CS 2019.02.01.28
Files > Conference Series > 2019 > Humboldt Kolleg 2019
Bionatura Conference Series Vol 2. No 1. 2019
“Breaking Paradigms: Towards a Multi-, Inter- and Transdisciplinary Science” In commemoration of the 250th Anniversary of Alexander von Humboldt
Gangliosides generalities and role in cancer therapies
Pasquel-Dávila Daniela S.1, Yánez-Vaca Sabrina A.2, Espinosa-Hidalgo Nicole D.3, Cuadros-Buenaventura Evelin G.4
*The work was done in an equitable way by the authors.
available in: http://dx.doi.org/10.21931/RB/CS/2019.02.01.28
ABSTRACT
Gangliosides are located in the plasma membrane; this confers them the ability to interact with other molecules in order to participate in important cellular processes. Some gangliosides presence or absence in the cell surface is associated with either normal condition or pathologies. Particularly in cancer, gangliosides play a critical role in pathological events like cellular malignancy, tumor formation, and metastasis, defining gangliosides as good candidates to be used as cellular markers. When specific gangliosides are exhibited, immunotherapy could be applied in order to inhibit tumorigenesis or induce an immunogenic response. Novel cancer treatments such as NGcGM3/VSSP vaccines, valproic acid, BMS-345541 inhibitor of GD2 and immunotherapies using 1E10 and 14F7 monoclonal antibodies are described. On this review, there will be studied the gangliosides that allowed developing biological techniques that can give immunogenicity to cancer cells.
Keywords: Gangliosides, cellular markers, cancer, vaccines, immunotherapy.
INTRODUCTION
The study of gangliosides as membrane molecules is not a new scientific interest. In fact, they were discovered as membrane glycolipids in the 1930s by Professor Klenk, who also noted that the content of these glycolipids was higher in the grey matter than in the white matter of the brain. Klenk proposed these molecules as gangliosides, because they were mainly present in the central nervous system cells, ganglienzellen1,2. Today, however, gangliosides are seen as attractive molecules to target cancer cells through immunotherapy2. This is because of its association with different oncogenic cellular processes2–4. Despite being highly expressed in the plasma membrane of eukaryotic cells (a-series mostly) at normal conditions5,6, it has been reported that the expression of more complex gangliosides (b-series and c-series) increase in cancer cells, especially in those types of cancer from neuroectoderm origin, such as neuroblastoma, glioblastoma, melanoma, breast cancer, and lung cancer7–9.
In general, gangliosides have been used in immunotherapy as the principal component in different vaccines, whose objective is to increase the immune response of patients towards specific gangliosides that are overexpressed in the membranes of cancer cells10–12. Some of these studies have shown good results11–15. In consequence, we consider them as promising proposals in cancer treatment. For this reason, the paper presented here has the purpose to make a ganglioside review on their chemical structure, physiology, biosynthesis and specially focusing on gangliosides and cancer relationship and their uses as a target for cancer immunotherapy.
Definition, structure and gangliosides location
Gangliosides, unlike other glycosphingolipids, contain one or more additional sialic acid molecules, thus they are considered as a subclass of acidic glycolipids. They usually present two variants of sialic acid, the N-Acetylated (NeuAc), which is normally found in healthy tissues of the body, and the N-Glycolylated (NeuGc) which is the one that is overexpressed in the cell membranes of cancerous tissues16,17. Focusing on N-Glycolylated gangliosides, they are formed by one or more molecules of sialic acid, cholesterol, N-acetyl glucosamine molecules and negatively charged heads that are formed by oligosaccharides (Figure 1)18.
The location of gangliosides are in the plasma membrane by crossing it or parallel to it, and the anchoring occurs in the ceramide group that is linked to the glycol hydrophilic head, which has sialic acid residues. This binding left free an exposed glycan on the cell surface. Gangliosides opportune location confers them the ability to interact with several extracellular molecules such as lipids, proteins, among others and they can also act as important receptors which are able to be recognized by the variant site of specific antibodies2.
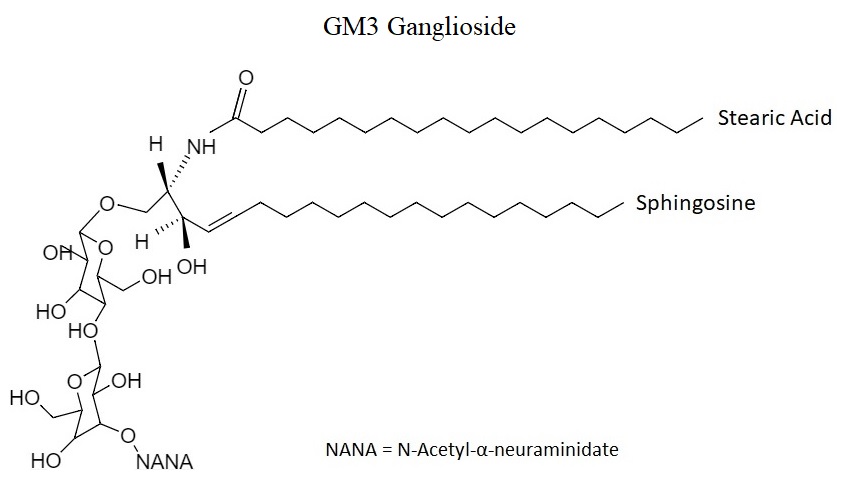
Figure 1: GM3 ganglioside structure.
Ganglioside biosynthesis pathways
Gangliosides are divided according to the number of sialic acid molecules that contain in their structure: 0-, a-, b- and c-series, which have 0, 1, 2 and 3 molecules of sialic acid respectively7. The synthesis of gangliosides starts in the endoplasmic reticulum, by the formation of ceramide (Cer synthase)19, which then is transported to Cis-Golgi. The next step is led by the glycosyltransferases, whose function is the addition of monosaccharides, either glucose (GlcCer) or galactose (GalCer), to the ceramide in the 1- hydroxy group. The formation of LacCer is then produced by LacCer synthase, and α2,3-sialyltransferase ST3 Gal V (GM3) will transfer the first sialic acid residue to the previous molecule. Then, depending on the final ganglioside structure, sialyltransferases ST8Sia I (GD3S) and ST8Sia V (GT3 synthase) will be responsible for adding additional sialic acid molecules. Finally, different glycosylation steps of gangliosides will be done7,9 (figure 2).
Some studies have suggested an association between the overexpression of complex gangliosides and the increase in the expression of the gene encoding GD3S9,20.
Figure 2: Ganglioside biosynthesis in eukaryotic cells, showing the intervention of different enzymes. The enzymes which transform Ceramide (Cer) to lactosylceramide (LacCer) (purple arrow), the enzymes (Glycotransferases add sugar and sialic acids) which form 0-series to c-series gangliosides (green arrow) and the enzymes which transfer the N-acetylgalactosamine (GalpNAc), Galp, and Neup5Ac residues (blue arrow).
Ganglioside functions in cells
Different gangliosides roles have been described in several studies, and there is evidence that cell function and phenotype are influenced by them21. There are events that can be triggered or inhibited by the interaction of gangliosides with extracellular molecules (sugars and proteins). For example, cell growth, differentiation, migration, adhesion, immune interactions, apoptosis, and even tumor aggressiveness and metastasis2.
Gangliosides fundamental role has been studied on knockout mice and it could reveal several vital functions, particularly in early stages of embryonic development. Studies made in mouse embryos where the glucosylceramide synthase gene was silenced, the resulting individuals have a low chance to survive more than 7.5 days. On the other hand, the ones that have a GM3 synthase knock-down showed a higher sensitivity to insulin and similar effects occur in a GM2 / GD2 synthase knock-down resulting in a lower capacity to repair nervous tissue2.
Principal gangliosides in cancer cells
Currently, it is well established that the principal difference between a normal and a tumoral cell is the pattern and structure of the carbohydrates that are expressed on their cellular membrane. One of the most evident differences could be found on the carbohydrates associated with ceramide such as glycosphingolipids3. Specifically, gangliosides, besides its importance in different cellular processes, they also play an important role in pathological events. Many gangliosides that are present in tumor cells are also found in healthy tissues but the pathogenicity occurs when there is an over-expression on the cell surface, producing malignant transformations due to changes in carbohydrate expression patterns. There is also evidence suggesting that some gangliosides modulate aggressive angiogenesis and tumor growth22.
Gangliosides are known to be suppressors of the cellular or humoral immune response, and some of them are reported to decrease antitumoral immune cells regulation such as T and B lymphocytes, dendritic cells recognition and also natural killer cytotoxic action2. Otherwise, some gangliosides can also have an inhibitory effect on tumor growth23. It is known that GM3 ganglioside can act as an inhibitor of the growth of tumor cells due to anti-angiogenesis or motility24.
Another ganglioside such as N-Glycolyl GM3 was found in colon adenocarcinoma and it also has a role in lung cancer25. There is a restricted expression of gangliosides like GM3, GD3, and their derivatives 9-O-acetyl-GD3 and 9-O-acetyl-GT3, in normal breast tissues but there is overexpression of 50 % of them in cancer tissue, in the same way, there is an overexpression of 100% of N-Glycolyl-GM3 ganglioside in breast cancer26. Four types of gangliosides have been found in human melanoma cells, among them GM3 and GD3 which have been identified as main components and GM2 and GD2 as minor components27. The following table resumes the principal gangliosides: GM1, GM3, GD3, GD2, GT3, among others, which are present in colon, breast, melanoma, lung and nervous system cancer (Table 1).
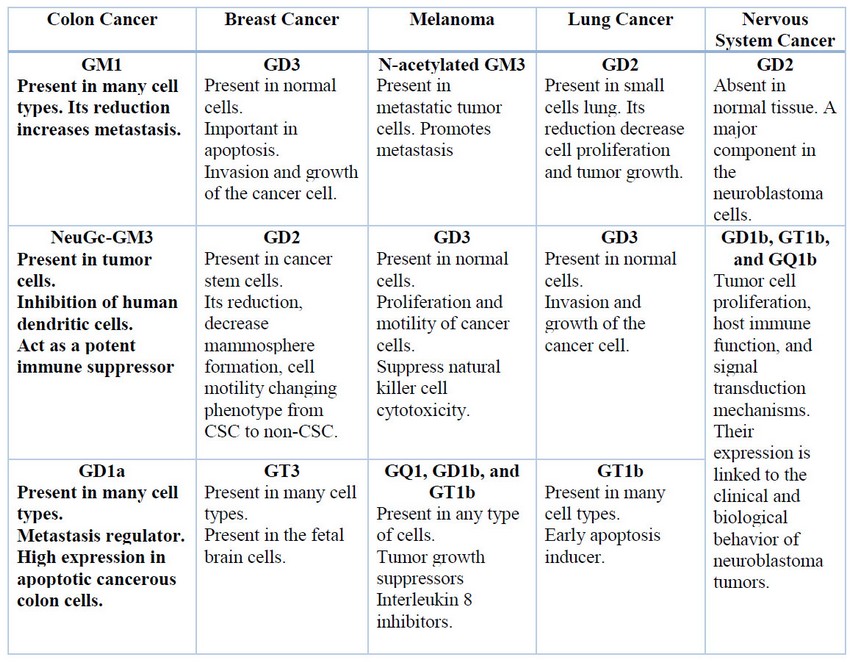
Table 1: Summary of the principal Gangliosides present in a different type of cancer.
Ganglioside therapy techniques
Even though gangliosides are overexpressed in oncogenic processes, they generate a low immune response, mainly thymus independent, since they are of saccharide origin and self-antigen nature. For this reason, different strategies are needed to be used in immunotherapies. Examples of those are the use of monoclonal antibodies, adjuvants and/or liposomes11,13,16,28.
NeuGcGM3/VSSP/Montanide ISA 51 vaccine.
NeuGcGM3 gangliosides in combination with a complex of outer membrane proteins of the gram-negative bacteria Neisseria meningitidis that form very small size proteoliposomes (VSSP) and Montanide ISA 51 as an adjuvant are the components of a vaccine (NeuGcGM3 / VSSP / Montanide ISA 51) that has been used in immunotherapies against breast cancer. In phase I of a clinical trial, this vaccine induced a strong immune humoral response in patients with stage III and IV breast cancer. It was reported that after immunization, in 100% of patients with stage III breast cancer, a high response of anti-NeuGcGM3 IgM was shown. Also, in the case of IgG, It was induced in 90% of patients. In addition, it was also shown that the toxicity of the vaccine NeuGcGM3 / VSSP / Montanide ISA 51 was within the limits established by the World Health Organization (WHO)11.
On the other hand, a phase III clinical trial study showed similar results in the adjuvant setting in patients in stage I and II of breast cancer. Since there was a significant increase in the concentration of anti-NeuGcGM3 IgM and IgG after immunization in both groups of patients (stage I and II). In addition, the majority of patients' hyperimmune sera, not only they could recognize the tumor cells that expressed the NeuGcGM3 ganglioside, but they also had cytotoxic activity on these cells (they killed the cells)13.
1E10 antibody: active immunotherapy treatment.
The use of anti-idiotypic antibodies against gangliosides present in malignant cell membranes has been recently studied. NeuGcGM3 is an attractive ganglioside which is highly expressed in tumor cells and is undetectable in healthy human tissues29. This ganglioside is expressed on breast cancer cells12 and in advanced lung cancer cells30. For that, this ganglioside is considered as a blank for immunotherapy. One approach for producing a long-lasting immune response against GM3 is the use of anti-idiotype antibodies as a way to mimic it. In studies with animal models, BALB/c mice were used, which were immunized with liposomes containing GM3. Then, monoclonal antibodies called P3 (mAb) were obtained. These IgM type antibodies are capable of efficiently recognizing the ganglioside present in tumor cells. Subsequently, immunization of BALB / c mice with mAb P3 was carried out together with Keyhole limpet hemocyanin (KLH) and in this way, it was obtained the 1E10 (mAb)14.
Racotumomab is the name of 1E10 mAb which is used in the anti-idiotypic vaccines that are conjugated with alumina. During the development of this anti-idiotypic vaccine, specifically on phase I, the principal goals was to identify the dosage, the immunity, and the toxicity12. The results suggest that the vaccine was well tolerated and immunologically active28. The first fact is appraised because the adverse effects were classified as grade I and II for the National Center Institute Toxicity Criteria (NCIC)12, whereas the last one is supported because the vaccine displays antibodies specific response against monoclonal antibody 1E1028. Despite these facts, anti-idiotypic vaccination could provide survival advantages12.
In particular, patients immunized with Racotumomab has demonstrated low toxicity and high immunogenicity to this anti-idiotype vaccine showing a strong antitumor and antimetastatic effect in syngeneic and allogeneic models which evidence that it is able not only to mimic GM3(Neu5Gc) but also to activate idiotypically cells that secrete antibodies against this antigens14.
Valproic acid: chemical treatment.
The degree of GM3 ganglioside expression and the type of tumor cells can produce different effects. In astrocytoma, neuroblastoma, sarcoma, thyroid carcinoma and cutaneous melanoma with high levels of gangliosides suggest aggressive behavior where patients had lower survival. On the other hand, in oral mucosa melanoma, the expression of GM3 (Neu5Gc) was associated with lower risk recurrence and a better prognosis14. Additionally, a larger amount of GM3 and a greater amount of GM2 gangliosides control the metastatic cell line, producing a low metastatic cell line in the first case and a high metastatic cell line in the second case31.
GM3 ganglioside (II3NeuAcLacCer) inhibits epidermal growth factor (EGF) dependent receptor autophosphorylation and cell growth, whereas de-N-acetyl-GM3 (deNAcGM3; II3NeuNH2Lac-Cer) promotes these processes32. Epidermal growth factor receptor (EGFR) at membrane microdomains plays an essential role in the growth control of epidermal cells, including cancer cells derived therefrom33. GM3 can bind to the receptor by two ways: the union of GM3 and basic amino acid residues of the receptor as it has been found in the case of insulin receptor or GM3 binding via carbohydrate-carbohydrate interaction as it has been observed between GM3 and N-glycans of EGFR23.
Valproic acid, a histone deacetylase (HDAC) inhibitor, is used as an antiepileptic drug and mood stabilizer, this acid has been used in clinical patients since 196715. Epileptic patients receiving VPA have significantly improved hemoglobin F levels, supporting the hypothesis that non-toxic levels of VPA can induce cell differentiation15. It was conducted a study using cancer cell lines founding that VPA stimulates the expression of ST3GAL5 gene that encodes GM3 synthase, the increase in GM3 synthase expression causes an increase in GM3 levels on the cell surface resulting in an inhibition of EGFR phorlation23. Consequently, the growth signal is reduced, and cell proliferation is inhibited. The expression of the ganglioside will depend on the level of induction of its synthase gene. It was reported that the treatment of gliomas with valproic acid and radiochemotherapy were well tolerated in children with encouraging response rates15. In preclinical studies, VPA inhibited the growth of glial tumor cells in humans and rodents and induced a different mature glial phenotype. In the same way using valproic acid in human tissue with cancer cells (human retinal pigment epithelial cells, carcinoma, and neuroblastoma), resulted in the increase of GM3 expression and reduction of cancer cells proliferation23,34,35. Evidence has also been presented that histone deacetylase HDAC inhibitors can sensitize malignant cells to radiotherapy and chemotherapy15.
14F7 antibody: passive immunotherapy treatment.
One alternative for an active treatment is the use of immunohistochemistry targeting of GM3. The absence of GM3 ganglioside in normal human cells36 and the presence of the GM3-which is highly specific in cancer cells in different types such as breast37 , brain38, melanoma37, digestive system36, lung39, and bladder40 are the reasons why NeuGcGM3 is one of the most useful and interesting targets for cancer cells in passive immunotherapy36. The treatment uses 14F7 which is an antiganglioside monoclonal antibody (mA14F7) of mice. 14F7 mA is specifically defined as a murine immunoglobulin G1 able to recognize the NeuGcGM341. In other words, 14F7 mAb is produced by immunizations of mice of GM3 with human lipoproteins, so it has a specific variable region of isotype and it does not react with other gangliosides37.
It was demonstrated in vitro with experimental tissue that 14F7 mAb is able to recognize the majority of cancer cells by the presence of GM3 ganglioside in a different type of cancers: digestive system and lung cancer42,43. The 14F7 mAb binds to the NeuGcGM3 at the cancer cells and through reactions and complement arrangements killing these cells41. The antibody binding has a cytotoxic effect44, the loss of membrane integrity and cytoskeleton constitution changes, which conduce to cell death. First, most of the fragments (fab regions) are recognized by the antigen even when they were compared to another P3 antibody, it was shown to have a high binding capacity and cytotoxicity. Second, the membrane constitution suffers swelling and change in diameter. Third, the activation of FAS receptors and caspases produce cell apoptosis.
BMS-345541 inhibitor of GD2.
A typical neuroectodermal antigen that can be expressed and has relevance in a variety of cancers is the disialoganglioside GD2; this b-series ganglioside can be found on small cell lung cancer, glial tumors, and different sarcomas (bones, bladder)45–47. However, it is commonly expressed in neuroectodermal tumors such as skin and uveal melanoma. The study of GD2 is important due to the fact that it is related to cell-to-cell adhesion and in signal transduction. When there is pathology like cancer, this molecule will be involved in processes such as proliferation, neoangiogenesis, immune-scape, and invasion46.
Different studies have demonstrated that GD2 ganglioside can be found in metaplastic carcinoma samples and in stem cells in breast cancer46,48. At this point, it is important to know that GD2 is obtained by the precursor GD3, this molecule comes from the synthesis of the GD3S (GD3 synthase) a highly expressed enzyme on breast cancer cells that has disialoganglioside (GD2+ cells)47,48.
Orsi et.al in 2017 identified that the inhibition of GD3S on breast cancer stem cells prevents metastasis on in-vivo mouse models46. This conclusion was achieved due to a deeper analysis of GD2+ cells that shows that they have active signaling of the transcription factor NF-kB. However, when the action of NF-kB is impeded by the inhibitor BMS-345541, the important consequences are the reduction of GD2+ cells and the inhibition of GD3S expression on breast cancer stem cells and cell migration. Those facts contribute to establishing that the inhibitor BMS-345541 suppresses the tumorigenic function of breast cancer stem cells48.
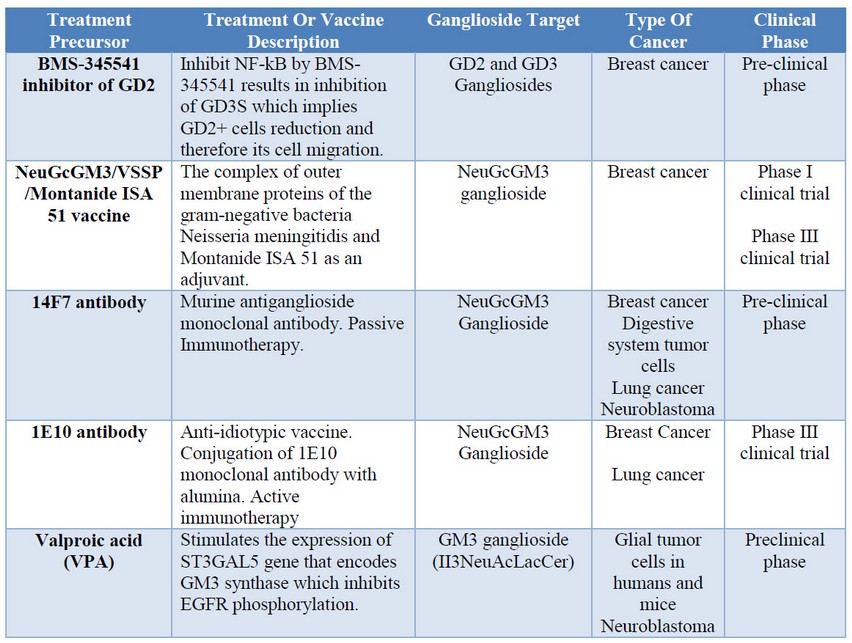
Table 2: Cancer treatment based on gangliosides.
CONCLUSIONS
Gangliosides research as target molecules in immunotherapies and treatments for cancer has been developed since the important, evident, and useful aspects of the role of gangliosides in the regulation of cell proliferative signals. Different immunotherapies and treatments with gangliosides have been developed. Examples of that are BMS-345541 inhibitor of GD2, 14F7 antibody, and valproic acid which are still on the preclinical phase; whereas NeuGcGM3/VSSP/Montanide ISA 51 vaccine and 1E10 antibody have already reached phase III on clinical trials. These achievements are important because it shows the potential application of gangliosides as targets to deal with cancer cells. By this way, recognizing the gangliosides that are present on certain cell lineage will allow developing new biomarkers, antibodies or metabolic pathways that could enhance the immune response in a specific and less aggressive way. Therefore, this fact could increase the survival rates of cancer patients.
REFERENCES
1. Yamakawa T. GANGLIOSIDE STRUCTURE , FUNCTION , AND BIOMEDICAL POTENTIAL [Internet]. 1st ed. Ledeen RW, Yu RK, Rapport MM, Suzuki K, editors. New York; 1984. 649 p. Available from: https://b-ok.cc/book/2249097/d3c588)
2. Krengel U, Bousquet PA. Molecular recognition of gangliosides and their potential for cancer immunotherapies. Front Immunol. 2014;5(JUL):1–11.
3. Hakomori, S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: Overview and perspectives. Cancer Res. 1985;45(June):2405–14.
4. Rabu C, McIntosh R, Jurasova Z, Durrant L. Glycans as targets for therapeutic antitumor antibodies. Futur Oncol. 2012;8(8):943–60.
5. Fishman PH, Brady RO. Biosynthesis and function of gangliosides. Science (80- ). 1976;194(4268):906–15.
6. Tadashi Yamashita, Ryuichi Wada, Teiji Sasaki, Chuxia Deng, Uwe Bierfreund, Konrad Sandhoff and RLP. A vital role for glycosphingolipid synthesis during development. Proc Natl Acad Sci USA. 1999;96(August):9142-9147.
7. Steenackers A, Vanbeselaere J, Cazet A, Bobowski M, Rombouts Y, Colomb F, et al. Accumulation of unusual gangliosides G Q3 and G P3 in breast cancer cells expressing the G D3 synthase. Molecules. 2012;17(8):9559–72.
8. Wiesner DA, Sweeley CC. Circulating gangliosides of breast‐cancer patients. Int J Cancer. 1995;60(3):294–9.
9. Groux-Degroote S, Rodríguez-Walker M, Dewald JH, Daniotti JL, Delannoy P. Gangliosides in Cancer Cell Signaling. Prog Mol Biol Transl Sci. 2018;156:197–227.
10. Bitton RJ, Guthmann MD, Gabri MR, Carnero AJL, Alonso DF, Fainboim L, et al. Cancer vaccines: An update with special focus on ganglioside antigens (Review). Oncol Rep. 2002;9(2):267–76.
11. Camacho R, del Carmen Arango M, Pérez R, Carr A, Rodríguez E, Fernández LE, et al. Immunotherapy of Advanced Breast Cancer With a Heterophilic Ganglioside (NeuGcGM3) Cancer Vaccine. J Clin Oncol. 2003;21(6):1015–21.
12. M.D. G, M.A. C, G. C, C. V, L. K, R.J. B, et al. Cellular and humoral immune response to N-glycolyl-GM3 elicited by prolonged immunotherapy with an anti-idiotypic vaccine in high-risk and metastatic breast cancer patients. J Immunother [Internet]. 2006;29(2):215–23. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed7&NEWS=N&AN=2006242637
13. A. V-Z, Z. G, V. M, A.M. V, K. P, P. L-L, et al. Immunologic Response Elicited in Breast Cancer Patients Receiving a NeuGcGM3-based Vaccine as Adjuvant Therapy. J Immunother [Internet]. 2017;40(8):289–301. Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L616798404%0Ahttp://dx.doi.org/10.1097/CJI.0000000000000175
14. Labrada M, Dorvignit D, Hevia G, Rodríguez-Zhurbenko N, Hernández AM, Vázquez AM, et al. GM3(Neu5Gc) ganglioside: an evolution fixed neoantigen for cancer immunotherapy. Semin Oncol [Internet]. 2018;45(1–2):41–51. Available from: https://doi.org/10.1053/j.seminoncol.2018.04.003
15. Masoudi A, Elopre M, Amini E, Nagel ME, Ater JL, Gopalakrishnan V, et al. Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res. 2008;28(4 C):2437–42.
16. Mulens V, Marinello P, Carr A, Mazorra Z, Fernández LE. Gangliósidos en la biología e inmunoterapia del cáncer: la experiencia cubana. Cancerelogía. 2009;4(January):155–67.
17. Alonso DF, Fernandez LE, Gabri MR, Guthmann MD, Gomez RE, Gold S, et al. NGcGM3 ganglioside: A privileged target for cancer vaccines. Clin Dev Immunol. 2010;2010.
18. Lopez PH, Schnaar RL. Gangliosides in cell recognition and membrane protein regulation. Curr Opin Struct Biol. 2009;19(5):549–57.
19. Stiban J. Sphingolipids as Signaling and Regulatory Molecules. 2010;688(January). Available from: http://link.springer.com/10.1007/978-1-4419-6741-1
20. Furukawa K, Horie M, Okutomi KI, Sugano S, Furukawa K. Isolation and functional analysis of the melanoma specific promoter region of human GD3 synthase gene. Biochim Biophys Acta - Gene Struct Expr. 2003;1627(2–3):71–8.
21. Regina Todeschini A, Hakomori S itiroh. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim Biophys Acta - Gen Subj. 2008;1780(3):421–33.
22. Yu RK, Tsai Y, Ariga T, Yanagisawa M. Structures , Biosynthesis , and Functions of Gangliosides-an Overview. 2011;544(10):537–44.
23. Kawashima N, Nishimiya Y, Takahata S, Nakayama KI. Induction of glycosphingolipid gm3 expression by valproic acid suppresses cancer cell growth. J Biol Chem. 2016;291(41):21424–33.
24. Wang XQ, Sun P, Paller AS. Ganglioside GM3 blocks the activation of epidermal growth factor receptor induced by integrin at specific tyrosine sites. J Biol Chem. 2003;278(49):48770–8.
25. Wang XQ, Sun P, O’Gorman M, Tai T, Paller AS. Epidermal growth factor receptor glycosylation is required for ganglioside GM3 binding and GM3-mediated suppression of activation. Glycobiology. 2001;11(7):515–22.
26. Oliva JP, Valdés Z, Casacó A, Pimentel G, González J, Álvarez I, et al. Clinical evidences of GM3 (NeuGc) ganglioside expression in human breast cancer using the 14F7 monoclonal antibody labelled with 99mTc. Breast Cancer Res Treat. 2006;96(2):115–21.
27. Tai T, Cahan LD, Tsuchida T, Saxton RE, Irie RF, Morton DL. Immunogenicity of melanoma‐associated gangliosides in cancer patients. Int J Cancer. 1985;35(5):607–12.
28. Toledo, D; Alfonso, S; Santiesteban, E; Aguirre, F; Hernandez, A; Mazorra, Z; Vásquez, A; Crombet, T; Macías A. La Vacuna Anti-idiotipo 1E10: Experiencias en la Inmunoterapia del Cáncer Avanzado. Cancerología [Internet]. 2009;4:143–6. Available from: papers3://publication/uuid/B1C6B35C-2E06-4F56-A3A9-F39BB8479857
29. Cacciavillano W, Sampor C, Venier C, Gabri M, T.G. de Dávila M, Galluzo ML, et al. A Phase I of the Anti-Idiotype Vaccine Racotumomab in Neuroblastoma and Other Pediatric Malignancies. Pediatr Blood Cancer [Internet]. 2015;62(1):2120-21–4. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1751722207003393%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/9840377%0Ahttp://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12134909%0Ahttp://dx.doi.org/10.1016/j.ctrv.2010.02.006%0Ahtt
30. Sanchez L, Muchene L, Lorenzo-Luaces P, Viada C, Rodriguez PC, Alfonso S, et al. Differential effects of two therapeutic cancer vaccines on short- and long-term survival populations among patients with advanced lung cancer. Semin Oncol [Internet]. 2018;45(1–2):52–7. Available from: https://doi.org/10.1053/j.seminoncol.2018.04.005
31. Huang X, Li Y, Zhang J, Xu Y, Tian Y, Ma K. Ganglioside GM3 inhibits hepatoma cell motility via down-regulating activity of EGFR and PI3K/AKT signaling pathway. J Cell Biochem. 2013;114(7):1616–24.
32. Zhou Q, Hakomori SI, Kitamura K, Igarashi Y. G(M3) directly inhibits tyrosine phosphorylation and de-N-acetyl-G(M3) directly enhances serine phosphorylation of epidermal growth factor receptor, independently of receptor-receptor interaction. J Biol Chem. 1994;269(3):1959–65.
33. Yoon S-J, Nakayama K -i., Hikita T, Handa K, Hakomori S -i. Epidermal growth factor receptor tyrosine kinase is modulated by GM3 interaction with N-linked GlcNAc termini of the receptor. Proc Natl Acad Sci. 2006;103(50):18987–91.
34. Song N, Kim SJ, Kwon HY, Son SW, Kim KS, Ahn HB, et al. Transcriptional activation of human GM3 synthase (hST3Gal V) gene by valproic acid in ARPE-19 human retinal pigment epithelial cells. BMB Rep. 2011;44(6):405–9.
35. Kwon HY, Kang NY, Dae HM, Kim KS, Kim CH, Do S Il, et al. Valproic acid-mediated transcriptional regulation of human GM3 synthase (hST3Gal V) in SK-N-BE(2)-C human neuroblastoma cells. Acta Pharmacol Sin. 2008;29(9):999–1005.
36. Santana RB, Arias MC, González CER, Irene R, Goyanes Á, Tamayo YQ, et al. Expresión del gangliósido N-glicolil GM3 en tumores malignos : un estudio inmunohistoquímico empleando el AcM 14F7 . :1–14.
37. Carr A, Mullet A, Mazorra Z, Vázquez AM, Alfonso M, Mesa C, et al. A Mouse IgG 1 Monoclonal Antibody Specific for N -Glycolyl GM3 Ganglioside Recognized Breast and Melanoma Tumors . Hybridoma. 2002;19(3):241–7.
38. Dufresne M, Guneysu D, Patterson NH, Marcinkiewicz MM, Regina A, Demeule M, et al. Multimodal detection of GM2 and GM3 lipid species in the brain of mucopolysaccharidosis type II mouse by serial imaging mass spectrometry and immunohistochemistry. Anal Bioanal Chem [Internet]. 2017;409(5):1425–33. Available from: http://dx.doi.org/10.1007/s00216-016-0076-x
39. Gu Y, Zhang J, Mi W, Yang J, Han F, Lu X, et al. Silencing of GM3 synthase suppresses lung metastasis of murine breast cancer cells. Breast Cancer Res. 2008;10(1):1–12.
40. Satoh M, Ito A, Nojiri H, Handa K, Numahata K, Ohyama C, et al. Enhanced GM3 expression, associated with decreased invasiveness, is induced by brefeldin A in bladder cancer cells. Int J Oncol. 2001;19(4):723–31.
41. Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, Sampor C, et al. Detection of N-Glycolyl GM3 Ganglioside in Neuroectodermal Tumors by Immunohistochemistry: An Attractive Vaccine Target for Aggressive Pediatric Cancer. Clin Dev Immunol. 2011;2011:1–6.
42. Blanco R, Rengifo E, Cedeño M, Rengifo CE, Alonso DF, Carr A. Immunoreactivity of the 14F7 Mab Raised against N-Glycolyl GM3 Ganglioside in Epithelial Malignant Tumors from Digestive System. ISRN Gastroenterol. 2010;2011:1–8.
43. Blanco R, Rengifo CE, Cedeño M, Frómeta M, Rengifo E, Carr A. Immunoreactivity of the 14F7 Mab (Raised against N-Glycolyl GM3 Ganglioside) as a Positive Prognostic Factor in Non-Small-Cell Lung Cancer. Patholog Res Int. 2012;2012:1–12.
44. Roque-Navarro L, Chakrabandhu K, de Leon J, Rodriguez S, Toledo C, Carr A, et al. Anti-ganglioside antibody-induced tumor cell death by loss of membrane integrity. Mol Cancer Ther. 2008;7(7):2033–41.
45. Orlandi R. Engineering mouse monoclonal antibodies for cancer immunotherapy. Year Immunol [Internet]. 1993;7(0014):69–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8372514
46. Orsi G, Barbolini M, Ficarra G, Tazzioli G, Manni P, Petrachi T, et al. GD2 expression in breast cancer. 2017;8(19):31592–600.
47. Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev [Internet]. 2018;69(July):152–63. Available from: https://doi.org/10.1016/j.ctrv.2018.07.004
48. Battula VL, Nguyen K, Sun J, Pitner MK, Yuan B, Bartholomeusz C, et al. IKK inhibition by BMS-345541 suppresses breast tumorigenesis and metastases by targeting GD2+ cancer stem cells. Oncotarget. 2017;8(23):36936–49.
Received: 1 April 2019
Accepted: 28 May 2019
Pasquel-Dávila Daniela S.1, Yánez-Vaca Sabrina A.2, Espinosa-Hidalgo Nicole D.3, Cuadros-Buenaventura Evelin G.4
1,2,3,4 School of Biological Science and Engineering, Yachay Tech University, Urcuquí – Ecuador
1https://orcid.org/0000-0002-4258-6009, 2https://orcid.org/0000-0002-9677-6852, 3https://orcid.org/0000-0003-0316-7757,4https://orcid.org/0000-0002-6579-6252.
*The work was done in an equitable way by the authors.
Corresponding author: [email protected]
