CS 2019.02.01.4
Files > Conference Series > 2019 > Humboldt Kolleg 2019
Bionatura Conference Series Vol 2. No 1. 2019
“Breaking Paradigms: Towards a Multi-, Inter- and Transdisciplinary Science” In commemoration of the 250th Anniversary of Alexander von Humboldt
INVESTIGATION / RESEARCH
The importance of mycorrhiza in agroforestry systems for the stability of ecosystems in semi-arid zones and simple quantification of soil fungi - A study at the Mollesnejta research center for Andean Agroforestry in the valley of Cochabamba, Bolivia
Benjamin Ungar¹, Jakob Vögerl², Nora Medrano Mercado³,*Noemi Stadler-Kaulich4
available in: http://dx.doi.org/10.21931/RB/CS/2019.02.01.4
ABSTRACT
Soil erosion and poor production conditions in developing countries are a major problem for local primary care. It is therefore even more important to ensure a functioning and stable ecosystem from which agricultural plants profit, too. Trees have found a brilliant way to gather enough nutrients for their survival. They enter a symbiosis with special types of fungi, the so-called mycorrhiza. This leads to more resistance of the crops, especially against drought. There is a method that can be used with the simplest means to prove that mycorrhizae are found in tree roots and thus assure improvement of soil fertility.
Keywords: Mycorrhiza; Agroforestry; Soil fertility
INTRODUCTION
Improved cultivation conditions thanks to mycorrhiza
In developing countries, the soil is often severely eroded, such as in Africa or in large parts of South America. In these areas, it is difficult to farm and in more extreme cases this leads to hunger, malnutrition and secondary diseases, or even death. In such regions, people can greatly benefit from supplementing arable crops with mycorrhiza-promoting tree and plant species. The positive effects of mycorrhiza on cultivation and ecosystems are explained below.
Mycorrhiza is a group of fungi in the soil that forms a symbiotic connection with plant roots, whereby the fungus facilitates the nutrient uptake for the plant and the host in turn supplies carbohydrates to the fungus 1. However, the fungi do not only supply the trees with the most important plant nutrients, such as phosphorus and nitrogen, but also water. This is related to the fact that the mushroom roots, so-called hyphae, have a much smaller diameter (up to 1 μm) than plant roots and can, therefore, penetrate the smallest cavities and pores and absorb nutrients much more efficiently 2. In addition, the root network enlarged by the fungus also leads to overall improved nutrient uptake in the soil 3. Mycorrhiza can be classified into two main groups:
Ectomycorrhiza
Ectomycorrhiza surrounds the fine roots of a plant with their hyphae in reticular form without penetrating the rhizome layer of their symbiosis partner. Sometimes, however, it can happen that the outer layers of cells are permeated by the fungus hyphae 4. The ectomycorrhizae occur mainly in trees and shrubs of the temperate zone, but also in subtropical and tropical plants 2.
Endomycorrhiza
The fungal mycelium of endomycorrhiza is embedded in the root tissue. The most important symbiotic form of endomycorrhiza is the vesicular-arbuscular mycorrhiza (AM or VAM), although only about 160 different species are known in this subgroup. In this symbiotic form, the fungus hyphae penetrate the rhizome layer of the plant and grow in and between the root cells 5,3. In this case, the fungus forms root-like structures (so-called arbuscules) and vesicles (bladder-like structures) in and outside the cortex cells (see Figure 1). At the arbuscules, an exchange of nutrients for sugar takes place, whereas fats and oils are stored in the vesicles 2.
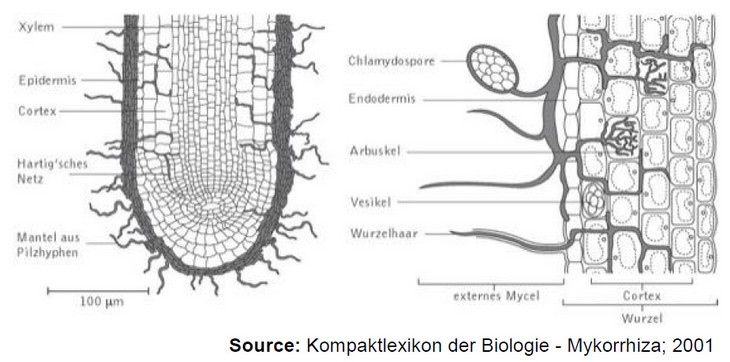
Figure 1: On the left is a schematic representation of ectomycorrhiza and the netlike structure around the plant root. On the right is an example of endomycorrhiza with vesicular (vesicular) and arbuscular (root-like) formations 2.
In total, there are several thousands of symbiotic fungi species. The number of ectomycorrhiza fungi species is estimated at about 5,000 6. It is believed that most terrestrial plants are mycorrhizal, but not every tree species can enter a symbiosis with mycorrhiza 3. On the one hand, this could be due to a dense layer of the rhizome that is unable to penetrate every arbuscular fungus species 7. On the other hand, this could also be since some plant species do not tolerate the colonization by AM and prevent it in different stages of development 8. However, plants can recognize the fungi by certain signal substances and due to altered gene expressions in any case. This is also important because it allows the plant to differentiate between pathogenic and beneficial organisms 10.
Mycorrhiza promote more resilient ecosystems
Mycorrhizae have been well researched for several decades. There are already many studies that show that mycorrhizae result in increased and healthier growth of crop plants 11,12. The significantly improved resistance of plants and cultivation systems in symbiosis with mycorrhizal fungi could be observed 13. This is true for biotic factors (e.g., pathogen infestation by fungi) 14 as well as abiotic factors (e.g., drought stress) that would be more detrimental without such plant-fungi symbiosis 9,15,16.
Biodiversity is also positively influenced by mycorrhizal fungi. Although competitive interactions between different plant species play a role in the development of an ecosystem, little attention has been given to the effects of interactions between microbes and plants for a long time. In a study (2002) 17 a totally new approach was tested: plant seeds were collected and AM fungi isolated. When the plant seeds of 11 different plants without mycorrhiza were exposed in a greenhouse, it was found that 8 of these plants in the grassland simulating microcosm were almost completely dependent on the presence of mycorrhiza. Furthermore, it became clear that in this environment without fungus, only the species produced the most biomass, which also did not enter a symbiosis with mycorrhiza.
The results showed that the presence of mycorrhizal fungi is required to maintain a basic level of plant biodiversity and that, at low AMF diversity, an alteration in the composition and number of AMF taxa can lead to large fluctuations in the structure and composition of the plant community. 17
Due to the presence of mycorrhiza, the ecosystem becomes more stable in addition to the improved nutrient uptake of the plants and plant diversity on the area also increases to a certain extent.
The targeted use of this knowledge in the promotional form of mycorrhizal fungi is favored to reduce fertilizer costs and improve overall plant health. The greening of arid or salty areas, areas contaminated with heavy metals or waste dumps, where stones from the mining industry were deposited, are potential application sites for an upgrade with mycorrhizal fungi 18. Some types of mycorrhiza are even already commercially marketed 34.
Most farmers in developing countries cannot afford those mycorrhiza kits, but so-called pioneer plants native to the area can be used as a substitute as most of them are already mycorrhizal 19. The advantage of mycorrhiza is that they can be found in all climatic zones of the world and many plants can form a symbiosis 18. Thus, trees, especially in forest-like communities, promote mycorrhiza networks by serving as starting points for the mycorrhizal fungi. This is one of the many benefits that are exploited in agroforestry systems.
Agroforestry and its contribution to nature
Agroforestry systems and their positive impact on the soil and the high erosion protection against wind, dehydration and nutrient flushing (especially on slopes) have been widely recognized by now. With this method, the progress of desertification can be slowed down or reversed especially in densely populated, humid to (sub-) tropical regions 20. From the perspective of land improvement, such use of trees is optimal for the regeneration of tired soils, as well as for numerous ecosystem services, such as water filtration 21. This combination of tree rows and agricultural purposes is also of interest regarding biodiversity, soil fertility, yield and ecosystem stability 22. But what exactly is agroforestry?
Agroforestry refers to land use systems that combine both shrubs (trees or bushes) and arable crops and/or animal husbandry, which leads to positive ecological and economic interactions 23. Simplified, in addition to common crops such as cereals, (row-shaped) perennial trees such as fruit trees/nut trees or other useful woods are established (see figure 2).

Figure 2. Own footage, Benjamin Ungar, 2019; Mollesnejta
Through such a windbreak not only wind erosion is prevented, but also improves the growth conditions for the arable crops. Namely, near a natural windbreak, higher and longer-lasting soil moisture and dew formation occur (see Figure 3). In addition, it should be noted that the crop species and the distance to the windbreak hedge plays a role in the yield 24.
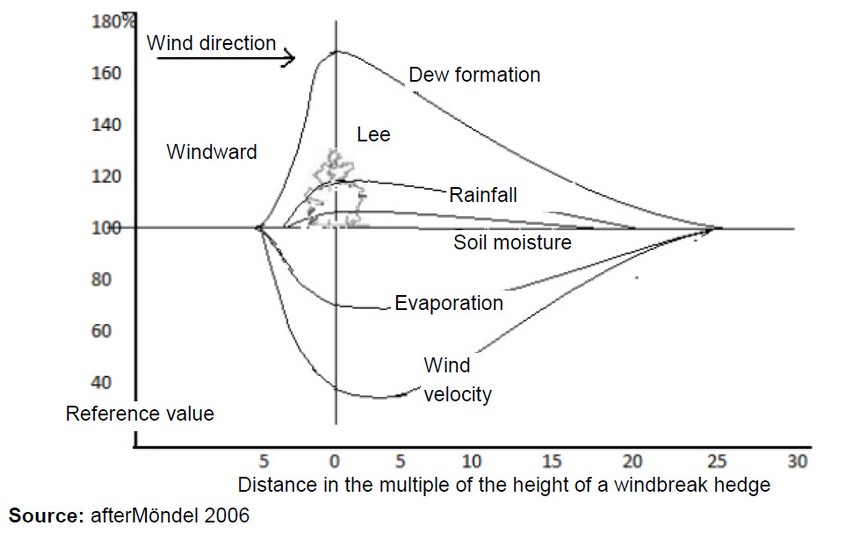
Figure 3: Source: after Möndel 2006. Representation of the course of individual climate values in relation to a windbreak hedge, the cross-sectional view (Translated from German by B. Ungar).
Furthermore, the inclusion of trees in an agricultural system increases the biodiversity, which makes it possible to use the wood strips for closing gaps in biotope composite systems. This, in turn, means that threatened animal and insect species find shelter there, so even predators of pests can do so. It is well-known that a lack of biodiversity increases the pest pressure as well as the occurrence of plant diseases 25. Thus, increasing biodiversity to some degree means that fewer amount of pesticides are needed than in traditional cropping systems, which negatively affect soil organisms, and slow down soil regeneration 26,27.
Also, this form of land use can buffer any environmentally harmful effect in sensitive biotopes of the environment, for example by nutrients from fertilizers leaching into the groundwater and surface waters are enormously mitigated. In addition, woody strips contribute to climate relief by fixing more carbon. The yield on the land also increases with a certain amount of biodiversity compared to monoculture cultivation 21,28,29.
By pruning the trees, the trimmed biomass can be applied to the field as a natural fertilizer in chopped form, closing the nutrient cycle and reducing fertilizer requirements over time. A general advantage of trees is that their root system allows them to penetrate deeper soil layers to transport nutrients upwards and make them accessible to the crops with their fine roots. In addition, this also applies to deeper groundwater reservoirs. At unfavorable times, however, a short-term competition between the plants could occur 30. In summary, it can be said that agroforestry systems provide almost countless advantages as a land use system.
Trees serve, as already mentioned, as starting points of mycorrhiza. When trees enter such a connection with the fungi, the other plants in the vicinity, such as crops, benefit from an enlarged root network 31. Because the root system is greatly expanded by the fungi, which allows the plants to communicate with other actors or to send nutrients to other plants when for some reason they should not have enough for themselves 32. This increases productivity as well as the stability and robustness of an ecosystem.
Over time all these factors lead to an increase in revenue for farmers, as more crops can be cultivated on less land and in the following years harvested trees provide fruits, and fuelwood 33. Finally, yields remain stable and do not decline as rapidly as traditional crops, as such permaculture systems also minimize the leaching of nutrients 34.
Experimental procedure for mycorrhiza counting on Mollesnejta
At the end of November 2018, an agroforestry plot was implemented for test purposes as part of this experiment. In total, three rows of trees were planted with 3.8 m space in between and with 0.75 m to the next plant within the row. In total, about 150 trees were planted. One half of the experimental area is Tagasaste (Cytisus proliferus), the other half is Ch'acatea (Dodonaea viscosa). The two species were separated by an uncovered open space of 15 m (see Figure 4)
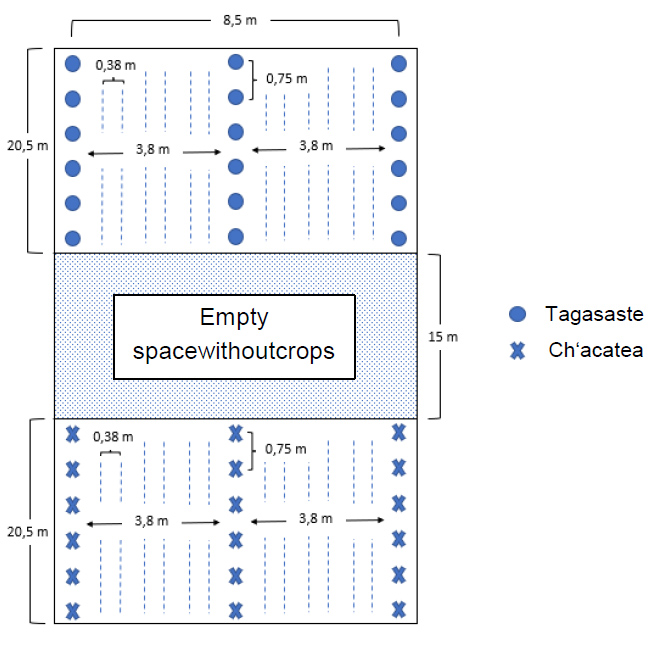
Figure 4: The trial area at Mollesnejta
The plot was designed to find out how the number of mycorrhiza populations on the tree roots in this agroforestry plot will change over time and how this will impact the crop yields. Root samples were first taken for analysis in the laboratory. It should also be determined if there is a simple, quick and inexpensive method for detecting mycorrhiza in tree roots and at the same time allowing for a count of symbiotic fungal hyphae.
Experimental setup for mycorrhiza counting
For counting, the mycorrhizal roots must first be stained before they become visible under the microscope. This was the result of a study by Vierheilig (1998)35 Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi which now has been supplemented by concrete ratios and sizes for this procedure.
This guarantees that in the future comparable trials can be carried out for the Mollesnejta experimental site in Cochabamba. With the method described in more detail below, meaningful results could be obtained with the simplest means and chemicals: the visualization of the mycorrhizal hyphae in the root samples under the microscope was contrasted with the root parts even at a 10-fold magnification. The equipment for the experiment was provided by the "Universidad Mayor de San Simón" (UMSS) in Cochabamba. The following materials were used for this experiment:
Biological material
Five root samples each (eight weeks ago planted, about 6 months old trees of the species Ch'acatea (Dodonaeaviscosa) and Tagasaste (Cytisusproliferus) from the upper soil layer (up to 30 cm)
Chemicals
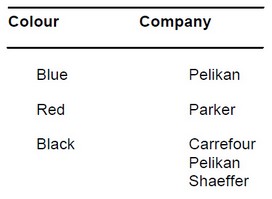
Ink (black, company Pelikan)
Vinegar (5 percent solution of household vinegar from the supermarket)
10% Potassium hydroxide (KOH)
Note: Not all ink brands and colors are suitable for staining mycorrhiza. Other inks that were used by Vierheilig et al. (1998) are:
Experimental procedure
First, the small plant roots were washed thoroughly with sieve and tap water to eliminate all soil particle. Then each root sample was placed in a test tube and completely covered with 10% of potassium hydroxide solution. The test tube was then held over the flame of a Bunsen burner with the help of a tube clamp. The solution was cooked for 15 minutes, due high risk of boiling delays caution is needed.
Subsequently, the individual samples were rinsed with distilled water three times in the test tube by gentle shaking. After that, the second phase began. For this purpose, the color solution was prepared (100 mL household vinegar and 2 mL black ink from Pelikan). The root samples, which had previously been heated in potassium hydroxide solution, were then boiled for three minutes in this dye solution using the Bunsen burner. Also, during that step boiling delays may occur.
Finally, the root samples in the test tubes were rinsed three times with distilled water (mixed with a few drops of vinegar) to rinse off excess ink. The pouring was facilitated by pouring the water from the test tube through a sieve so that no root material was lost. The rinsing process needs to be done very cautiously to not destroy the root material.
Evaluation and conclusion
By boiling in the 10% potassium hydroxide solution, the pigments are withdrawn from the roots. Therefore, under the microscope, it is easy to see the ink-colored mycorrhiza at 10x magnification and the grown fungal network between the individual cells of the tree roots (see figure 5)). It should be noted here that one can only recognize mycorrhizal hyphae under the microscope if the root appears completely decolorized, i.e. white. Roots that are still dark after the potassium lye are therefore not suitable for this experiment.
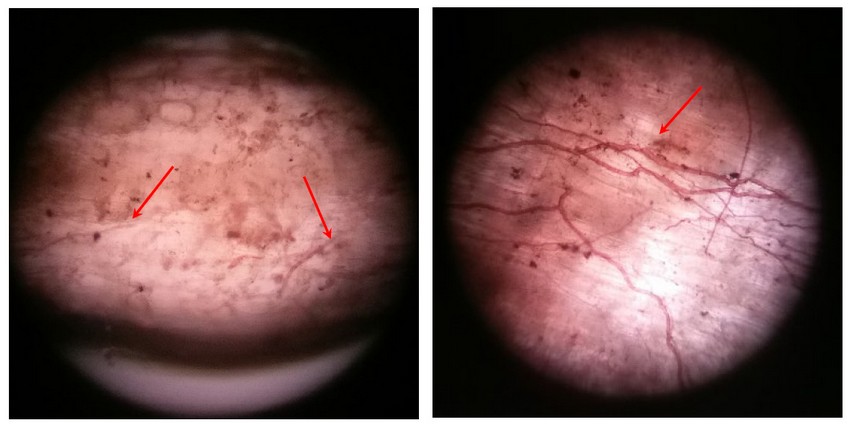
Figure 5: Microscopic view of colored vain-like mycorrhiza networks in tree root samples. 10x magnification. On the left, you can see the roots of a young Ch’acatea and one of a 20-year-old Ch’acatea on the right.
All hyphae strands of a sample were counted under the microscope at the end, which was visible within a microscope section (0.20 mm²) at 10x magnification. At random, a group of 2-3 spots was selected, which presented an intact piece of the root so that one could count the mycorrhizal hyphae. Because the root pieces are not straight, it was only possible to estimate what percentage of the area (0.20 mm²) was occupied by a root fragment. By this approximation, the root area could be calculated. Subsequently, all main hyphae were counted and the sum of all branching on this main strand. This is illustrated by an example in re 5.
Since the root samples came from very young trees that had been planted only a few weeks earlier, it was assumed that there were no or very few mycorrhizal hyphae in these tree roots. This assumption was confirmed in this experiment. Five roots of young Tagasaste trees and Ch'acatea trees were examined under the microscope. Ten (countable) microscope sections have been examined at 10x magnification per plant species. Note: At 40x magnification, many more mycorrhizal hyphae have been detected, but counting at this magnification was not feasible, or only with a tremendous amount of time, and was therefore decided to enumerate at 10X magnification, not least to be able to achieve comparable results to the test. The results are shown in Table 1:
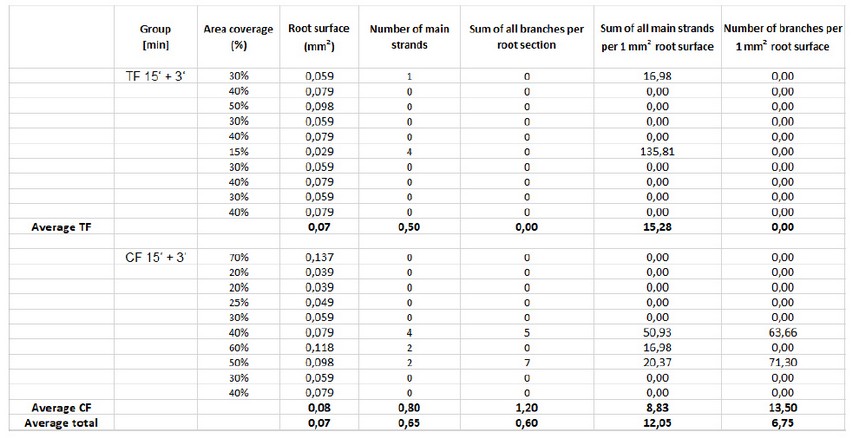
Table 1: Counting of mycorrhizal hyphae visible at 10x magnification. "TF" stands for Tagasaste of the test area and "CF" for Ch'acatea of the test area. The counted hyphae main strands and all branches were extrapolated to the conclusion in the last two columns on each one mm2.
On the one hand, the mycorrhizal hyphae in these roots were present in lower numbers, on the other hand, they were not as clearly visible as in the test experiment (see Figure 5). This may be since the fungal cells were still in the developmental stage and therefore were subjected to different properties than mature fungal hyphae.
CONCLUSION
We are curious how the mycorrhiza values will develop over the next few years and hope that the topic of agroforestry will reach more and more people. Looking around the neighborhood in the small village of Combuyo, it quickly becomes clear that the Mollesnejta estate is a green island in the middle of degraded soil. All that is needed is time and the proper care of the plants using natural tools such as mycorrhiza - without agrochemicals.
REFERENCES
1. Smith SE, Read DJ. Mycorrhizal symbiosis. 3rd ed. Amsterdam, Boston: Elsevier/Academic Press; 2008. Available from: URL: http://search.ebscohost.com/login.aspx?direct=true&scope=site&db=nlebk&db=nlabk&AN=230784.
2. Ge. Kompaktlexikon der Biologie - Mykorrhiza; 2001 [cited 2019 Jan 28]. Available from: URL: https://www.spektrum.de/lexikon/biologie-kompakt/mykorrhiza/7904.
3. Stahr K, Kandeler E., Hermann L, Streck T. Bodenkunde und Standortlehre; 2008 [cited 2019 Jan 28].
4. Marmeisse R., Guidot A., Gay G., Lambilliotte R., Sentenac H., Combier J.-P., Melayah D., Fraissinet‐Tachet L., Debaud J.C. Hebelomacylindrosporum– a model species to study ectomycorrhizal symbiosis from gene to ecosystem - Marmeisse - 2004 - New Phytologist - Wiley Online Library; 2004 [cited 2019 Jan 26]. Available from: URL: https://nph.onlinelibrary.wiley.com/doi/full/10.1111/j.1469-8137.2004.01148.x.
5. Kuhn H, Kloppholz S, Helber N, Requena N. ArbuskuläreMykorrhiza: Zuckerbrot und Peitsche. Biospektrum 2012 [cited 2019 Jan 26]; 18(2):149–51. Available from: URL: https://link.springer.com/content/pdf/10.1007%2Fs12268-012-0155-2.pdf.
6. Graf F. Was wäre der Baum one Mykorrhiza, ETH Zürich Research collection, 2002. Available from: URL: https://www.research-collection.ethz.ch/bitstream/handle/20.500.11850/146161/eth-25107-01.pdf?sequence=1&isAllowed=y [cited 2019 Jan 31]
7. Bonfante P PS. Strategies of arbuscular mycorrhizal fungi when infecting host plants 1995.
8. Morte A, Varma A. Root Engineering. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. ( vol 40).
9. Harrison MJ. Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 2005; 59:19–42.
10. Maillet F, Poinsot V, Andre O et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 2011.
11. Brechelt A: Wirkung verschiedener organischer Düngemittel auf die Effizienz der VA‐Mykorrhiza 1987.
12. Karagiannidis N., Nikolaou N., Mattheou A. Wirkung dreier VA-Mykorrhizapilze auf Ertrag und Nahrstoffaufnahme von drei Unterlagen und einer Tafeltraubensorte. National Agricultural Research Foundation, Soil Science Institute of Thessaloniki, Greece 1994 [cited 2019 Jan 28].
13. Bethlenvalvay G.J. SH, editor. Arbuscular mycorrhizas and agrosystem stability. In: Impact of arbuscular mycorrhizas on sustainable agriculture and natural ecosystems: Birkhäuser, Basel; 1994.
14. Paszkowski U. A journey through signaling in arbuscular mycorrhizal symbioses; 2006 [cited 2019 Jan 28]. Available from: URL: https://nph.onlinelibrary.wiley.com/doi/full/10.1111/j.1469-8137.2006.01840.x.
15. Strack D, Fester T, Hause B, Walter MH. Die arbuskuläre Mykorrhiza: Eine unterirdische Lebensgemeinschaft. Biologie in unserer Zeit 2001; 31(5):286–95.
16. van der Heijden, M. G. A., Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998 [cited 2019 Jan 29]; 396(6706):69–72.
17. Geue H. Molekularbiologische Untersuchungen zum Nachweis arbuskulärer Mykorrhizapilze bei Wildpflanzenpopulationen landwirtschaftlicher Nutzflächen 2002.
18. Das unterirdische Geheimnis von Steinpilz und Trüffel; 2015 [cited 2019 Jan 29]. Available from: URL: https://www.waldwissen.net/wald/pilze_flechten/lwf_mykorrhiza/index_DE.
19. Young A. Agroforestry for soil management. Wallingford: CAB INTERNATIONAL; 1997.
20. Jose S. Agroforestry for ecosystem services and environmental benefits: an overview. Agroforest Syst 2009; 76(1):1–10.
21. Haselwandter K, Bowen GD. Mycorrhizal relations in trees for agroforestry and land rehabilitation. Forest Ecology and Management 1996 [cited 2019 Jan 30]; 81(1-3):1–17.
22. Nair PK. An introduction to agroforestry // Working with mycorrhizas in forestry and agriculture. Dordrecht: Kluwer Acad. Publ; Australian Centre for International Agricultural Research; 1993. Available from: URL: http://www.loc.gov/catdir/enhancements/fy0822/92046550-b.html.
23. Gesellschaft für Pflanzenbauwissenschaften e. V., editor. Anforderungen an den Pflanzenbau in einer sich urbanisierenden Welt: Kurzfassungen der Vorträge und Poster; 2017.
24. Charles R., Cholley E., Frei P., Mascher F. Krankheiten beim winterweizen: Einfluss des Anbausystems und Auswirkungen auf den Ertrag. Agrar Forschung Schweiz 2011; (6).
25. van der Sluijs JP, Amaral-Rogers V, Belzunces LP, van Bijleveld Lexmond MFIJ, Bonmatin J-M, Chagnon M et al. Conclusions of the Worldwide Integrated Assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environ Sci Pollut Res Int 2015; 22(1):148–54.
26. Liess M. Vom Labor ins Freiland — Aspekte der Bewertung von Pflanzenschutzmitteln (PSM) in der Umwelt. UWSF - Z Umweltchem Ökotox 1997; 9(1):1–2.
27. Unseld R, Reppin N, Eckstein K, Zehlius-Eckert W, Hoffmann H, Huber T. Leitfaden Agroforstsysteme: Möglichkeiten zur naturschutzgerechten Etablierung von Agroforstsystemen: BN; 2011.
28. Paul C, Knoke T., Weber M. Aufforstung in Panama: Agroforstsysteme zur Verbesserung der Wertholzproduktion in den Tropen 2014 [cited 2019 Jan 30]. Available from: URL: https://mediatum.ub.tum.de/doc/1233973/file.pdf.
29. Bender B. et al. Moderne Agroforstsysteme mit Werthölzern: Leitfaden für die Praxis. 1.th ed.; 2009 [cited 2019 Jan 31].
30. Honegger A., Wittwer R. et al. Auswirkungen langjähriger biologischer Landwirtschaft 2014 [cited 2019 Jan 30].
31. OEL_164_20092012 [cited 2019 Jan 31].
32. Plieninger T. et al. Chancen von Agroforstwirtschaft für die ländliche Entwicklung: Ergebnisse aus dem AGROFORWARD-Projekt 2018.
33. Nair VD, Nair PKR, Kalmbacher RS, Ezenwa IV. Reducing nutrient loss from farms through silvopastoral practices in coarse-textured soils of Florida, USA. Ecological Engineering 2007 [cited 2019 Jan 31]; 29(2):192–9.
34. e.g. on this webpage: https://inoq.de/produkte-service/mykorrhiza-produkte/?lang=en.
35. Vierheilig H. et al., 1998; Centre de Recherche enbiologieforestière, Université Laval, Québec.
Received: 3 March 2019
Accepted: 28 May 2019
Benjamin Ungar¹, Jakob Vögerl², Nora Medrano Mercado³, *Noemi Stadler-Kaulich4
Former Student of Technische Universität München, Germany (1), University of Applied Sciences Rhine-Waal, Kleve, Germany (2), Lab. De Chagas e Inmunoparasitologia-Depto. De Biologia- Fac. de Ciencias y Tecnologia-Univ Mayor de San Simon, Cochabamba-Bolivia (3),Mollesnejta - Andean Agroforestry Center, Cochabamba-Bolivia(4)
