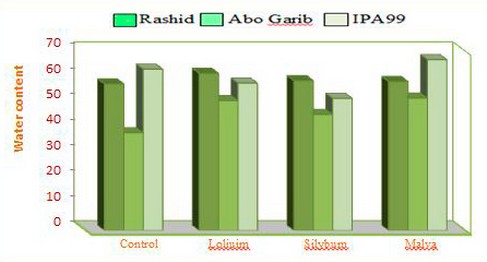
Figure 5. Histogram of the water content of 100 grains of three cultivars of wheat plants treated with three weeds( Silybum marianum L. and Malva parviflora L. and Loliuim rigidum L.).
Relationship between phenotypic and genotypic results
This study showed significant positive predictive values as phenotypic results associated with specific molecular outcomes(table2). In term of wheat length, there was a significance increasing percentage in Rashid cultivar after treat-ing with Loliuim rigidum L. and Malva parviflora L.(L-Treated(T),45cm: control (c),34cm and M-treated,44cm: control,34cm resp.), these results were confirmed with TaGA3ox2-1gene expression related with the same case(L-t,5.1043:c,0.870 67 and M-t,3.96014:c,0.87067resp.)other outcomes showed high expression with previous gene (T,7.96787:c,0.942034), that associated with phenotypic finding(T, 55:c,35) after treating of Abo Grib cultivar with Malva parviflora L. as well as, growth value was increased in IPA99 cultivar(T,44:c,21), which agreed with that documented genotypically (T,6.85702:c,1.01002). Spike weight showed the highest increasing value phenotypically in Rashid(t,55gm:c35, gm)after treating Abo Grib cultivar with Malva parviflora L., which in turn agreed with Ta14S-R1 gene expression(T,6.71807:c,1.01065).
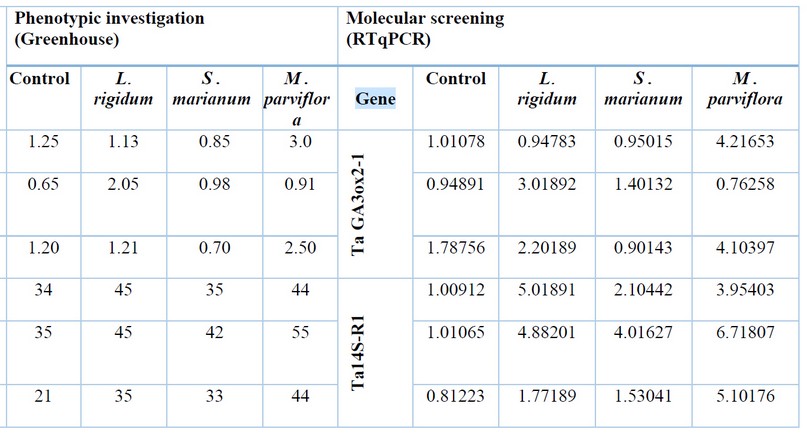
Table 2. Comparison between phenotypic results and molecular screening outcomes.
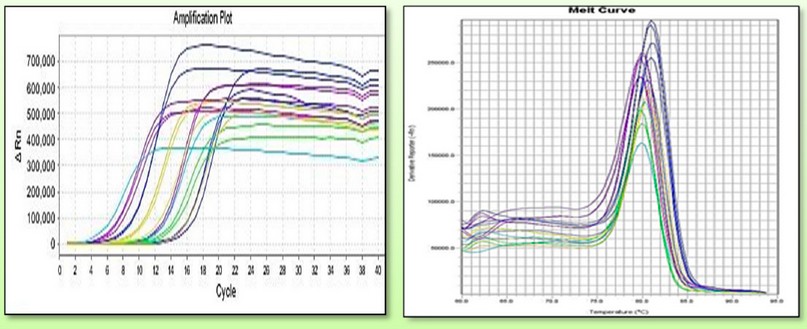
Figure 6. Shows the events of the TaGA3ox2-1gene amplification curve via RTqPCR, in which different values of previous gene expression were documented.
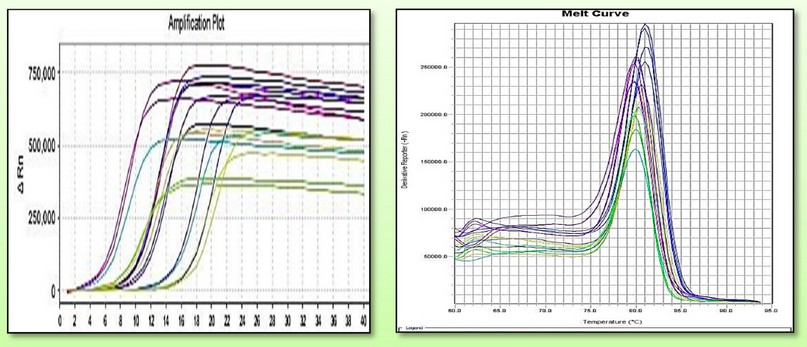
Figures 7. Comparative curves of TaGA3ox2-1gene. Right, amplification curves and left melting curves.
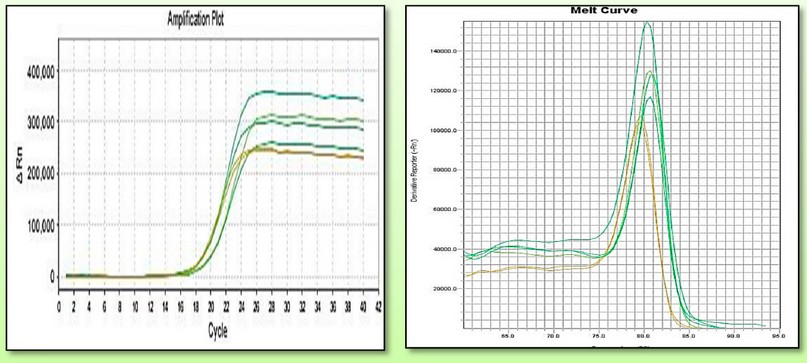
Figure 8. Showed the events of B-actin gene amplification curve via RTq- PCR as a reference gene.
DISCUSSION
Weeds leave massive amounts of residue in the field, affecting the linked crops and subsequent crops in various cropping systems. Allelochemicals released by weeds impact agricultural plant germination, stand establishment, growth, yield, and physiology. By regulating enzyme activity, protein synthesis, photosynthesis, respiration, cell division, and enlargement, among other physiological functions, they produce significant reductions in crop germination and growth, resulting in a significant drop in crop production. Allelopathic weeds, in short, are a possible hazard to crop plants and can result in financial losses25
The present results showed a synergistic relationship between utilizing weed residues and wheat cultivars growth in which the studied parameters were increased. In turn, these outcomes agreed with the documented results of many researchers and disagreed with others. According to to26, some plant species have a more considerable inhibitory impact when used as residues due to higher allelochemical extraction during the decomposition process. Flaveria bidentis L., an invasive weed, was shown to exude allelopathic phenolic chemicals, and residues from this weed hindered the growth and biomass of cotton seedlings. There was the highest decrease in root fresh (64%) and dry biomass (64%) and root/shoot ratio(64%)after being treated with V. sativa raw residues. The outcomes showed that the interaction of leaches and residues could lead to a synergetic effect in nature. In turn, this causes significant economic and environmental disposal of waste and organic materials accumulate-tion27.
On the other hand,28 used various concentrations of aqueous extracts(5,10 and 15g/l)of the three weeds, significantly reducing the percent germination of Pirsabaq cultivar. In pot culture, root and shoot length and dry seedling biomass of the three wheat varieties showed differential responses to different weeds. Aqueous extract at 15g/l of A. fatua increased the source, shot height, and dry weight of Pirsabaq cultivar11.
CONCLUSION
In terms of molecular investigation, many studies were achieved in this field over the world22 noticed that among the 18genes analyzed, genes encoding enzymes that promote the synthesis of bioactive gibberellin like TaGA20ox-2, TaGA20oxD, TaGA3ox2-1, TaGA3ox2-2, TaGA3ox2-2, these genes act as positive components in its response pathways were up-regulated in hybrid cultivars. A previous study showed that three Ta14S homoeologous genes have regulatory roles in seed development and germination via synthesis of 14-3-3 proteins are involved in signal transduction pathways with significant roles in late embryo development in seeds and germination, which represented a high transcription of Ta14S-2B 29.
Funding: self-funding
Acknowledgments: In this section, we acknowledge any person who supports us to complete this project.
Conflicts of Interest: there is no conflict
REFERENCE
1- Majeed, A.; Muhammad, Z; Hussain, M.and Ahmad, H. In vitro allelopathic effect of aqueous sugarcane extracts on wheat germination parameters, Acta Agric. Slov.؛2017. 109. 349–356.
2- Muhammad , Z. and Abdul Majeed. Allelopathic effects of aqueous extracts of sunflower on wheat (Triticum aestivum L.) and maize (Zea mays L.). Pakistan Journal of Botany؛2014. 46(5):1715-1718.
3-Javed, K.ALLELOPATHY;A BRIEF REVIEW. J Nov. Appl Sci؛2020..,9 (1): 1-12.Journal of Agricultural Sciences, 3(3): 235-341.
4-Dmitrović,S.;Simonović,A.;Mitić,N.;Savić,J.; Cingel,A.;Filipović,B.;and Ninković,S. Hairy root exudates of allelopathic weed Chenop odium mur ale L.induce oxidative stress and down-regulate core cell cycle genes in Arabidopsis and wheat seedlings. Plant Growth Regulation2014.,V.75, P.365-382.
5- Al-Johani ,N.S.; Aytah, A.A., and Boutraa ,T.Allelopathic impact of two weeds, Chenopodium murale and Malva parviflora on growth and photosynthesis of barley (Hordeum vulgare L.). Pakistan Journal of Botany.2012. 44(6):1865-1872.
6- Modafer, A .; Mahdir, Shaker, M . S. and Mariam A. I. and Ammar ,W. M .Allelopathic Effect of some Winter Weeds on Germination and Growth of Wheat (Tritrcum aestivum L.). Tikrit J.A.2017. Vol.17 No 2.
7- Zohaib ,A.; Tabassum,T.;, Ali Zohaib , Abbas,T.and Tassadduq Rasool . Influence of water soluble phenolics of Vicia sativa L. on germination and seedling growth of pulse crops. Sci Agric.2014. 8:148-51.
8- Asad, M.; Iqbal, M.N.;Ashraf, A. et al. Effect of Post-emergence Herbicides to Control Broad-leaved Weeds in Wheat Under Rainfed Conditions. PSM Biol. Res.,2016. 01(1): 16-21.
9- - Ijaz A. and Bakhtiar, G..Competitive Ability of Wheat Crop against Different Densities of Avena fatua and Silybum marianum. Sarhad Journal of Agriculture.2017. Vol.37, Iss. 2, Pages 331-713.
10- Siyar,S.;Chaudhry,Z.;Hussain,F.;Hussain,Z. and Majeed,A. Allelo-pathic Effects of Some Common Weeds Prevailing in Wheat Fields on Growth Characteristics of Wheat (Triticum aestivum L.) PSM Biol. Res. 124-127.PSM Biological Research 2017; 2(3): 124-127.
11- Siyar, S. ; Abdul Majeed ; Zahir M. ; Hazrat, A. and Naila, I. Allelopathic effect of aqueous extracts of three weed species on the growth and leaf chlorophyll content of bread wheat. Acta Ecologica Sinica - International Journal.2019;Vol.39 No.1 pp.63-68.
12- Hedden, P.; Phillips, A.L. Gibberellin metabolism: new insights revealed by the genes. Trends in Plant Science,2000; 5:523-530.
13- Sakamoto, T.; Miura, K.; Itoh ,H.; Tatsumi, T.;Ueguchi-Tanaka, M. ; Ishi-yama,, K.; Kobayashi, M.; Agrawal ,G.K; Takeda, S.; Abe, K.; Miyao, A; Hirochika, H.; Kitano, H.; and Ashikari ,M.; Matsuoka, M. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiology,2004; 134:1642-1653.
14- Zhu, Y.Y.; Nomura ,T.; Xu ,Y.H.; Zhang, Y.Y.; Peng ,Y.; Mao, B.Z.;, Hanada ,A.; Zhou ,H.C.; Wang ,R.X.; Li , PJ; Zhu, X.D.; Mander, L.N.; Kamiya, Y.J.; and Yamaguchi, S.; He, Z.H. Elonkgated Uppermost Internode encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice. Plant Cell,2006; 18:442-456.
15- Rood, S.B.; Buzzeli, R.I.; Mander, L.N.; Pearce, D. and Pharis, R.P.Gibberellins: a phytohormonal basis for heterosis in maize. Science,1988; 241:1216-1218.
16- Rood , S.B. and Zanewich , K. P.Vernalization and Gibberellin Physiology of Winter Canola (Endogenous Gibberellin (GA) Content and Metabolism of [3H]GA1 and [3H]GA20. Plant Phvsiol.1995; 108: 61 5-621.
17-Xing, u.o.;Wang, Y.; Wang, R.;Xiao et al.Molecular characterization and expression analysis of three homoeologous Ta14S genes encoding 14-3-3 proteins in wheat (Triticum aestivum L.). The crop journal,2016; 4.188-198.
18- Yuan,J.S. and Stewart,C.N.Real-time PCR statistics. PCR Encycl-opedia,2005; 1:101127-49.
19- ISTA. Intension rules for seed testing. Seed Sci. and Tech.1976; 34.
20- Chung, I.M.;Ahn, JK and Yun,S.J .Assessment of allelopathic potential of Coastal Bremoda grass.Agro.J.,2001;80:557-560.
21- Turner,N.C.Techniques and experimental approaches for the measu-rement of plant water status. Plant and soil,1981; volume 58, pages339–366.
22- Zhang, Y.i.; Zhongfu, N.i. and Yingyin ,Yao et al.Gibberellins and heterosis of plant height in wheat (Triticum aestivum L.). MC Genetics 2007; 8:40.
23- Drebee, H. A.The Effect of Cultivated Area and the Prices of Buying the Rice on Its Production in AL- Qadisiyah Province-Iraq During (1990-2014) by Using VECM. Q. J A. S,2017; volume7,Issue1,Pages 123-135.
24- Livak, K.J. and Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods,2001; 25:402-408.
25- Zohaib, A.1.; Tabassum, T.1 .; Aanjum, S.A.1.;Abbas, T.1 and Nazir, U.1. Allelopathic Effect of some associated weeds of wheat on germination ability and biomass production of wheat seedlings.2017.
26- Zhang, F.-J., Guo, J.-Y., Liu, W.-X., Wan, F.-H. Influence of coastal plain yellowtops(Flaveria bidentis) residues on growth of cotton seedlings and soil fertility. Arch. Agron. Soil Sci.2012; 58, 1117e1128.
27- Majeed, A., Muhammad, Z., Ahmad, H. Allelopathic effects of leaf extracts of three agroforestry trees on germination and early seedling growth of wheat (Triticum aestivum L.). Azarian J. Agric.,2017; 4(3): 69-73.
28- El-Rokiek , K. G.; Saad El-Din, S. A. ; El-Sawi S. A. ; and Ibrahim , M. E. , . Contr-lling Anagallis arvensis and Malva parviflora Associated Wheat Using Some Essential Oils. I-Using Citrus sinensis Peel Oil. Egypt. J. Plant Prot. Res.2018; Inst,1 (2):96-111.
29- Wang X., Wang Y., Xiao R. et al.. Molecular characterization and expression analysis of three homoeologous Ta14S genes encoding 14-3-3 proteins in wheat (Triticum aestivum L.).The crop journal.2016; V.4,Is.3, P. 188-198.
Received: September 22, 2022 / Accepted: October 18, 2022 / Published:15 November 2022
Citation: Al-Doree E K, Al-Sffar R S, Jasim I R. Phenotypic and genotypic investigation of three weeds residues allelopathic effect on the growth of three hybrid wheat cultivars. Revis Bionatura 2022;7(4) 64. http://dx.doi.org/10.21931/RB/2022.07.04.64
